Abstract
Studies on residue levels and accumulation profiles of persistent organic pollutants (POPs) in human adipose tissues of Korean populations are scarce. In this study, concentrations and accumulation features of polychlorinated biphenyls (PCBs), organochlorine pesticides (OCPs), and polybrominated diphenyl ethers (PBDEs) were measured in adipose tissues of Korean women age 40–68 years. The highest concentrations were found for PCBs and DDTs, which were 1–2 orders of magnitude greater than the concentrations of hexachlorocyclohexanes, chlordanes, and PBDEs. The concentrations of PCBs and OCPs were lower than those reported for other countries. However, PBDE concentrations were greater than those reported for other countries, suggesting that ongoing exposure to PBDEs is a concern in Korea. The profiles of PBDEs were characterized by the predominance of BDE 209, followed by nona- and octa-BDEs, which are consistent with the consumption patterns of products containing PBDEs in Korea. The concentrations of PCBs and some OCPs were significantly correlated with each other, whereas PBDEs showed low or moderate correlations with other POPs, suggesting differences in exposure routes and biotransformation potentials of the compounds studied. The concentrations of organochlorines and PBDEs were not correlated with subjects’ age and body mass index. The results of this study provide baseline information on POPs in adipose tissues of the general population in Korea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Polybrominated diphenyl ethers (PBDEs) have been used as brominated flame retardants (BFRs) in many products, such as electronics, plastics, paints, textiles, and building materials (Watanabe and Sakai 2003). Environmental contamination by PBDEs is of global concern due to their persistence, bioaccumulation, and long-range transport, traits that contribute to PBDE’s occurrence in various environmental compartments and in human tissues around the world (Law et al. 2006; Wang et al. 2007; Shaw and Kannan 2009). PBDEs exert neurodevelopmental and endocrine-disrupting effects in laboratory animals (Birnbaum and Staskal 2004; Costa and Giordano 2007; Messer 2010). In 2009, selected mixtures of PBDEs were listed as persistent organic pollutants (POPs) under the Stockholm Convention (United Nations Environment Program 2009).
BFRs were not produced in Korea but were imported from other countries. The total consumption of BFRs in Korea in 2002 was 49,050 tons (Moon et al. 2007). A major share of the BFR consumption in Korea was deca-BDE, which accounted for 25% (12,324 tons) of the total BFR market, whereas penta- and octa-BDEs accounted for only a minor proportion (0.2% [84 tons]) (Moon et al. 2007). Considering the rapid growth of the electronics market in Korea and the associated increase in demand for PBDEs, it is important to investigate exposure of the Korean general population to PBDEs.
Concentrations of organochlorines (OCs), including polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs), have generally been decreasing in the environment and in humans during the past few decades (Hagmar et al. 2006; Rappolder et al. 2007; Helgason et al. 2008; Lignell et al. 2009). In Korea, studies on PCBs and OCPs have been focused on residue levels and sources in environmental matrices, such as air, soil, or sediment, and wildlife (Kim and Smith 2001; Hong et al. 2006; Choi et al. 2008; Moon et al. 2009; Park et al. 2010).
Human specimens, such as breast milk, serum, and adipose tissue, have been used in biomonitoring the extent of human exposure to organohalogen contaminants (Covaci et al. 2008; Jin et al. 2009; Hardell et al. 2010). Measurement of contaminants in adipose tissue can provide information on steady-state concentrations and integrated levels of accumulation over time. Although the collection of blood and breast milk samples is relatively simple and less invasive than the collection of adipose tissues, residual levels of lipophilic contaminants in whole blood, sera, or plasma may fluctuate with surges in blood lipids (Johnson-Restrepo et al. 2005). Thus, for assessing human exposure to lipophilic contaminants, adipose tissue is preferable when available.
Although human exposure to OCs and PBDEs is of great concern (Kang et al. 1997; Moon et al. 2009), few studies have examined residue levels in blood of the Korean population (Lee et al. 2007; Kang et al. 2008). Moreover, before this study, no studies have examined PBDE accumulation in human adipose tissues from Korea. The objective of this study was to determine the concentrations and accumulation features of OCs and PBDEs in adipose tissues of Korean women.
