Abstract
Siloxanes are used in a wide range of industrial applications, such as fuel additives, automotive polishes and waxes, and antifoaming agents, as well as in personal care products and biomedical devices. Despite the potential for environmental occurrence, few studies have reported sources and pathways of siloxanes in the environment. In this study, we determined concentrations of four cyclic siloxanes, namely, octamethylcyclotetrasiloxane (D4), decamethylcyclopentasiloxane (D5), dodecamethylcyclohexasiloxane (D6), and tetradecamethylcycloheptasiloxane (D7) as well as 13 linear siloxanes (L4 to L16), in sediments sampled from the Songhua River and in sewage sludge sampled from treatment plants that discharge wastewater into the Songhua River in northeastern China. Siloxanes were found in all of the sediment samples at total concentrations (sum of all cyclic and linear siloxanes) as high as 2050 ng/g dry weight (dw). The respective total concentration ranges for cyclic (D4, D5, D6, and D7) and linear (L4 to L16) siloxanes in sewage sludge were 602 to 2360 and 98 to 3310 ng/g dw. Cyclic siloxanes were found at greater concentrations than linear siloxanes in sediment samples. However, the summed concentrations of linear siloxanes were higher than the summed concentrations of cyclic siloxanes in sludge samples. Among cyclic siloxanes, D7 and D5 were the dominant compounds in both sediment and sludge. Among linear siloxanes, L10 and L11 together accounted for 55% of the total concentrations in sludge samples, and L6 accounted for 30% of the total concentration in sediment samples. To our knowledge, this is the first report to document concentrations and compositions of organosiloxanes in sediments and sewage sludge from China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Siloxanes are polymeric organic silicon molecules consisting of a backbone of alternating silicon–oxygen [Si–O] units with organic side-chains attached to each silicon atom (Hamelink 1992; Allen et al. 1997). During the last three decades, organosilicones comprising both cyclic and linear siloxanes (e.g., polydimethylsiloxane or PDMS) have been widely used in the manufacture of electronics, furniture, cookware, cosmetics, healthcare products, and medical devices because siloxanes have low surface tension, high thermal stability, and smooth texture and are believed to be chemically inert. Annual production of octamethylcyclotetrasiloxane (D4, where D refers to the number of Si–O units that make up the chain), which is used as a base material for the production of polymeric silicones, is 100 to 500 million pounds (www.ewg.org/reports/cheminventory). There is a great potential for dispersal of siloxanes into the environment from consumer products and from process aids that are discharged into wastewater (Allen et al. 1997), or are land filled (Hamelink 1992).
Siloxanes have low water solubility (0.002 to 0.056 mg/l at 25°C) (Varaprath et al. 1996) and have been reported to elicit toxic effects in aquatic organisms (Fendinger et al. 1997a; Watermann et al. 1997; Stevens and Annelin 1997). Furthermore, cyclic siloxanes have been implicated in endocrine disruption, connective tissue disorders, adverse immunologic responses, and fatal liver and lung damage in exposed laboratory animals (Hayden and Barlow 1972; Granchi et al. 1995; Lieberman et al. 1999; He et al. 2003; Quinn et al. 2007). The study of oral exposure to D4 in mice showed weak estrogenic activity, mediated through estrogen receptor-alpha (He et al. 2003); D4 has been shown to bind to estrogen receptor-alpha, but not to estrogen receptor-beta, in studies in vivo and in vitro (Quinn et al. 2007). Inhalation exposure of rats to cyclic siloxanes for 3 months at doses greater than several hundreds of parts per million in air caused an increase in liver weight and a decrease in ovary weight (Burns-Naas et al. 1998, 2002).
