Abstract
In the present article, the efficiency of biological treatment of landfill leachates was evaluated by implementation of physicochemical characterisation and a complex toxicity assessment. An array of toxicity tests using bacterium Vibrio fischeri, alga Desmodesmus subspicatus, crustacean Daphnia magna, and embryo of fish Danio rerio, as well as unconventional methods using biochemical biomarkers (protein content, enzymes cholinesterase, and glutathione-S-transferase), were employed. Toxicity of leachates varied depending on the season of collection in relation to their different physicochemical characteristics. Uncommon effects of leachates on organisms, such as hormetic-like increases of algal growth and reproduction of daphnids, were identified. New approaches using the activities of enzymes were found unsuitable for routine hazard assessment of leachates. Although physicochemical parameters and toxicity decreased significantly after biological treatment, the effluents did not meet the demands of the current Slovenian legislation; thus, the existing biological treatment was found inappropriate. The development of advanced treatment techniques for landfill leachates is thus encouraged.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Land-filling is still a most common way of municipal solid-waste treatment (Kjeldsen et al. 2002; Thomas et al. 2009). According to EUROSTAT statistics from the year 2007, approximately 106 million tons of municipal waste was land-filled in the whole European Union; among these in Slovenia were 690,000 tons, yielding 522 kg/capita in the whole European Union region and 441 kg/capita in Slovenia alone (Eurostat 2010). Leachates from such landfills, which are generated by excess rainwater percolating through waste layers, represent a grave environmental threat to the environment because they represent a major source of contamination to groundwater and surface waters (Isidori et al. 2003).
Landfill leachates are a complex mixture of inorganic and organic substances. Kjeldsen et al. (2002) summarized the most common constituents of such leachates based on several chemical analyses performed on landfill leachates from different origins. Among these are dissolved organic matter, inorganic macrocomponents (e.g., Ca2+, Mg2+, K+, Fe2+, etc.), heavy metals, and xenobiotic organic compounds originating from household or industrial chemicals (e.g., aromatic hydrocarbons, phenols, chlorinated aliphatics, pesticides, etc.) (Baun et al. 2004). In general, they may contain high concentrations of dissolved organic matter and inorganic macrocomponents, but these may vary considerably depending on the stabilization stage of the landfill and seasonal variations. Many papers have reported high concentrations of ammonia (500 to 2000 mg l−1), which is formed mainly by the decomposition of proteins. The concentrations of heavy metals in leachates are variable and usually fairly low (Jensen and Christensen 1999; Kjeldsen et al. 2002).
Currently biological treatment still remains the most widely applied technique to treat landfill leachates; however, it has proven not to be the most successful one (Goi et al. 2010). Despite the fact that the combined approach using physicochemical techniques and toxicity assessment to evaluate the efficiency of the treatment is now well recognized (Kjeldsen et al. 2002; Thomas et al. 2009), this approach has rarely been implemented in routine practice. In addition, toxicity assessments in practice rarely include a battery of tests with a variety of test species from different trophic levels and various exposure periods, which could lead to misevaluation of treatment efficiency (Bernard et al. 1996).
In ecotoxicity studies, new tools to identify the hazard of chemicals are constantly being developed (Jemec et al. 2010). Among these are also biochemical biomarkers, which include the activities of enzymes and are generally considered a more sensitive and sometimes more specific measure of toxic exposure and effect than survival or growth. Among more commonly applied enzymes are glutathione S-transferase (GST) and cholinesterase (ChE). The former is involved in cellular detoxification of xenobiotics, including organophosphorus pesticides, as well as defense processes against oxidative stress (Booth and O’Halloran 2001). In case of exposure to such substances, GST activities are presumably increased. ChEs involved in neurotransmission and are specifically inhibited by organophosphorus and carbamate pesticides as well as metals and detergents. The protein content of an organism has also been used as a biomarker of chemical exposure and reflects the entire physiological state of the organism (Knowles and McKee 1987). These new approaches have been commonly used to study the effects of pure chemicals on organisms (Jemec et al. 2007a, b, 2010), but they have not often been applied for routine identification of the hazard of wastewaters.
In the present study, the toxicity of municipal landfill leachates before and after biological treatment was assessed. The integrated toxicity approach using an array of tests with organisms from different trophic levels (e.g., bacterium Vibrio fischeri, alga Desmodesmus subspicatus, crustacean Daphnia magna, and fish Danio rerio) and physicochemical characterization was applied. In addition, newer techniques, such as the use of biochemical biomarkers, were also implemented and will be discussed. The aim of this study was (1) to evaluate the treatment efficiency of an existing biological wastewater treatment plant and the suitability of landfill leachates to be discharged into the environment and (2) to estimate the sensitivity and suitability of each toxicity test for hazard identification of landfill leachates.