Materials and Methods
Sample Collection
The use of human tissues for this study was approved by the Ethics Committee of the Kyungpook National University Institutional Review Board. A total of 53 female myoma patients who were undergoing laparoscopy-assisted surgery at Kyungpook National University Hospital agreed to provide adipose tissue samples. Omental fat samples were obtained from the donors between May 2007 and May 2008. All samples were stored at −70°C until analyses. Participant’s age, body mass index (BMI), and extractable lipid are listed in Table 1. The age of the subjects ranged from 40 to 68 years (average 47), and lipid content of the adipose tissue samples ranged from 63 to 83% (average 73%).
Chemical Analysis
We analyzed 22 PCB congeners (PCBs 8, 18, 28, 29, 44, 52, 87, 101, 105, 110, 118, 128, 138, 153, 170, 180, 187, 194, 195, 200, 205 and 206), 23 PBDE congeners (BDEs 17, 28, 47, 49, 66, 71, 85, 99, 100, 119, 126, 138, 153, 154, 156, 183, 184, 191, 196, 197, 206, 207 and 209), and 14 OCP compounds in 53 adipose tissues. Dichlorodiphenyltrichloroethanes (DDTs) analyzed include p,p′-DDE, p,p′-DDD, o,p′-DDD, p,p′-DDT, and o,p′-DDT; chlordanes (CHLs) included oxy-chlordane, trans-chlordane, cis-chlordane, trans-nonachlor, and cis-nonachlor; and hexachlorocyclohexanes (HCHs) included α-, β- and γ-HCH. Hexachlorobenzene (HCB) was also analyzed. Internal standards of 13C-labeled PBDEs (BDEs 28, 47, 99, 153, 154, 183, 197, 207 and 209) and 13C-labeled PCBs (PCBs 28, 52, 101, 138, 153, 180 and 209) were purchased from Wellington Laboratories (Guelph, ON, Canada). All solvents were of ultratrace residue-analysis grade (J. T. Baker, Phillipsburg, NJ).
Analyses of PBDEs and OCs in adipose tissue samples were performed according to methods described elsewhere (Moon et al. 2009; 2010; Park et al. 2010). In brief, samples were homogenized with anhydrous Na2SO4 and extracted for 20 h in 400 ml a 3:1 mixture of dichloromethane (DCM) and hexane using a Soxhlet apparatus. Before extraction, surrogate standards PCBs 103, 198, and 209 were spiked into the samples. Aliquots of extracted samples were subsampled for lipid measurement. Lipids were removed from the extracts by gel permeation chromatography using Bio-beads S-X3 (Bio-Rad, Hercules, CA). The columns were eluted with a mixture of 50% DCM in hexane (flow rate 5 ml/min). The first 100 ml of eluant was discarded and the resultant 150-ml fraction, which contained PBDEs and OCs, was collected and passed through a cartridge packed with 0.5 g silica gel (neutral, 70–230 mesh; GL Sciences, Tokyo, Japan). The eluant was concentrated to 10 ml and spiked with internal standards, 13C-labeled PBDEs, and 13C-labeled PCBs. The extracts were cleaned by passage through a multilayer silica gel column with 150 ml 15% DCM in hexane using the Dioxin Cleanup System (DAC695/DPU8; GL Sciences). The eluant was concentrated to approximately 1 ml and then evaporated at room temperature to 50–100 μl. The residues were dissolved in 100 μl n-nonane for instrumental analysis.
A high-resolution gas chromatographer (GC)/mass spectrometer (JMS 800D, Jeol, Tokyo, Japan) was used for identification and quantification of PBDEs based on the relative response factors of individual congeners. The high-resolution mass spectrometer was operated in the electron ionization mode, and ions were monitored by selected ion monitoring. PBDE congeners were quantified separately for tri- to hepta-BDEs and octa- to deca-BDEs using a DB5-MS capillary column (15-m length, 0.25-mm inner diameter, 0.1-μm film thickness; J & W Scientific, Palo Alto, CA). PCBs and OCPs were determined using a gas chromatographer (Agilent 6980 N) coupled to a mass spectrometer (JMS GC Mate II; Jeol). The GC/MSD was operated in the electron impact (70 eV) and selected ion monitoring modes for most intensive ions of the molecular ion cluster of individual compounds. A DB5-MS capillary column (30-m length, 0.25-mm inner diameter, 0.25-μm film thickness; J & W Scientific) was used for analyses of PCBs and OCPs. Quantification of each compound was performed by external standard method.