The low water solubility of siloxanes suggests that these compounds will be partially removed from the aqueous phase during sewage treatment by adsorption onto sludge (Watts et al. 1995; Fendinger et al. 1997b). Some siloxanes are released within the wastewater stream as suspended solids; in the receiving waters they are eventually deposited into sediments. Consequently, high concentrations of siloxanes are expected to exist in sediments in areas affected by or that receive wastewater treatment plant (WWTP) effluents. However, limited information is available on the concentration ranges, distribution, and fate of siloxanes in surface sediments. In this study, concentrations and distribution of siloxanes were determined in surface sediments collected along the Songhua River, which is the third largest river in China. Sewage sludge samples collected from WWTPs located along the Songhua River were also analyzed to enable characterization of cyclic and linear siloxanes in sludge. The data are useful for further evaluation of potential risks and pathways of siloxanes in the riverine ecosystem. Since 2009, Chinese siloxane monomer production capacity has expanded to 1 million tons/y as many new silicone projects were put into operation (Bai 2010). To our knowledge, this is the first study to report siloxanes in sediments and sludge from China.
Materials and Methods
Study Area and Sampling Locations
The Songhua River is the largest tributary of the Heilong River in northeastern China and flows for 1927 km from the Changbai Mountains through Heilongjiang and Jilin provinces; both of the latter have a long history of intensive industrial operations. The Songhua River has two main tributaries, the Nen River and the Secondary Songhua River, with a total watershed area of 550,000 km2. The Songhua River flows into the Heilong River at the border between China and Russia.
Twenty-five sediment samples were collected downstream of major cities and main tributaries of the Songhua River (Fig. 1). Eight sludge samples were collected from eight WWTPs serving seven large cities located along the river: Harbin (HRB; the capital of Heilongjiang Province; population 4.89 million), Jiamusi (JMS; population 0.82 million), Mudanjiang (MDJ; a major city located along the Mudan River, which is a tributary of the Songhua River; population 0.8 million), Qiqihar (QQHR; population 1.44 million), Changchun (CC; the capital of Jilin Province, population 3.68 million), Jilin (JL; population 2 million), and Songyuan (SY; population 0.52 million), in Heilongjiang and Jilin Provinces, during July 2009. Surface sediment (0 to 20 cm) and sludge samples were collected and stored in aluminum containers, freeze dried, and kept at −20°C in dark. Sediment samples taken from several spots (three to four within 10 m) at a given sampling location were pooled to obtain a representative sample. Total organic carbon (TOC) of sediment and sludge samples was determined by TOC analyzer (TOC-VCPH, Shimadzu, Japan).
Reagents and Standards
Octamethylcyclotetrasiloxane (D4), decamethylcyclopentasiloxane (D5), and dodecamethylcyclohexasiloxane (D6) standards were obtained from Tokyo Chemical Industry (Wellesley Hill, MA). PDMS 200 fluid (viscosity of 5 cSt), which contains tetradecamethylcycloheptasiloxane (D7) and linear siloxanes (L4 to L16), was obtained from Sigma-Aldrich (St. Louis, MO). Tetrakis(trimethylsiloxy)-silane (M4Q; purity 97%) was from Aldric, and was used as surrogate standard. PCB-30 was purchased from AccuStandard (New Haven, CT) and was used as internal standard. Organic solvents used in this study were of analytic grade and were purchased from Mallinckrodt Baker (Phillipsburg, NJ).
Extraction and Cleanup
Four cyclic and 13 linear solixanes were determined in sediment and sludge samples according to a method described previously (Horii and Kannan 2008) with slight modifications. Five grams of sediment or 1 g sludge (previously freeze dried and homogenized) was taken in a 50-ml polypropylene tube, and 500 ng M4Q were spiked as a surrogate standard. After 1 h of equilibration, 25 ml of a mixture of ethyl acetate/n-hexane (1:1 v/v) was added, shaken for 30 min, and centrifuged at 3000 rpm for 5 min, and the solvent layer was then transferred into a round-bottom flask. The extraction was repeated two more times, and the extracts were combined into the round-bottom flask. The extract was concentrated by rotary evaporation to approximately 2 to 3 ml, and 5 ml isooctane was added and evaporated under a gentle stream of nitrogen to approximately 1 ml. The extract was further purified by passage through a silica gel (0.5-g, Grade 634, pore size 60 A, 100–200 mesh; Sigma-Aldrich, St. Louis, MO) packed glass column, which was eluted with 12 ml dichloromethane/n-hexane (1:4 v/v). The eluate was concentrated, and 10 ng PCB-30 was spiked as an internal standard for gas chromatograph (GC)–mass spectrometer (MS) analysis.