Materials and Methods
Wastewater Samples
The investigated leachates originate from a regional municipal landfill, which is divided in two parts. The first part covers 10,800 m2 and was closed in 2006 after 20 years of waste disposal. At that time, no recycling and waste-separation practice had been adopted. Since then, waste has been deposited to a new part of the landfill, which has been estimated to contain 160,000 m3 of waste by the time it closes in 2013. The collection area covers approximately 35,000 inhabitants. Leachates from both parts of the landfill are mixed before treatment in an egalization basin (36% [v/v] of leachate from the old part of the landfill and 64% [v/v] leachate from the new part). Mixed leachate is currently settled in a primary sedimentation tank, whereas biodegradation takes place in the second sequenced batch reactor (SBR). Samples of the leachates were collected in high-density polyethylene containers (3 l) (1) from drainage pipes that lead to an egalisation basin at the existing SBR treatment plant and (2) from the existing treatment plant after treatment before their release into a nearby stream.
The samples were collected during different periods of the year: November 2007 and March 2008. Each time, influent to the wastewater treatment plant and effluent from the plant were taken. The samples were stored in the dark at 4°C for analyses, which was performed within 3 days, or frozen at −18°C until use. All toxicity tests were performed using the samples without any pretreatment. The samples were designated as follows: sample 1 = influent from November 2007; sample 2 = effluent from November 2007; sample 3 = influent from March 2008, and sample 4 = effluent from March 2008.
Physicochemical Characterisation
Analytical control of leachates characterization and monitoring of treatment efficiency included pH, biochemical oxygen demand (BOD5), chemical oxygen demand (COD), dissolved organic carbon (DOC) (Shimadzu TOC 5000A Analyzer 1998), and nitrogen as Kjeldahl nitrogen and ammonium. Phosphate, nitrite, nitrate, and chloride were determined in the filtered samples by chemically suppressed ion chromatography (DIONEX 4000) using a 0.2-μm filter. International Organisation for Standardisation (ISO) standards used are listed in Žgajnar Gotvajn et al. (2009).
Toxicity Tests
Conventional Toxicity Tests
Toxicity tests were performed according to the protocols and ISO standards, which were previously described in detail by Tišler et al. (2009) and Jemec et al. (2007a). ISO standards are listed in these publications; therefore, they are omitted from this section. At least one preliminary and two definitive trials for each test species were conducted.
Luminescence of Vibrio fischeri NRRL-B-11177 was measured using a LUMIStox 300 luminometer (Dr. Lange GmbH, Düsseldorf, Germany). Reactivated liquid-dried bacteria were exposed to the following concentrations of all four samples: 1.56, 3.13, 6.25, 12.5, 25, 50, and 80% (v/v). The percentage of luminescence inhibition after 30 min was calculated for each concentration relative to the control.
Water fleas D. magna Straus 1820 were obtained from the Institut für Wasser, Boden und Lufthygiene, des Umweltbundesamtes (Berlin, Germany). They were cultured in 2.5 l modified M4 media at 21 ± 1°C and 16:8-h light-to-dark regime (1800 lux) with a diet of alga D. subspicatus Chodat 1926 corresponding to 0.13 mg carbon/daphnia/day. Neonates <24 h old, derived from the second to fifth brood, were exposed to the following concentrations: 2, 2.5, 3, 3.5, and 4% (v/v) of sample 1; 14, 16, 18, 20, and 25% (v/v) of sample 2; 4, 4.2, 4.6, 4.8, and 5% (v/v) of sample 3; and 8.6, 12.1, 18.1, 25.3, and 35.4% (v/v) of sample 4.
Chronic (21-day) toxicity to daphnids was evaluated using a semistatic exposure system. Individual daphnids < 24 hours old were placed in 50 ml test solution; 10 test containers/ concentration and a control were prepared. The criteria used to evaluate reproduction after 21 days was the number of neonates per adult. The animals were fed daily a diet of D. subspicatus (0.13 mg carbon/daphnia/day), and the newly born neonates were counted and removed. The immobility of adult daphnids during 21 days was also monitored. The concentrations tested were as follows: 0.0007, 0.0141, 0.0281, 0.0563, 0.1125, and 0.225% (v/v) of sample 1; 0.938, 1.875, 3.75, and 7.5 15% (v/v) of sample 2; 0.31, 0.63, 1.25, 2.5, and 5% (v/v) of sample 3; and 1.56, 3.13, 6.25, and 12.5% (v/v) of sample 4.
The green, unicellular algae D. subspicatus Chodat 1926 (CCAP 276/22; Culture Collection of Algae and Protozoa, Cumbria, United Kingdom) were cultured according to Jaworski (Tišler et al. 2009) on an orbital shaker at 150 rpm (alternating 15 min of agitation and rest) at a constant room temperature of 21 ± 1°C and fluorescent illumination (4000 lux). The algal density and growth rate were determined after 72 h by counting the algal cells in a Bürker counting cell. Growth was also estimated by determining the amount of chlorophyll-a according to ISO 10260 (1992).
The effect of leachate samples on the growth of algae D. subspicatus during the daphnid-reproduction test was also investigated. Different concentrations of sample 1 (shown in Fig. 5) were prepared in duplicates in the medium used for the daphnid-reproduction test. The experiment lasted for 2 days. Daphnids were not present to avoid the removal of algae due to feeding. Each day the same amount of algae as in the reproduction test (0.13 mg carbon) was added to 50 ml medium. Afterward, the algae were filtered through a 0.2-μm filter, and chlorophyll-a was determined as described previously.