Procedural blanks (n = 8) were processed in the same way as the samples and were included after every seventh sample. With the exception of the presence of deca-BDE (approximately 1 ng/g) in the blanks, they did not contain quantifiable amounts of the target compounds. The respective recoveries of PCBs 103, 198 and 209, spiked into all samples before extraction, ranged from 86 ± 9% (average ± SD), 87 ± 10%, and 86 ± 11%. Recoveries of 13C-labeled PBDEs and PCBs were 87 ± 15% and 91 ± 11%, respectively. The calculated limits of detection (signal-to-noise ratio = 3) were 0.1–0.5 pg/g for tri- to hepta-BDEs, 1–5 pg/g for octa- to deca-BDEs, 0.05–0.5 ng/g for individual PCB congeners, 0.5 ng/g for DDTs, 0.1 ng/g for CHLs, 0.2 ng/g for HCHs, and 0.05 ng/g for HCB. To assess the quality of the analytical procedures and instrumental conditions, we analyzed standard mussel (Mytilus edulis) reference material (SRM 2974; National Institute of Standards and Technology [NIST]; Gaithersburg, MD) and cod liver oil (SRM 1588b; NIST) for OCs and tri- to hepta-BDE congeners, respectively. Our values (n = 4) were 76–115% of the certified values for PBDEs, 78–95% of the certified values for PCBs, and 72–104% of the certified values for OCPs.
Statistical Analyses
Kolmogorov–Smirnoff and Shapiro–Wilk tests were performed to assess normality of concentrations of PCBs, OCPs, and PBDEs in the adipose tissues. Spearman’s correlation analysis was performed to investigate the relations between the chemical concentrations and demographic factors, such as BMI and age. All analyses were performed using SPSS software version 18.0 (SPSS, Chicago, IL) for Network.
Results and Discussion
Concentrations of PCBs, OCPs, and PBDEs
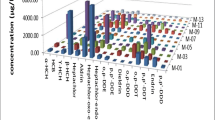
Concentrations of PCBs, OCPs, and PBDEs in adipose tissues of Korean women are listed in Table 1. Among OCs, concentrations of PCBs (270 ± 140 ng/g lipid wt) and DDTs (250 ± 210 ng/g lipid wt) were the highest. Concentrations of CHLs (18 ± 14 ng/g lipid wt), HCHs (12 ± 11 ng/g lipid wt), and HCB (8.6 ± 4.7 ng/g lipid wt) were 1–3 orders of magnitude lower than the concentrations of PCBs and DDTs. This pattern is similar to that reported for fish, shellfish, squids, and cetaceans from Korean coastal waters (Moon et al. 2009, 2010; Won et al. 2009; Park et al. 2010). Although the consumption of HCHs is greater than that of DDTs in northeast Asian countries (Li 1999; Yang 2008), HCH concentrations were lower than those of DDTs in adipose tissues of Korean women. This is due to the greater bioaccumulation potential of DDTs than HCHs (Loganathan and Kannan 1994) and the rapid evaporation of HCHs to the atmosphere after their application (Ramesh et al. 1991). The distribution of concentrations of HCHs in adipose tissues was different from that of other POPs, with majority of samples showing concentrations <2 ng/g lipid wt (Fig. 1).
Most of the adipose tissue samples contained PBDE concentrations in the range of 4–20 ng/g lipid wt. The highest PBDE concentration (149 ng/g lipid wt) was found in sample from a 50-year-old woman; this was considered as an outlier value in subsequent statistical analysis. The concentrations of PBDEs (16 ± 20 ng/g lipid wt) in adipose tissue samples were similar to those of CHLs and HCHs but were greater than those of HCB. Although HCB was never used as pesticide in Korea, this compound was found in adipose tissues from Korean women.
Comparison of PCBs, OCPs, and PBDEs in Human Adipose Tissues with Those Reported by Other Studies
Although the sampling campaign was different among countries investigated, the overall concentrations of OCPs (e.g., DDTs, CHLs, HCHs, and HCB) in adipose tissues of Korean women were lower than those reported for other countries (Table 2). In particular, residual levels of DDTs, CHLs, HCHs, and HCB in samples from Korea were the lowest among the reporting countries. The concentrations of PCBs (270 ± 140 ng/g lipid wt) in our study were lower than those measured in adipose tissues from Italy (1550 ± 1532 ng/g lipid wt [Schiavone et al. 2010]), Japan (850 ± 600 ng/g lipid wt [Kunisue et al. 2007]), the Czech Republic (626 ± 376 ng/g lipid wt [Pulkrabová et al. 2009]), and Belgium (490 ± 341 ng/g lipid wt [Covaci et al. 2008]). However, PCB concentrations in our study were greater than those reported for adipose tissues from women in Singapore (68 ± 87 ng/g lipid wt [Tan et al. 2008]), Brazil (80 ± 70 ng/g lipid wt [Kalantzi et al. 2009]), and New York, United States (157 ± 152 ng/g lipid wt [Johnson-Restrepo et al. 2005]).