Instrumental Analysis
Because some siloxanes are found in components of the GC and in the stationary phase of the capillary chromatograph column, steps were taken to decrease the instrumental background as described in our earlier study (Horii and Kannan 2008). Concentrations of linear and cyclic siloxanes were determined by Agilent 6890 GC interfaced with an Agilent 5973 MSD (Agilent Technologies, Foster City, CA). GC separation was accomplished by use of a 30-m Rxi-5MS fused silica capillary column (0.25-mm inner diameter and 0.25-μm film thickness; Restek, Bellefonte, PA). One microliter of the aliquot was injected in splitless mode at 200°C. The column oven temperature was programmed from 40°C for 2 min, to 220°C at a rate of 20°C min−1, and to 280°C at 5°C min−1, and held for 10 min; post-runtime at 300°C was 5 min. The MS was operated in an electron impact selected ion monitoring mode. The ions were monitored at m/z 281 for D4; m/z 255 and 267 for D5; m/z 341 and 429 for D6; m/z 281, 147, and 341 for D7; m/z 207 and 295 for L4; m/z 281, 147, and 369 for L5; m/z 221, 281, and 355 for L6; m/z 221, 147, and 295 for L7 to L16; m/z 281, 369, and 147 for M4Q; and m/z 256 for PCB-30. A PDMS mixture with known composition and content of linear siloxanes was used for the identification and quantification of linear siloxanes, as reported in our earlier study (Lykissa et al. 1997; Horii and Kannan 2008; Lu et al. 2010).
Quality Assurance and Quality Control
Because organosilicone compounds are present in many consumer products, the analyst took care to avoid the use of hand lotions or other possible sources of contamination before or during the analysis. Before analysis, solvents and standards were injected into the GC as a check of column performance, instrumental background, resolution, and sensitivity. With each batch of 10 samples analyzed, a procedural blank, a spike blank, a matrix spike (100 ng g−1 for D4, D5, D6 and M4Q and 1000 ng g−1 for PDMS), and a matrix spike duplicate were analyzed in sequence to check for contamination and for peak identification and quantification. Mean recoveries were 78.7% ± 11.3% for all target compounds in the matrix spike, and the relative SD (n = 4 analyses) of duplicate analyses was 5 to 12%.
A series of injections of target compounds at various concentrations (2, 5, 10, 20, 50, 100, and 200 ng ml−1 for D4, D5, D6 and 20, 50, 100, 200, 500, 1000, and 2000 ng ml−1 for PDMS), with a constant level of the internal standard (PCB-30; 100 ng ml−1), were performed to determine the dynamic range of the instrument. Good linearities were observed, with correlation coefficients >0.994 for all target compounds. Calibration curves were obtained by linear regression analysis to calculate concentrations. The limit of quantification (LOQ) was set to be three times the levels found in procedural blanks: 0.5 ng g−1 for D4 and D6, and 1.0 ng g−1 for D5 and D7. None of the 13 linear siloxanes was found in procedural blanks. The LOQ was 0.28 ng g−1 for L4, 0.86 ng g−1 for L5, 0.35 ng g−1 for L6 to L10, 0.52 ng g−1 for L11 to L13, and 2.0 ng g−1 for L14 to L16. A quality-control standard mixture of target compounds and n-hexane (solvent) was injected after every five samples to check for instrumental background, carryover, and stability. The term “total siloxane concentration” refers to the sum of the 4 cyclic and 13 linear siloxanes analyzed in this study.