Breeding zebrafish to obtain eggs was performed as described in Kammann et al. (2004) and Tišler et al. (2009). Fertilized eggs in the four- to eight-cell stages were placed in 24-well plates; each well contained 1 mL synthetic ISO medium (Tišler et al. 2009) with different concentrations of samples: 2, 2.5, 3, 3.5, and 4% (v/v) of sample 1; 10, 15, 20, 25, and 30% (v/v) of sample 2; 5.5, 6, 6.5, 6.7, 6.8, and 7% (v/v) of sample 3; and 23% through 27% (v/v) of sample 4. For each experiment, a control containing only synthetic ISO medium was prepared. After 24 and 48 h of exposure at 26°C, lethal malformations, i.e., egg coagulation, missing heartbeat, missing somites, and missing tail detachment from the yolk sac, were evaluated (Tišler et al. 2009).
Biochemical Biomarkers
Biochemical biomarkers, e.g., the activities of ChE and GST, were assessed in adult daphnids chronically exposed to landfill leachates. After exposure, four to five adult daphnids (depending on the amount surviving) per concentration were combined into one enzyme sample; thus, two samples were prepared for each concentration. The preparation of homogenates for enzyme measurements was as described in Jemec et al. (2007a). Samples were snap frozen in liquid nitrogen and stored at −80°C until measurements were taken.
ChE and GST activities were determined using microtiter plates (PowerWave XS; Bio-Tek Instruments) as previously described by Jemec et al. (2007b). 1-Chloro-2,4-dinitrobenzene was used as substrate for GST. One enzyme unit (EU) was determined as the amount of ChE that hydrolyses 0.01 nmol acetylthiocholine min−1 mg protein−1 (ε412 = 13600 M−1 cm−1) and the amount of GST that conjugates 1 nmol reduced glutathione min−1 mg protein−1 (ε340 = 9600 M−1 cm−1). These enzyme units were chosen to facilitate comparison of both enzyme activities for each sample.
Protein concentration was determined using a BCAProtein Assay Kit (Pierce, Rockford, IL). Average protein content per animal was expressed in mg protein ml−1 per animal−1 (the concentration of proteins in homogenate was divided by the number of animals combined in the homogenate).
Statistical Analyses
Linear regression analysis was used to calculate the EC x /IC x values for V. fischeri, D. subspicatus, and D. rerio. The 30-min IC20, IC50, and IC80 values for luminescence bacteria were calculated using linear regression analysis supported by computer software (Dr. Lange LUMISsoft 4 software, version 1.001, Düsseldorf, Germany). The percentages of zebrafish embryos with lethal malformations (48-h LC10, LC50, and LC90) and inhibition of algal growth (72-h IC10, IC50, and IC90) were calculated using Excel software (Microsoft). Algal growth was calculated according to Tišler et al. (2009). The percentages of immobile daphnids were analysed with probit analysis to determine the effective EC10, EC50, and EC90 values with corresponding 95% confidence limits. The results were also expressed as toxic units (TUs). These were calculated as 100 divided by the obtained EC50/IC50/LC50 values (Tišler and Zagorc-Končan 2007).
Differences in enzyme activities and protein content compared with controls were calculated by Kruskal–Wallis analysis and nonparametric Mann–Whitney U test (P < 0.05). Differences in the reproduction of daphnids were calculated by one-way analysis of variance (P < 0.05), and Dunnett’s test. All tests were performed using Statgraphics software (Statgraphics Plus for Windows 4.0; Statistical Graphics Corporation).
Results and Discussion
Physicochemical Characteristics of Leachates
Based on physicochemical characterization, the properties of influents collected at different periods of the year vary considerably (Table 1). Influent (sample 1) from the first sampling in autumn 2007 was more polluted than influent (sample 3) from the second sampling in spring 2008. Comparing raw leachates [samples 1 and 3 (Table 1)], a significant difference between COD, DOC, BOD5, and concentrations of NH4 +-N and nitrate N can be noticed. Raw leachates also expressed low biodegradability in terms of BOD5/COD ratio (0.06). The effluent (sample 4) from the treatment plant was less polluted than the influent (sample 3).
Acute Toxicity
Results of the toxicity tests are presented as effective (EC x ), inhibition (IC x ), and lethal (LC x ) concentrations (Table 2) and as TUs (Fig. 1), with the latter being used to allow for easier comparison with published data. In general, influents to the wastewater treatment plants (samples 1 and 3) were significantly more toxic than effluents (samples 2 and 4). For example, the influent from the first sampling was 87% more toxic to V. fischeri, 80% more toxic to daphnids, and 86% more toxic to D. rerio than the effluent from the same sampling. Slightly smaller differences between the samples were observed for the second sampling (sample 3 was 64% more toxic to V. fischeri, 74% more toxic to daphnids, and 74% more toxic to D. rerio than sample 4).