PBDE concentrations (16 ± 20 ng/g lipid wt) found in our study were comparable with those reported for Italy (11 ± 10 ng/g lipid wt [Schiavone et al. 2010]) and Singapore (10 ± 21 ng/g lipid wt [Tan et al. 2008]). Although the average concentrations of PBDEs (253 ± 639 ng/g lipid wt [Johnson-Restrepo et al. 2005]) in adipose tissues from women in New York City were approximately 15 times greater than those measured in our study, adipose tissues from women in countries such as Japan (Kunisue et al. 2007), the Czech Republic (Pulkrabová et al. 2009), Belgium (Covaci et al. 2008), Spain (Fernandez et al. 2007), and Brazil (Kalantzi et al. 2009) had concentrations lower than those reported in our study, <10 ng/g lipid weight. Large amounts of deca-BDEs were consumed in Korea in 2001, accounting for approximately 50% (12,324 tons) of the total deca-BDE demand in Asia (Watanabe and Sakai 2003; Moon et al. 2007). Our results suggest that high levels of PBDE contamination in Korea is a great concern. In fact, environmental monitoring of POPs in Korean coastal waters has shown significant contamination by PBDEs (Moon et al. 2010; Park et al. 2010).
Distribution Profiles of OCPs, PCBs, and PBDEs
The distribution profiles of OCPs, PCBs, and PBDEs in adipose tissues of Korean women are shown in Fig. 2. Among OCPs, the major compound was p,p′-DDE, which accounted for 80% of the total OCP concentrations. The greater proportion of metabolites, such as p,p′-DDE, compared with the parent compound, p,p′-DDT, suggests historical sources from technical mixtures of DDT used in Korea. Some amounts of p,p′-DDT (and o,p′-DDT) were detected in adipose tissue samples, which may be associated with exposure through seafood consumption, which is a major DDT exposure route for the general Korean population (Moon et al. 2009). The proportion of p,p′-DDT in seafood commonly consumed in Korea was >10% of the total OCP concentrations (Moon et al. 2009). Although α-HCH comprises the majority of HCH technical mixtures, ranging from 55 to 80% (Willett et al. 1998), only β-HCH was detected in adipose tissue samples due to its greater persistence compared with other HCH isomers (Loganathan and Kannan 1994). The major CHL compound identified in this study was trans-nonachlor, accounting for >60% of total CHL concentrations, followed by oxychlordane (30%).
Hexa- and hepta-chlorobiphenyls constituted the greatest proportions (>75%) of total PCB concentrations. Pentachlorobiphenyls accounted for a small portion (approximately 10%) of total PCBs. For all tissue samples, the major congeners were PCBs 153, 138, 180, 187, 118, and 170, which collectively accounted for 80% of total PCB concentrations. The accumulation profiles of OCPs and PCBs in adipose tissues are similar to the results previously reported for seafood (Moon et al. 2009), cetaceans (Park et al. 2010), and human sera (Kang et al. 2008) from Korea.
Several studies have reported PBDE concentrations in human samples from other countries, but those studies measured only tetra- to hepta-BDE congeners, such as BDEs 28, 47, 99, 100, 153, 154 and 183, which are the major components of penta- and octa-BDE technical mixtures (La Guardia et al. 2006). However, data on more highly brominated octa- to deca-BDE congeners in human samples are scarce. In our study, BDE 209 (deca-BDE) was a predominant PBDE congener, accounting for 25 ± 15% of total PBDE concentrations. In addition, more highly brominated congeners, such as octa- to nona-BDEs (BDEs 197 and 207), accounted for 35% of the total PBDE concentrations. This result is likely related to the high consumption of deca-BDE technical mixtures in Korea and its ongoing release into the environment (Thuresson et al. 2006). Similar to our study, some studies showed the dominance of BDE 209 in human serum, breast milk, and adipose tissues from Japan (Takasuga et al. 2004; Inoue et al. 2006; Kunisue et al. 2007) and China (Jin et al. 2009). Koh et al. (2010) reported a shift in the congener pattern of PBDEs found in breast milk from Taiwanese women between 2000 and 2001 and 2007 and 2008. During the study period, the results showed a significant increase in the proportions of more highly brominated congeners, such as BDEs 183 and 209, to total PBDE concentrations in breast milk of Taiwanese women.