Result and Discussion
Levels and Geographic Distribution in Sediments
The geographic distribution of concentrations of total siloxanes (Σ cyclic and linear siloxanes) in sediments from the Songhua River is shown in Fig. 2. Mean, median, and range of concentrations of cyclic and linear siloxanes in sediment and sewage sludge are listed in Table 1 and Fig. 3a. The cyclic siloxanes were detected in all 25 sediment samples from the Songhua River. Concentrations of D4, D5, D6, and D7 were in the ranges of 1 to 33, 3 to 155, 2 to 527, and 2 to 1350 ng g−1 dry weight (dw), respectively. The sum of cyclic siloxane concentration (ΣD4, D5, D6, and D7) ranged from 8 to 2040 ng g−1 dw (mean 259). Among linear siloxanes, L15 and L16 were not detected in any of the sediment samples analyzed, and L4, L5, and L14 were rarely detected in sediments. The sum of linear siloxane concentrations (ΣL4 to L16) ranged from 1 to 80 ng g−1 dw (mean 13). Six of the 25 sediment samples were from HRB, the largest city located along the Songhua River. Concentrations of total siloxanes in sediments varied widely from 10 to 2050 ng g−1 dw. Concentrations of cyclic and linear siloxanes found in sediments in our study are similar to concentrations reported from sediments in Denmark (Kaj et al. 2005); the highest concentration of cyclic siloxanes reported for the Danish sediments was 2300 ng g−1 dw, and the highest concentration of linear siloxanes was 87 ng g−1 dw (Kaj et al. 2005). A study of freshwater and marine sediments sampled downstream of municipal WWTPs in the United States (Powell et al. 1999) showed linear siloxane concentrations ranging from lower than the method detection limit (200 ng g−1 dw) to 34,200 ng g−1 dw. Concentrations of linear siloxanes reported for sediments collected from several locations in North America, Europe, and Japan were in the range of 600 to 16,600 ng g−1 dw (Fendinger et al. 1997a).
a Box plot of concentrations of individual cyclic and summed linear siloxanes in surface sediment samples (n = 25) and b WWTP sludge samples (n = 8) from the Songhua River. The lower and upper boundaries of the box represent the 25th and 75th percentiles, and the line within the box is the median concentration. The dots are individual concentration values
The highest concentrations of cyclic and linear siloxanes were 2040 and 14 ng g−1 dw, respectively, found in sediment collected downstream of JL, which has several chemical industrial complexes; Jilin Chemical Industry produces 150,000 tons of silicone/year. The surface sediment sampled downstream of JL (viz., TLZ and SY), also contained high concentrations cyclic and linear siloxanes. The mean concentrations of D4, D5, D6, D7, and ΣL4 to L16 (sum of linear) in sediments from JL, TLZ, and SY (collectively, the Secondary Songhua River area) were 7, 134, 373, 668, and 24 ng g−1 dw, all of which were higher than overall mean concentrations (Table 1) for the entire study area, except for D4. Generally, concentrations of siloxanes were higher in sediment collected in locations downstream of cities than in locations upstream, suggesting that urban areas contribute to contamination of siloxanes in rivers. Furthermore, the concentrations of siloxanes were higher in sediments collected downstream of large cities than downstream of small cities.