A significant difference in toxicity of the influents from two different samplings was observed. The influent from the first sampling (sample 1) was more toxic (41% for V. fischeri 29% for daphnids, and 49% for D. rerio) than the influent from the second one (sample 3), whereas effluents exhibited quite similar toxicity. This relation between toxicity of samples is even more obvious when presented as TUs (Fig. 1). For influents (samples 1 and 3), the difference in toxicity is in accordance with the difference observed in physicochemical characterization (Table 1).
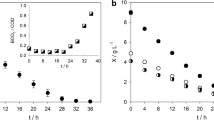
In a recent extensive review by Thomas et al. (2009), the toxicity of leachates from different landfills to several test organisms was reviewed. The toxicity of 10 untreated leachates toward V. fischeri ranged from 1 to 6.6 TU (Rutherford et al. 2000; Slomczynska et al. 2004; Ward et al. 2002) 1 to 34.4 TU for D. magna (acute 48-h test) exposed to 18 untreated leachates (Lambolez et al. 1994; Rutherford et al. 2000; Slomczynska et al. 2004), and 1 to 294 TU for alga Pseudokirchneriella subcapitata (72-h exposure) exposed to 22 untreated leachates (Baun et al. 2004; Bernard et al. 1996; Lambolez et al. 1994; Thomas et al. 2009) (Fig. 2). It was concluded that landfill toxicity varies greatly depending on the type of waste and consequently physicochemical properties of the leachates (Thomas et al. 2009). TU values derived from untreated samples in the present study (samples 1 and 3) for V. fischeri (5.5–9.3 TU), D. magna (48-h exposure; 20.8–29.4 TU), and D. subspicatus (cell count: 4.7–15.2 TU; chlorophyll-a: 9.3–20.8 TU) (Figs. 1, 2) are in range of literature data.
Toxicity data expressed in TU adopted from literature data (Thomas et al. 2009) and this study. In case of algae, our data are provided for D. subspicatus, and literature data are based on P. subcapitata. Data for D. magna are based on 48-h values
Chronic Toxicity and Hormetic-Like Effects
Chronic toxicity assessed by alga D. subspicatus showed the same relation between the samples as acute toxicity. Again, influents to the wastewater treatment plant (samples 1 and 3) were significantly more toxic than effluents (samples 2 and 4). In addition, the difference in the toxicity of influents from two samplings was observed for algae; influent from the first sampling was 61 and 55% more toxic, respectively, than influents from the second sampling when assessed by cell count and chlorophyll-a, respectively. Comparing IC50 values, both effluents were similarly toxic to algae when assessed by cell count, but sample 4 was more toxic if determined by chlorophyll-a (Table 2; Fig. 1).
When algal growth between the control and different concentrations of samples was compared, an interesting increase of growth was found at lower concentrations of samples 1 through 3, whereas at greater concentrations growth was inhibited (Fig. 3). Increased growth was more prominent when assessed by chlorophyll-a. Such increased growth was not taken into account when IC x values are calculated for hazard-assessment purposes, but this information is valuable when the mode of action of toxicants is investigated.
Chronic toxicity was also assessed in a 21-day reproduction test with D. magna. We observed increased reproduction, which was more prominent in the case of samples 1 through 3. However, no inhibition of reproduction was observed in any of the samples (Figs. 4a–d). The problem was that usually at high concentrations, at which inhibition of reproduction would be expected, adult daphnids died by day 21; therefore, reproduction at this concentration could not be evaluated. Due to these findings, we were not able to determine the 21-day LOEC (lowest observed-effect value), which is used for hazard assessment. We could determine these values based on concentrations that induced reproduction, but this is not a common approach.
The reproduction of daphnids (mean number of neonates per female daphnid) and mortality of adult daphnids (%) exposed to different concentrations of samples 1, 2, and 4 for 21 days (a, b, d) and sample 3 for 16 days (c). *Significant changes compared with control. SEs are shown for the reproduction data
The immobilty of adult daphnids during 21 days was also noted. We determined the concentration range at which 21-day LC50 would occur: for sample 1 it occurred at 0.113–0.225% (v/v), for sample 2 at 7.5–15% (v/v), for sample 3 at <0.31% (v/v), and for sample 4 at 3.13–6.25% (v/v). We have had problems performing the reproduction test with sample 3, because despite several repetitions, daphnids survived only until day 16; afterward all daphnids died at 0.31% (v/v) (Fig. 4c). Therefore, in the case of sample 3, we provide data on reproduction after 16 days; as a result, the number of neonates per female in controls is lower than in the case of the 21-day exposure.