As mentioned previously, the major source of PBDE contamination in Korea is associated with the extensive use of deca-BDE technical mixtures (Moon et al. 2007), which are composed primarily of BDE 209 but also include minor proportions of nona-BDEs 206 and 207. In our study, BDEs 49 and 66 were detected in almost all adipose tissue samples (100% for BDE 49 and 80% for BDE 66), which is different from the results of previous studies that have rarely found BDEs 49 and 66 in biotic compartments (Stapleton et al. 2006; Shaw et al. 2009). Results from this study are consistent with penta-BDE, comprising a small portion (<0.2%) of technical mixtures used in the Korean BFR market. The proportion of BDEs 49 and 66 in penta-BDE technical mixtures is only 0.6–1.3% of total PBDEs (La Guardia et al. 2006), consistent with the pattern observed (0.9 ± 0.5% for BDEs 49 and 66) for human adipose tissues in our study.
Correlations Among POPs
Previous studies have reported age-dependent accumulations of some legacy POPs in human adipose tissue samples (Johnson-Restrepo et al. 2005; Kunisue et al. 2007; Covaci et al. 2008). However, in this study no age-dependent accumulation of PCBs and OCPs were found, except for CHLs (r = 0.341, p < 0.05) (Fig. 3). The departure from previously reported results is likely attributable to the age of the subjects, which ranged narrowly from 40 to 70 years, as well as the sex. Adipose tissues were sampled only from adult women, and women can eliminate POPs through maternal transfer and lactation (Hardell et al. 2010; Tsang et al. 2011). Similar to PCBs and OCPs, PBDEs were not correlated with subjects’ age, which is consistent with data reported in previous studies (Johnson-Restrepo et al. 2005; Fernandez et al. 2007; Kunisue et al. 2007; Covaci et al. 2008; Pulkrabová et al. 2009; Petreas et al. 2011). In particular, greater levels of PBDEs were found in the youngest age group (40 years old), suggesting that PBDEs have an additional exposure route to humans other than food consumption. Johnson-Restrepo and Kannan (2009) showed house dust to be the major source of human exposure to PBDEs in the United States. Karlsson et al. (2007) showed a positive correlation between PBDE concentrations in house dust and human serum. Whereas the major source of human exposure to legacy POPs, such as PCBs, is dietary (Kannan et al. 1992; Arnich et al. 2009), ingestion of dust is the major pathway for human exposure to PBDEs (Frederiksen et al. 2009). Further studies are needed to assess the magnitude of exposure to PBDEs by way of dust ingestion by the Korean population.
We recently reported concentrations of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) (Moon et al. 2011a), polycyclic aromatic hydrocarbons (PAHs), and synthetic musk compounds (Moon et al. 2011b) in adipose tissue samples from the same set of samples. Relation between BMI and organohalogen contaminants in adipose tissue was investigated using Spearman’s correlation analysis. There were no significant correlations between BMI and concentrations of PCBs, PBDEs, and OCPs, except for HCHs (r = −0.330) and PAHs (r = −0.335) (Table 3). Previous studies showed positive (Arrebola et al. 2010) or negative (Fernandez et al. 2008) correlations between BMI and PCB levels. Wolff et al. (2005) reported a negative correlation between BMI and OC levels in pregnant women from New York in 1998 to 2001. Given the range of results found in previous studies, it appears that BMI is not a sole predictor of OC levels in humans.
Concentrations of PCBs, DDTs, CHLs, HCB, and PCDD/Fs (r = 0.402–0.834) were significantly correlated with each other but not with HCHs. These correlations suggest that the contaminants share similar exposure routes, such as food ingestion and bioaccumulation in human bodies. PBDEs showed low and moderate correlation with other POPs, indicating that their exposure routes and bioaccumulation potential are different. We also investigated the relationship of POPs with synthetic musks. Concentrations of synthetic musks had no relations or negatively correlated with concentrations of POPs, suggesting that musks have different exposure routes, such as dermal exposures (Reiner and Kannan 2006).