Concentrations and Profiles of Siloxanes in WWTP Sludge
In WWTP sludge samples, the range of concentrations of total cyclic siloxanes (D4, D5, D6, and D7) was 602 to 2360 ng g−1 dw (mean 997). The concentrations of L4 and L5 in sludge samples were lower than the LOQ; the respective concentrations of L6 through L7, L8, L9, L10, L11, L12, L13, L14, L15, and L16 were <0.35 to 13, 6 to 89, <0.35 to 248, 3 to 494, 30 to 972, 34 to 900, 14 to 401, 7 to 161, 5 to 84, <2 to 70, and <2 to 72 ng g−1 dw, respectively. The concentrations of total siloxanes in sludge were in the range of 1410 to 3920 ng g−1 dw (mean 2740). The distributions of concentrations of D4 to D7 and detectable L6 to L16 in sludge are shown in Fig. 3b. The concentrations of D5 and D7 were greater than the concentrations of D4 and D6. For linear siloxanes, mean concentrations increased with increasing chain length from L6 to L10, but they decreased from L11 to L16. Concentrations of cyclic siloxanes that have been reported for sewage sludge from some European countries were significantly higher than the concentrations found in our study. The average concentration of the sum of D4, D5 and D6 was 26,000 ng g−1 dw in sludge from Scandinavian countries (Kaj et al. 2005). Sludge sample from a sewage treatment plant in Finland contained the highest concentration of D4 to D6, i.e., 100,000 ng g−1 dw (Kaj et al. 2005). Concentrations of D4 to D6 found in sludge from two small Swedish sewage treatment plants were 5500 to 6700 ng g−1 dw. Concentrations of linear siloxanes (MM, MDM, MD2M, and MD3M) measured in sludge from the Scandinavian countries were markedly lower than concentrations of the cyclic siloxanes, with an average concentration of 110 ng g−1 dw (sum of four compounds) (Kaj et al. 2005). That pattern differed from the pattern in our study: We found higher concentrations of linear siloxanes than cyclic siloxanes in sludge.
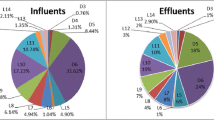
The composition profile of cyclic and linear siloxanes in our study differed between sludge and sediment (Fig. 4a). In the sediment samples, D4, D5, D6, and D7, respectively, accounted for 3.8, 26, 22 and 32% of the total siloxane content, whereas linear siloxanes (ΣL4 to L16) accounted for only 4.9% of the total siloxane content. In sludge samples, D4, D5, D6, and D7, respectively, accounted for 2.2, 11, 4.7, and 16% of the total siloxane content, and linear siloxanes (ΣL4 to L16) accounted for 67% of the total siloxane content. Among the four cyclic siloxanes that we analyzed, the concentration of D5 was higher than the concentrations of D4 and D6 in both sludge and sediment samples. Such a pattern could suggest either a high degree of absorption of D5 to sludge or else greater production and use of this compound than the other siloxanes. In a previous study of American and Japanese consumer products, the highest concentrations of D5 and D6 were found in cosmetics (e.g., liquid foundation), whereas the highest concentrations of D4 and D7 were found in household cleaning products (e.g., furniture polish) and baby bottle nipples, respectively. The frequency of occurrence and concentrations of D5 were higher than for other cyclic siloxanes (Horii and Kannan 2008). A similar pattern was found in our Chinese sediments, with higher median concentrations of D5 than of the other three cyclic siloxanes. Because of the differences in the abundance of linear siloxanes between sediment and sludge, we examined the profiles of linear siloxanes in sediment and sludge separately (Fig. 4b). In sediments, L6 (29.9%) was the most abundant linear siloxane, followed by L7 (18.1%), L8 (8.93%), L9 (12.0%), L10 (12.2%), and L11 (11.8%); the abundances of the remaining linear siloxanes were much lower (0.8 to 2.5%). In sludge, L10 and L11 were the major linear siloxanes, accounting for 29.5 and 25.3% of the total linear siloxane concentrations, respectively; L4 and L5 were not detected in sludge. The profile of linear siloxanes in sludge samples was similar to that previously reported for personal care and household products, with high L11 concentrations followed by lower L10, L12, and L9 concentrations (Horii and Kannan 2008).
a Profiles (%) of cyclic siloxanes (D4, D5, D6, and D7) and linear siloxanes (ΣL4 to L16) and b profiles (%) of individual linear siloxanes (L4 to L16) in total concentration linear siloxanes in sediment and sludge samples from northeastern China. The upper boundaries represent mean abundance, and the whiskers represent the SD
Relation of Siloxanes with Total Organic Carbon
A significant positive correlation existed between the concentrations of cyclic siloxanes and the concentrations of linear siloxanes in sediment samples (Fig. 5a). This correlation suggests that cyclic and linear siloxanes derive from similar sources. However, a negative correlation existed between the concentrations of cyclic siloxanes and those of linear siloxanes in sludge samples (Fig. 5b). TOC in sludge varied from 136 to 324 mg g−1. The correlation coefficient (R 2) between TOC and linear siloxane concentrations in sludge was 0.40 (Fig. 6). This correlation indicates that linear siloxanes bind to organic matter. There was no correlation between TOC and cyclic siloxane concentrations in sludge. The pattern of high concentrations of linear siloxanes in sludge, but the lower composition of these compounds in sediment, suggests more efficient removal of linear siloxanes than cyclic siloxanes in wastewater-treatment processes. Linear siloxanes may have strong binding affinity to particulate matter and TOC such that they partition to sludge more strongly than do cyclic siloxanes. We also sought a relation between pH and siloxane concentrations in sediments of the Songhua River, but no significant associations were found.