Increase of algal growth and reproduction of daphnids could be considered a hormetic response, which by definition a biphasic dose-response phenomenon characterised by low-dose stimulation and high-dose inhibition. Hormesis is an organismal strategy for optimal resource allocation that ensures maintenance of homeostasis (Calabrese 2008). By definition, maximum stimulatory response is in general 30–60% greater than the control value, which was also the case in daphnid reproduction. However, algal growth increased by more than presumed maximum stimulatory response, and up to now no agreement has been reached whether this can still be regarded as hormesis (Calabrese 2008). Increased algal growth (Ward et al. 2002) and daphnid reproduction (Dave and Nilsson 2005) under exposure to low levels of landfill leachates has previously been observed. Increased daphnids’ reproduction was also observed in wastewaters, i.e., those from pharmaceutical industry (Tišler and Zagorc-Končan 1999), wastewaters from metal surface coating, and steel pipe manufacturing (Rodriguez et al. 2006). Individual substances, such metals and pharmaceuticals, were also shown to induce daphnid reproduction (Bodar et al. 1988; Flaherty and Dodson 2005). Because detailed chemical analysis of leachates was not performed, it is hard to elucidate the inducer of hormesis. Furthermore, it cannot be predicted how a complex mixture of contaminants present in these samples might influence hormesis.
In our experiments, most probably a direct relation between the increased algal growth and increased daphnid reproduction exists. Namely, daphnids were fed daily with alga D. subspicatus. We showed that landfill leachate (sample 1) also induces the growth of algae in the medium used for the daphnid reproduction test (Fig. 5). Therefore, daphnids with increasing concentrations of sample had more food available. It is known that there is a direct relation between food supply and daphnid reproduction. When the food supplies more energy than the required maintenance, most of the excess is converted into egg production (Hebert 1978). One can also notice that the significance of increased reproduction correlates with increased algal growth, meaning that reproduction was more significantly increased in samples in which algal growth was more promoted (Figs. 3, 4).
In risk assessment, decreased daphnid reproduction is regarded as an adverse effect, but an increased reproduction is commonly overlooked. Hammers-Wirtz and Ratte (2000) showed that increased daphnid reproduction has adverse effects on offspring quality (smaller size, greater mortality); because of this fitness of the next generations of daphnids is affected. They suggested that all significant deviations from the control should be considered because positive effects also indicate changes in daphnid physiology, resulting in changes in following generations of daphnids.
Biochemical Biomarkers as Tools to Identify Sublethal Effects
Biochemical biomarkers were assessed in adult daphnids after a 21-day exposure in samples 1, 2, and 4 and after a 16-day exposure in sample 3. Statistically significant increases of protein content per animal were observed in samples 1 and 2, where it increased with increasing concentrations of samples, whereas in sample 3 proteins did not change in a dose- dependent manner (e.g., greater increase at 0.6% than at 1.3%), No statistically significant protein changes in sample 4 were found (Fig. 6). The increased protein content in samples 1 and 2 correlated with increasing number of neonates per female found in these samples. In addition, in sample 4 no change of protein content is consistent with no significant increase in the number of neonates found (Fig. 4). High protein content per adult might therefore be due to larger amount of neonates still present in adult female daphnids after the experiment or larger size of adult female daphnids. The latter could not be determined because the animals were used for further enzyme analysis immediately after the end of experiment. The protein content proved to be a reliable tool to assess the physiological condition of daphnids, which was also shown by Knowles and McKee (1987).
Biochemical biomarkers in adult daphnids after 21-day exposure to different concentrations of samples 1, 2, and 4, and 16-day exposure to sample 3. *Significant changes compared with control. Enzyme unit for ChE = 0.01 nmol acetylthiocholine min−1 mg protein−1 and for GST = 1 nmol reduced glutathione min−1 mg protein−1
No general trend of increased or decreased GST and ChE activities was observed in samples 1 through 3, but some minor exceptions were observed at certain concentrations. In animals exposed to sample 4, in contrast, an increase of both activities was found (Fig. 6). Based on literature data, both increased or decreased GST could be expected in chemically stressed organisms (Brown et al. 2004). An increase would be expected at lower concentrations, where this would be a response to detoxification. At greater concentrations, antioxidant enzymes can be inhibited due to general cell dysfunction (Brown et al. 2004). For ChE, it is more generally expected that these enzymes are inhibited by certain substances (Guilhermino et al. 1998); however, under some exposure conditions this enzyme can also be induced (Jemec et al. 2007b; Romani et al. 2003). In complex mixtures, such as landfill leachates, it is impossible to explain enzyme changes by the presence of certain pollutants. The general conclusion from the results presented here is that the two enzymes measured in the present study are not more sensitive and specific measure of toxicity than higher-level changes, such as mortality or reproduction. Namely, at concentrations where no enzyme changes were measured, reproduction and survival were already affected. This has also been observed with some pure chemicals, where enzyme changes were found to be dependent on the species being investigated, the periods of exposure, and the class of chemicals (Jemec et al. 2010).