Conclusion
The results of this study provide valuable baseline information on exposure to OCs and PBDEs in the Korean general population. The concentrations of legacy POPs, such as PCBs and OCPs, in adipose tissues of Korean women were lower than those reported for other countries, whereas PBDE concentrations were greater than those reported for East Asian and European countries. The accumulation profiles of PBDEs were characterized by the dominance of BDE 209, followed by nona- and octa-BDE congeners, consistent with the consumption pattern of BFRs in Korea. The concentrations of legacy POPs in adipose tissues were correlated with each other, whereas PBDEs showed only moderate correlations with other POPs, suggesting different exposure routes and biotransformation potentials in the human body. The concentrations of OCs and PBDEs in adipose tissues were not correlated with age and BMI of donors.
References
Arnich N, Tard A, Leblanc J-C, Le Bizec B, Narbonne J-F, Maximilien R (2009) Dietary intake of non-dioxin-like PCBs (NDL-PCBs) in France: impact of maximum levels in some foodstuffs. Regul Toxicol Pharmacol 54:287–293
Arrebola JP, Fernazndez MF, Porta M, Rosell J, de la Ossa RM, Olea N, Martin-Olmedo P (2010) Multivariate models to predict human adipose tissue PCB concentrations in Southern Spain. Environ Int 36:705–713
Birnbaum LS, Staskal DF (2004) Brominated flame retardants: cause of concern? Environ Health Perspect 112:9–17
Choi S-D, Baek S-Y, Chang Y-S (2008) Atmospheric levels and distribution of dioxin-like polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in the vicinity of an iron and steel making plant. Atmos Environ 42:2479–2488
Costa LG, Giordano G (2007) Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology 28:1047–1067
Covaci A, Voorspoels S, Roosens L, Jacobs W, Blust R, Neels H (2008) Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in human liver and adipose tissue samples from Belgium. Chemosphere 73:170–175
Fernandez MF, Araque P, Kiviranta H, Molina-Molina JM, Rantakokko P, Laine O et al (2007) PBDEs and PBBs in the adipose tissue of women from Spain. Chemosphere 66:377–383
Fernandez MF, Kiviranta H, Molina-Molina JM, Laine O, Lopez-Espinosa MJ, Vartiainen T et al (2008) Polychlorinated biphenyls (PCBs) and hydroxyl-PCBs in adipose tissue of women in southeast Spain. Chemosphere 71:1196–1205
Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE (2009) Human internal and external exposure to PBDEs―a review of levels and sources. Int J Hyg Environ Health 212:109–134
Hagmar L, Wallin E, Vessby B, Jőnsson BAG, Bergman Å, Rylander L (2006) Intra-individual variations and time trends 1991–2001 in human serum levels of PCB, DDE and hexachlorobenzene. Chemosphere 64:1507–1513
Hardell E, Carlberg M, Nordström M, van Bavel B (2010) Time trends of persistent organic pollutants in Sweden during 1993–2007 and relation to age, gender, body mass index, breast-feeding and parity. Sci Total Environ 408:4412–4419
Helgason LB, Barrett R, Lie E, Polder A, Skaare JU, Gabrielsen GW (2008) Levels and temporal trends (1983–2003) of persistent organic pollutants (POPs) and mercury (Hg) in seabird eggs from Northern Norway. Environ Pollut 155:190–198
Hong SH, Yim UH, Shim WJ, Li DH, Oh JR (2006) Nationwide monitoring of polychlorinated biphenyls and organochlorine pesticides in sediments from coastal environment of Korea. Chemosphere 64:1479–1488
Inoue K, Harada K, Takenaka K, Uehara S, Kono M, Shimizu T et al (2006) Levels and concentrations ratios of polychlorinated biphenyls and polybrominated diphenyl ethers in serum and breast milk in Japanese mothers. Environ Health Perspect 114:1179–1185
Jin J, Wang Y, Yang C, Hu J, Liu W, Cui J et al (2009) Polybrominated diphenyl ethers in the serum and breast milk of the resident population from production area, China. Environ Int 35:1048–1052
Johnson-Restrepo B, Kannan K (2009) An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere 76:542–548
Johnson-Restrepo B, Kannan K, Rapaport DP, Rodan BD (2005) Polybrominated diphenyl ethers and polychlorinated biphenyls in human adipose tissue from New York. Environ Sci Technol 39:5177–5182