a Correlation between concentrations of total cyclic siloxane (D4 to D7) and concentrations of total linear siloxane (L4 to L16), in sediment samples from the Songhua River, northeastern China, and b correlation between concentrations of total cyclic siloxanes (D4 to D7) and concentrations of total linear siloxanes (L4 to L16) in WWTP sludge samples collected from northeastern China
We examined the relations among the concentrations of various siloxanes in sediment and sludge samples using Pearson correlation analysis (Table 2). Significant positive correlations (p < 0.01) were found between the pairs D5 and D6, D5 and D7, and D6 and D7 in surface sediments, and a significant positive correlation (p < 0.01) was found between pair D6 and D7 in sludge. A large number of siloxane blends have been developed for use in a variety of consumer products. The sources of siloxanes in the aquatic environment are effluents of the WWTPs (Allen et al. 1997). Positive correlations among D5, D6, and D7 in sediment, and the lack of correction between D5 and D6 or D5 and D7 in sludge, suggest the differential removal and possible degradation of these cyclic siloxanes in wastewater-treatment processes.
Conclusion
Cyclic and linear siloxanes were found in all of the sediment samples that we analyzed from the Songhua River and in all of sewage sludge samples collected from WWTPs in northeastern China. Total concentration ranges of cyclic (sum of D4, D5, D6, and D7) and linear siloxanes (sum of L4 to L16) were 7.94 to 2040 and 1.14 to 79.9 ng g−1 dw in sediments and 602 to 2360 and 97.7 to 3310 ng g−1 dw in sludge, respectively. Compared with the concentrations that have been reported for Scandinavian countries, total concentrations of siloxanes in sediment and sludge from the Songhua River watershed were lower. There was a strong geographic gradient in concentrations of cyclic siloxanes in sediments: Samples collected directly downstream of large cities contained higher concentrations of siloxanes. The composition of siloxanes differed considerably between sediment and sludge, suggesting differences in removal efficiencies for these two classes of siloxanes in WWTPs. D7 and D5 were the dominant cyclic siloxanes in all of the samples, whereas L10 and L11 were the dominant linear siloxanes (sum of two 54.8%) in sludge, whereas L6 dominated (29.9%) in sediment. To our knowledge, this is the first study to report concentrations and compositions of siloxanes in sediments and sludge from China.