Sensitivity of Toxicity Tests to Landfill Leachates
Toxicity tests exhibited differential sensitivity to landfill leachates. In general, 30-min V. fischeri and 72-h D. subspicatus tests were less sensitive than 48-h D. magna and D. rerio tests. No significant difference was observed between the V. fischeri and D. subspicatus tests when algal growth was assessed by cell counting, but the algal test using chlorophyll-a as an end point was more sensitive than the V. fischeri test. D. magna was in some cases slightly more sensitive than D. rerio (Fig. 1). Compared with toxicity tests using V. fischeri, D. magna, and D. subspicatus (Table 2), toxicity tests of daphnid reproduction proved to be the most sensitive (Fig. 4). These findings are in accordance with those of other investigators who found the V. fischeri test to be the least sensitive to landfill leachates compared with daphnid tests (D. magna, D. similis, and Ceridaphnia dubia) (Bernard et al. 1996; Isidori et al. 2003; Silva et al. 2004; Thomas et al. 2009; Ward et al. 2002). In addition, the algal test based on chlorophyll-a was more sensitive than the V. fischeri test as reported by other investigators for algae P. subcapitata, Scenedesmus quadricauda, and D. subspicatus (Bernard et al. 1996; Slomczynska et al. 2004; Ward et al. 2002). In contrast, the algal test using cell count to determine growth was not so sensitive, which indicates the need for the use of additional end points, such as chlorophyll-a, to determine the growth of algae. Some other test organisms, e.g., freshwater crustaceans Thamnocephalus platyrus, rotifers Brachionus calyciflorus (Isidori et al. 2003); protozoans Spirostomum ambiguum (Bernard et al. 1996), and plants Lemna minor (Clément and Merlin 1995) were found to be even more sensitive than daphnids. Different sensitivity of test organisms implies the need for a battery of tests for the most informative toxicity assessment of landfill leachates.
This study is among the first to report the use of D. rerio embryos to evaluate the toxicity of landfill leachates. Adult fish of this species were previously used and, similarly as in this work, high sensitivity to landfill leachates was found (Silva et al. 2004). There is currently a strong public and political pressure to replace adult fish tests with alternatives, among them fish cell lines and embryos (Lammer et al. 2009). It has already been proven that the fish embryo toxicity test is a possible surrogate for the acute fish-toxicity test (Lammer et al. 2009) and also presents an attractive model with a range of possible applications in environmental sciences (Scholz et al. 2008). We therefore suggest the use of this test system additionally for the evaluation of toxic potential of landfill leachates.
The correlation between the physicochemical properties and observed toxicity in this study cannot be reliably determined due to the small number of samples and limited amount of chemical parameters characterized. This relation for landfill leachates has previously been investigated in detail (Thomas et al. 2009), and the properties shown to influence the toxicity were chemical oxygen demand; ammonia (NH3), pH, alkalinity, and chloride ions (Assmuth and Penttilä 1995; Bernard et al. 1997; Clément and Merlin 1995).
Efficiency of Existing Biological Wastewater Treatment
The treatment efficiency of an existing SBR wastewater treatment plant was estimated based on the difference between EC50/IC50 values (Table 2) and the physicochemical properties of influents and effluents (Table 1). In the first sampling, the toxicity decreased by 80% for D. magna, by 87% for V. fischeri, by 86% for D. rerio, and by 89% for D. subspicatus (per both algal count and chlorophyll-a). The efficiency was lower in the second sampling: Toxicity decreased by 74% for D. magna, by 64% for V. fischeri, by 75% for D. rerio, and by 61% (algal count) and 30% (chlorophyll-a) for D. subspicatus. The reason for a lower decrease of toxicity in the second sampling most probably lies in the fact that the toxicity of influent from the second sampling was already significantly lower than the one from influent from the first sampling. The effectiveness of an existing SBR treatment plant based on physicochemical properties could only be calculated for the second sampling. It was observed that the existing SBR removed 54% of COD and 88% of DOC (Table 1). During biological treatment, concentration of ammonium nitrogen was also significantly decreased (73%), whereas concentration of nitrate N increased, indicating successful nitrification. Nitrification was also confirmed by decreased IC concentration (85%). It was concluded that the major presence of refractory compounds accomplished by their toxic impact tends to limit process effectiveness.
Despite the high decrease of both physicochemical parameters as well as toxicity of influent after treatment, both effluents are not suitable to be discharged into rivers according to the current Slovenian legislation (Official Gazette 2008). Leachate from nonhazardous landfill could be released into surface waters if COD was <300 mg l−1, BOD5 was ≤30 mg l−1, and ammonium and nitrate N were both <50 mg l−1. In terms of toxicity, effluent from the first sampling could not be released into rivers, whereas effluent from the second sampling would meet toxicity criteria (24-h TU for D. magna <4). Existing biological treatment can thus be considered inappropriate for these kinds of wastewaters. This is in accordance with estimations that biological treatment is most efficient only for “young leachates” with high BOD5-to-COD ratio (>0.6). The implementation of these techniques is limited in the case of high toxicity of leachates and low BOD5:COD ratio (Goi et al. 2010), which is also the case in our samples (BOD5-to-COD ratio = 0.06 for sample 3). Due to this reason, other more efficient physicochemical approaches are being developed, among them ozonation, Fenton oxidation, coagulation, absorption, precipitation, evaporation, and membrane filtration (Marttinen et al. 2002; Silva et al. 2004; Žgajnar Gotvajn et al. 2009).