Kalantzi OI, Brown FR, Caleffi M, Goth-Goldstein R, Petreas M (2009) Polybrominated diphenyl ethers and polychlorinated biphenyls in human breast adipose samples from Brazil. Environ Int 35:113–117
Kang Y-S, Matsuda M, Kawano M, Wakimoto T, Min B-Y (1997) Organochlorine pesticides, polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and dibenzofurans in human adipose tissue from western Kyungnam, Korea. Chemosphere 35:2107–2117
Kang J-H, Park H, Chang Y-S, Choi J-W (2008) Distribution of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in human serum from urban areas in Korea. Chemosphere 73:1625–1631
Kannan K, Tanabe S, Ramesh A, Subramanian AN, Tatsukawa R (1992) Persistent organochlorines in foodstuffs from India and their implications on human dietary exposure. J Agric Food Chem 40:518–524
Karlsson M, Julander A, van Bavel B, Hardell L (2007) Levels of brominated flame retardants in blood in relation to levels in household air and dust. Environ Int 33:62–69
Kim J-H, Smith A (2001) Distribution of organochlorine pesticides in soils from South Korea. Chemosphere 43:137–140
Koh T-W, Chen SC-C, Chang-Chien G-P, Lin D-Y, Chen F-A, Chao H-R (2010) Breast-milk levels of polybrominated diphenyl ether flame retardants in relation to women’s age and pre-pregnant body mass index. Int J Hyg Environ Health 231:59–65
Kunisue T, Takayanagi N, Isobe T, Takahashi S, Nose M, Yamada T et al (2007) Polybrominated diphenyl ethers and persistent organochlorines in Japanese human adipose tissues. Environ Int 33:1048–1056
La Guardia MJ, Hale RC, Harvey E (2006) Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical mixtures. Environ Sci Technol 40:6247–6254
Law RJ, Allchin CR, de Boer J, Covaci A, Herzke D, Lepom P et al (2006) Levels and trends of brominated flame retardants in the European environment. Chemosphere 64:187–208
Lee S-J, Ikonomou MG, Park H, Baek S-Y, Chang Y-S (2007) Polybrominated diphenyl ethers in blood from Korean incinerator workers and general population. Chemosphere 67:489–497
Li YF (1999) Global technical hexachlorocyclohexane usage and its contamination consequences in the environment: from 1948 to 1997. Sci Total Environ 232:121–158
Lignell S, Aune M, Darnerud PO, Cnattingius S, Glynn A (2009) Persistent organochlorines and organobromine compounds in mother’s milk from Sweden 1996–2006: compound-specific temporal trends. Environ Res 109:760–767
Loganathan BG, Kannan K (1994) Global organochlorine contamination trends: an overview. Ambio 23:187–191
Messer A (2010) Mini-review: polybrominated diphenyl ether (PBDE) flame retardants as potential autism risk factors. Physiol Behav 100:245–249
Moon H-B, Kannan K, Lee S-J, Choi M (2007) Polybrominated diphenyl ethers (PBDEs) in sediment and bivalves from Korean coastal waters. Chemosphere 66:243–251
Moon H-B, Kim H-S, Choi M, Yu J, Choi H-G (2009) Human health risk of polychlorinated biphenyls and organochlorine pesticides resulting from seafood consumption in South Korea, 2005–2007. Food Chem Toxicol 47:1819–1825
Moon H-B, Kannan K, Choi M, Yu J, Choi H-G, An Y-R et al (2010) Chlorinated and brominated contaminants including PCBs and PBDEs in minke whales and common dolphins from Korean coastal waters. J Hazard Mater 179:735–741
Moon H-B, Lee D-H, Lee YS, Kannan K (2011a) Concentrations and accumulation profiles of PCDDs, PCDFs and dioxin-like PCBs in adipose fat tissues of Korean women. J Environ Monit 13:1096–1101
Moon H-B, Lee D-H, Lee YS, Kannan K (2011b, submitted) Occurrence and accumulation patterns of polycyclic aromatic hydrocarbons and synthetic musk compounds in adipose tissues of Korean females. Chemosphere
Park B-K, Park G-J, An Y-R, Choi H-G, Kim GB, Moon H-B (2010) Organohalogen contaminants in finless porpoises (Neophocaena phocaenoides) from Korean coastal waters: contamination status, maternal transfer and ecotoxicological implications. Mar Pollut Bull 60:768–774
Petreas M, Nelson D, Brown FR, Goldberg D, Hurley S, Reynolds P (2011) High concentrations of polybrominated diphenyl ethers (PBDEs) in breast adipose tissue of California women. Environ Int 37:190–197