References
Allen RB, Kochs P, Chandra G (1997) Organosilicon Materials. In: Chandra G (ed) The handbook of environmental chemistry, vol 3. Part H. Springer-Verlag, Berlin, pp 1–25
Bai B (2010) Top 10 news of 2009. China Silicone News 3:4–8
Burns-Naas LA, Mast RW, Meeks RG et al (1998) Inhalation toxicology of decamethylcyclopentasiloxane (D5) following a 3-month nose-only exposure in Fischer 344 rats. Toxicol Sci 43:230–240
Burns-Naas LA, Meeks RG, Kolesar GB et al (2002) Inhalation toxicology of octamethylcyclotetrasiloxane (D4) following a 3-month nose-only exposure in Fischer 344 rats. Int J Toxicol 2:39–53
Fendinger NJ, Mcavoy DC, Lehmann RG, Mihaich EM (1997a) Polydimethylsiloxane. In: Chandra G (ed) The handbook of environmental chemistry, vol 3. Part H. Springer-Verlag, Berlin, pp 181–223
Fendinger NJ, Mcavoy DC, Eckhoff WS, Price BB (1997b) Environmental occurrence of polydimethylsiloxane. Environ Sci Technol 31:1555–1563
Granchi D, Cavedagna D, Ciapetti G et al (1995) Silicone breast implants: the role of immune-system on capsular contracture formation. J Biomed Mater Res 29:197–202
Hamelink JL (1992) Silicones. In: Hutzinger EO (ed) The handbook of environmental chemistry, vol 3. Part F. Springer-Verlag, Berlin, pp 383–394
Hayden JF, Barlow SA (1972) Structure-activity relationships of organosiloxanes and the female reproductive system. Toxicol Appl Pharmacol 21:68–79
He B, Rhodes-Brower S, Miller MR, Munson AE, Germolec DG, Walker VR, Korach KS et al (2003) Octamethylcyclotetrasiloxane exhibits estrogenic activity in mice via ER alpha. Toxicol Appl Pharmacol 192:54–261
Horii Y, Kannan K (2008) Survey of organosilicone compounds, including cyclic and linear siloxanes, in personal-care and household products. Arch Environ Contam Toxicol 55:701–710
Kaj L, Schlabach M, Andersson J, Palm Cousins A, Schmidbauer N, Brorstrom-Lunden E (2005) Siloxanes in the Nordic environment. TemaNord 593
Lieberman MW, Lykissa ED, Barrios R et al (1999) Cyclosiloxanes produce fatal liver and lung damage in mice. Environ Health Perspect 107:161–165
Lu Y, Yuan T, Yun SH, Wang W, Wu Q, Kannan K (2010) Occurrence of cyclic and linear siloxanes in indoor dust from China, and implications for human exposures. Environ Sci Technol 44:6081–6087
Lykissa ED, Kala SV, Hurley JB, Lebovitz RM (1997) Release of low molecular weight silicones and platinum from silicone breast implants. Anal Chem 69:4912–4916
Powell DE, Annelin RB, Gallavan RH (1999) Silicone in the environment: a worst-case assessment of poly(dimethylsiloxane) (PDMS) in sediments. Environ Sci Technol 33:3706–3710
Quinn AL, Regan JM, Tobin J, Marinik BJ, McMahon JM, Plotzke KP (2007) In vitro and in vivo evaluation of the estrogenic, androgenic, and progestagenic potential of two cyclic siloxanes. Toxicol Sci 96:145–153
Stevens C, Annelin RB (1997) Ecotoxicity testing challenges of organosilicone materials. In: Chandra G (ed) Organosilicone materials. Springer-Verlag, Berlin, p 83
Varaprath S, Frye CL, Hamelink J (1996) Aqueous solubility of permethylsiloxanes (silicones). Environ Toxicol Chem 15:1263–1265
Watermann B, Berger HD, Sonnichsen H, Willemsen P (1997) Performance and effectiveness of non-stick coatings in seawater. Biofouling 11:101–118
Watts RJ, Kong SH, Haling CS, Gearhart T, Frye CL, Vigon BW (1995) Fate and effects of polydimethylsiloxanes on pilot and bench-top active-sludge reactors and anaerobic-aerobic digesters. Water Res 29:2405–2411
Acknowledgments
We are grateful for financial support from the National Natural Science Foundation of China (Grant No. 50638020) and the State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin, China (Project 2008DX01) during the sampling portion of the study. Funding for the method development (analytic) portion was provided by a biomonitoring grant from Centers for Disease Control and Prevention (Atlanta, GA; 1U38EH000464-01) to the Wadsworth Center, New York State Department of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Z., Qi, H., Ren, N. et al. Survey of Cyclic and Linear Siloxanes in Sediment from the Songhua River and in Sewage Sludge from Wastewater Treatment Plants, Northeastern China. Arch Environ Contam Toxicol 60, 204–211 (2011). https://doi.org/10.1007/s00244-010-9619-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-010-9619-x