However, it is not yet clear how advanced techniques might affect the toxicity of wastewaters. For example, increased leachate toxicity to V. fischeri, D. magna, and algae Raphidocelis subcapitata was observed after ozonation (Marttinen et al. 2002; Silva et al. 2004). Increased toxicity was also observed to D. similis after ultrafiltration (Silva et al. 2004), and D. magna after ammonia stripping of landfill leachates (Marttinen et al. 2002). In experiments performed by Marttinen et al. (2002), biological treatment of leachates decreased their toxicity to daphnids and algae R. subcapitata, whereas toxicity was unchanged or even increased after physicochemical treatments. In contrast, in some studies, advanced techniques have already proven to be successful in decreasing the toxicity of leachates (Bila et al. 2005; Goi et al. 2009). This aspect requires further research before other techniques in addition to biological treatment are implemented in practice.
Conclusion
The toxicity of landfill leachates decreased after biological treatment, but the effluents still did not meet the environmental demands of the current Slovenian legislation. The toxicity of leachates was highly variable depending on the season of collection. Uncommon effects of leachates on organisms, such as hormetic-like increases of algal growth and daphnid reproduction, were identified. Some toxicity tests were found to be more sensitive than others; therefore, a battery of tests is recommended in future studies. However, the use of biochemical biomarkers was found to be unsuitable for routine hazard assessment of leachates. Because biological treatment was found inefficient for landfill leachates, we suggest further studies using advanced treatment techniques. The influence of such approaches on the toxicity of leachates also needs further attention before their application.
References
Assmuth T, Penttilä S (1995) Characteristics, determinants and interpretations of acute lethality in daphnids exposed to complex waste leachates. Aquat Toxicol 31:125–141
Baun A, Ledin A, Reitzel LA, Bjerg PL, Christensen TH (2004) Xenobiotic organic compounds in leachates from ten Danish MSW landfills: chemical analysis and toxicity tests. Water Res 38:3845–3858
Bernard C, Guido P, Colin J, Le Dû-Delepierre A (1996) Estimation of the hazard of landfills through toxicity testing of leachates. I. Determination of leachate toxicity with a battery of acute tests. Chemosphere 33:2303–2320
Bernard C, Colin JR, Le Dû-Delepierre A (1997) Estimation of the hazard of landfills through toxicity testing of leachates. 2. Comparison of physico-chemical characteristics of landfill leachates with their toxicity determined with a battery of tests. Chemosphere 35:2783–2796
Bila DM, Montalvao AF, Silva AC, Dezotti M (2005) Ozonation of a landfill leachate: evaluation of toxicity removal and biodegradability improvement. J Hazard Mater 117:235–242
Bodar CWM, Van Leeuwen CJ, Voogt PA, Zandee DI (1988) Effect of cadmium on the reproduction strategy of Daphnia magna. Aquat Toxicol 12:301–310
Booth LH, O’Halloran K (2001) A comparison of biomarker responses in the earthworm Aporrectodea caliginosa to the organophosphorous insecticides diazinon and chlorpyrifos. Environ Toxicol Chem 20:2494–2502
Brown RJ, Galloway TS, Lowe D, Browne MA, Dissanayake A, Jones MB et al (2004) Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquat Toxicol 66:267–278
Calabrese EJ (2008) Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem 27:1451–1474
Clément B, Merlin G (1995) The contribution of ammonia and alkalinity to landfill leachate toxicity to duckweed. Sci Total Environ 170:71–79
Dave G, Nilsson E (2005) Increased reproductive toxicity of landfill leachate after degradation was caused by nitrite. Aquat Toxicol 73:11–30
Eurostat (2010). Municipal waste generation and treatment, by type of treatment method (kg per capita). Available at: http://epp.eurostat.ec.europa.eu/portal/page/portal/waste/data/wastemanagement/waste_treatment. Accessed: January 15, 2010
Flaherty CM, Dodson SI (2005) Effects of pharmaceuticals on daphnia survival, growth, and reproduction. Chemosphere 61:200–207
Goi A, Veressinina Y, Trapido M (2009) Combination of ozonation and the Fenton processes for landfill leachate treatment: evaluation of treatment efficiency. Ozone: Sci Eng: J Int Ozone Assoc 31:28–36
Goi A, Veressinina Y, Trapido M (2010) Fenton process for landfill leachate treatment: evaluation of biodegradability and toxicity. J Environ Eng 136:46–53
Guilhermino L, Barros P, Silva MC, Soares AMVM (1998) Should the use of inhibition of cholinesterases as a specific biomarker for organophosphate and carbamate pesticides be questioned? Biomarkers 3:157–163
Hammers-Wirtz M, Ratte HT (2000) Offspring fitness in daphnia: Is the daphnia reproduction test appropriate for extrapolating effects on the population level? Environ Toxicol Chem 19:1856–1866
Hebert PDN (1978) The population biology of daphnia (Crustacea: Daphnidae). Biol Rev 53:387–426