Pulkrabová J, Hrádková P, Hajšlová J, Poustka J, Nápravníková M, Poláček J (2009) Brominated flame retardants and other organochlorine pollutants in human adipose tissue samples from the Czech Republic. Environ Int 35:63–68
Ramesh A, Tanabe S, Murase H, Subramanian AN, Tatsukawa R (1991) Distribution and behavior of persistent organochlorine insecticides in paddy soils and sediments in the tropical environment: a case study in South India. Environ Pollut 74:293–307
Rappolder M, Schröter-Kermani C, Schädel S, Waller U, Körner W (2007) Temporal trends and spatial distribution of PCDD, PCDF, and PCB in pine and spruce shoots. Chemosphere 67:1887–1896
Reiner JL, Kannan K (2006) A survey of polycyclic musks in selected household commodities from the United States. Chemosphere 62:867–873
Schiavone A, Kannan K, Horii Y, Focardi S, Corsolini S (2010) Polybrominated diphenyl ethers, polychlorinated naphthalenes and polycyclic aromatic musks in human fat from Italy: comparison to polychlorinated biphenyls and organochlorine pesticides. Environ Pollut 158:599–606
Shaw SD, Kannan K (2009) Polybrominated diphenyl ethers in marine ecosystems of the American continents: foresight from current knowledge. Rev Environ Health 24:157–229
Shaw SD, Berger ML, Brenner D, Kannan K, Lohmann N, Päpke O (2009) Bioaccumulation of polybrominated diphenyl ethers and hexabromocyclododecane in the northwest Atlantic marine food web. Sci Total Environ 407:3323–3329
Stapleton HM, Dodder NG, Kucklick JR, Reddy CM, Schantz MM, Becker PR et al (2006) Determination of HBCD, PBDEs and MeO-BDEs in California Sea lions (Zalophus californianus) stranded between 1993 and 2003. Mar Pollut Bull 52:522–531
Takasuga T, Senthilkumar K, Takemori H, Ohi E, Tsuji H, Nagayama J (2004) Impact of fermented brown rice with Aspergillus oryzae (FEBRA) intake and concentrations of polybrominated diphenyl ethers (PBDEs) in blood of humans from Japan. Chemosphere 57:795–811
Tan J, Li QQ, Laganath A, Chong YS, Xiao M, Obbard JP (2008) Multivariate data analyses of persistent organic pollutants in maternal adipose tissue in Singapore. Environ Sci Technol 42:2681–2687
Thuresson K, Hoglund P, Hagmar L, Sjödin A, Bergman Å, Jakobsson K (2006) Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect 114:176–181
Tsang HL, Wu S, Leung CKM, Tao S, Wong MH (2011) Body burden of POPs of Hong Kong residents, based on human milk, maternal and cord serum. Environ Int 37:142–151
United Nations Environment Program (2009) The 9 new POPs under the stock convention. http://www.chm.pops.int/. Accessed 4 April 2010
Wang Y, Jiang G, Lam PKS, Li A (2007) Polybrominated diphenyl ether in the East Asian environment: a critical review. Environ Int 33:963–973
Watanabe I, Sakai S-I (2003) Environmental release and behavior of brominated flame retardants. Environ Int 29:665–682
Willett KL, Ulrich EM, Hites RA (1998) Differential toxicity and environmental fates of hexachlorocyclohexane isomers. Environ Sci Technol 32:2197–2207
Wolff MS, Deych E, Ojo F, Berkowitz GS (2005) Predictors of organochlorines in New York City pregnant women, 1998–2001. Environ Res 97:170–177
Won JH, Hong SH, Shim WJ, Yim UH, Kim GB (2009) Persistent organochlorine pollutants in Korean offshore waters: squid (Todarodes pacificus) as a biomonitor. Mar Pollut Bull 58:1238–1244
Yang Y, Li D, Mu D (2008) Levels, seasonal variations and sources of organochlorine pesticides in ambient air of Guangzhou, China. Atmos Environ 42:677–687
Acknowledgments
We thank all of the women who donated tissues for this study. This study was funded by a grant from the National Fisheries Research and Development Institute (RP-2011-ME-011) and the Ministry of Land, Transport and Maritime Affairs, Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moon, HB., Lee, DH., Lee, Y.S. et al. Polybrominated Diphenyl Ethers, Polychlorinated Biphenyls, and Organochlorine Pesticides in Adipose Tissues of Korean Women. Arch Environ Contam Toxicol 62, 176–184 (2012). https://doi.org/10.1007/s00244-011-9679-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-011-9679-6