International Organisation for Standardisation (ISO) 10260 (1992) Water quality―Measurement of biochemical parameters―Spectrometric determination of the chlorophyll-a concentration. ISO, Geneva, Switzerland
Isidori M, Lavorgna M, Nardelli A, Parrella A (2003) Toxicity identification evaluation of leachates from municipal solid waste landfills: a multispecies approach. Chemosphere 52:85–94
Jemec A, Tišler T, Drobne D, Sepčić K, Fournier D, Trebše P (2007a) Comparative toxicity of imidacloprid, of its commercial liquid formulation and of diazinon to a non-target arthropod, the microcrustacean Daphnia magna. Chemosphere 68:1408–1418
Jemec A, Drobne D, Tišler T, Trebše P, Roš M, Sepčić K (2007b) The applicability of acetylcholinesterase and glutathione S-transferase in Daphnia magna toxicity test. Comp Biochem Physiol C 144:303–309
Jemec A, Drobne D, Tišler T, Sepčić K (2010) Biochemical biomarkers in environmental studies—lessons learnt from enzymes catalase, glutathione S-transferase and cholinesterase in two crustacean species. Environ Sci Pollut 17:571–581
Jensen DL, Christensen TH (1999) Colloidal and dissolved metals in leachates from four Danish landfills. Water Res 33:2139–2147
Kammann U, Biselli S, Hühnerfuss H, Reineke N, Theobald N, Vobach M et al (2004) Genotoxic and teratogenic potential of marine sediment extracts investigated with comet assay and zebrafish test. Environ Pollut 132:279–287
Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A, Christensen TH (2002) Present and long-term composition of MSW landfill leachate: a review. Crit Rev Environ Sci Technol 32:297–336
Knowles CO, McKee MJ (1987) Protein and nucleic acid content in Daphnia magna during chronic exposure to cadmium. Ecotox Environ Safe 13:290–300
Lambolez L, Vasseur P, Ferard JF, Gisbert T (1994) The environmental risks of industrial waste disposal: an experimental approach including acute and chronic toxicity studies. Ecotox Environ Safe 28:317–328
Lammer E, Carr GJ, Wendler K, Rawlings JM, Belanger SE, Braunbeck T (2009) Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp Biochem Physiol C 149:196–209
Marttinen SK, Kettunen RH, Sormunen KM, Soimasuo RM, Rintala JA (2002) Screening of physical-chemical methods for removal of organic material, nitrogen and toxicity from low strength landfill leachates. Chemosphere 46:851–858
Official Gazette of Republic of Slovenia (2008) Decree on the emission of substances in the discharge of landfill leachate, ULRS 62/2008 196–215 [in Slovene]
Rodriguez P, Martinez-Madrid M, Cid A (2006) Ecotoxicological assessment of effluents in the Basque country (Northern Spain) by acute and chronic toxicity tests using Daphnia magna Straus. Ecotoxicology 15:559–572
Romani R, Atognelli C, Baldracchini F, De Santis A, Isani G, Giovannini E et al (2003) Increased acetylcholinesterase activities in specimens of Sparus auratus exposed to sublethal copper concentrations. Chem Biol Interact 145:321–332
Rutherford LA, Matthews SL, Doe KG, Julien GRJ (2000) Aquatic toxicity and environmental impact of leachate discharges from a municipal landfill. Water Qual Res J Can 35:39–57
Scholz S, Fischer S, Gündel U, Küster E, Luckenbach T, Voelker D (2008) The zebrafish embryo model in environmental risk assessment: applications beyond acute toxicity testing. Environ Sci Pollut 15:394–404
Silva AC, Dezotti M, Sant’Anna GL (2004) Treatment and detoxification of a sanitary landfill leachate. Chemosphere 55:207–214
Slomczynska B, Wasowski J, Slomczynski T (2004) Effect of advanced oxidation processes on the toxicity of municipal landfill leachates. Water Sci Technol 49:273–277
Thomas DJL, Tyrrel SF, Smith R, Farrow S (2009) Bioassays for the evaluation of landfill leachate toxicity. J Toxicol Environ Health B 12:83–105
Tišler T, Zagorc-Končan J (1999) Toxicity evaluation of wastewater from the pharmaceutical industry to aquatic organisms. Water Sci Technol 39:71–76
Tišler T, Zagorc-Končan J (2007) The “whole-effluent” toxicity approach. Int J Environ Pollut 31:3–12
Tišler T, Jemec A, Mozetič B, Trebše P (2009) Hazard identification of imidacloprid to aquatic environment. Chemosphere 76:907–914
Ward ML, Bitton G, Townsend T, Booth M (2002) Determining toxicity of leachates from Florida municipal solid waste landfills using a battery-of-tests approach. Environ Toxicol 17:258–266
Žgajnar Gotvajn A, Derco J, Tišler T, Cotman M, Zagorc-Končan J (2009) Removal of organics from different types of landfill leachate by ozonation. Water Sci Technol 60:597–603
Acknowledgments
The authors thank Polona Zevnik, Emil Meden, and Tina Bobnar for technical assistance. The authors also gratefully acknowledge the financial support of the Ministry of Education, Science and Technology of the Republic Slovenia through research programs P2-0150 and P2-191.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jemec, A., Tišler, T. & Žgajnar-Gotvajn, A. Assessment of Landfill Leachate Toxicity Reduction After Biological Treatment. Arch Environ Contam Toxicol 62, 210–221 (2012). https://doi.org/10.1007/s00244-011-9703-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-011-9703-x