Abstract
The relative contributions of proximity to mercury sources and trophic ecology to realized axial muscle mercury concentrations were explored for three deep-water chondrichthyans (Etmopterus princeps, Centroscymnus coelolepis, and Hydrolagus affinis), two species of which are harvested for human consumption. Samples were taken at three North Atlantic Ocean locations: the Azores, the Charlie Gibbs Fracture Zone, and the Bear Seamount. Despite the long distances between anthropogenic sources and the sampling locations, all species from all locations had muscle mercury concentrations exceeding the United States human health screening value of 0.3 mg/kg wet weight. Proximity to anthropogenic sources was not an obvious determinant of these elevated concentrations. Generally, mercury concentrations appeared to increase with increased dependence on benthic versus pelagic food sources (as indicated by interspecies differences in δ13C), and with higher position in the trophic web (as indicated by differences in δ15N).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mercury is a major contaminant of concern because of its global atmospheric dispersion, its propensity to biomagnify after methylation, and its high toxicity. Numerous fish species have muscle mercury concentrations higher than consumption advisory limits (Kraepiel et al. 2003; Mason et al. 2006; Sunderland 2007), and health risk from fish consumption has been reported by Fujino (1994), Grandjean et al. (1997), and Crump et al. (2000). Consequently, understanding determinants of mercury concentration in fishes is important to human risk management.
Human exposure in any particular instance might involve an ill-defined mixture of mercury from natural and anthropogenic sources. Currently, natural mercury constitutes the smaller portion of total global atmospheric deposition (Ebinghaus et al. 1999; Pirrone et al. 2001), with the most prominent natural sources being volcanic activity, rock weathering, and oceanic emission. Most mercury is currently released into the environment from diverse human sources (Manohar et al. 2002). Inorganic mercury released from anthropogenic or natural sources is methylated and becomes available for biomagnification.
Although both anthropogenic and natural sources contribute to fish tissue mercury, their relative contributions vary widely among situations. In addition, the trophic position of finfish species is influential in determining mercury tissue concentrations as a consequence of methylmercury biomagnification (Cabana and Rasmussen 1994). Ruelas-Inzunza and Páez-Osuna (2005) measured high mercury concentrations in sharks from Altata-Ensensda del Pabellόn lagoon (Mexico), compared them with those of the same species from Guaymas Harbor, and concluded that Altata-Ensensda del Pabellόn lagoon had a higher input of anthropogenic mercury. Differences in shark trophic ecology at the two locations were not explored. Relative to accumulation from natural sources, high mercury concentrations were reported from deep-water fishes caught at the Mid-Atlantic Ridge, notionally as a consequence of hydrothermal vent mercury emissions (Martins et al. 2006). Elevated mercury concentrations have been observed in species inhabiting still other regions remote from anthropogenic sources, e.g., polar bears in the Arctic (Eaton and Farant 1982) and deep-water sharks from Northern Australia (Lyle 1986), tropical Queensland (Denton and Breck 1981), and the Mediterranean Sea (Hornung et al. 1993; Storelli et al. 2002).
In this study, mercury concentrations and stable nitrogen and carbon isotope ratios were measured in three deep-water chondrichthyans (Etmopterus princeps, Centroscymnus coelolepis, and Hydrolagus affinis) collected from three North Atlantic Ocean locations. Stable isotope ratios that suggest trophic position (δ13C and δ15N) and other features of trophic ecology were compared among species and locations to better understand general patterns of mercury accumulation in deep-water chondrichthyans from remote locations, and to gauge any associated human risk from consumption of deep-water fish.
Materials and Methods
Species Descriptions
Great lanternshark (E. princeps) and Portuguese dogfish (C. coelolepis) are sharks of the order Squaliformes (subclass Elasmobranchii). The third species, small-eyed rabbitfish (H. affinis), is a chimaera (subclass: Holocephali). The great lanternshark feeds on teleosts (46% of diet), cephalopods (46% of diet), and decapod crustaceans (8% of diet) (Cortés 1999) and has an estimated trophic position of 4.1 (Cortés 1999). Portuguese dogfish feed more diversely (79% cephalopods, 11% teleosts, 4% mammal, 4% decapod crustaceans, 1% other invertebrates, and 1% chondrichthyans) and has a slightly higher trophic position of 4.2 (Cortés 1999). In contrast, information from H. mirabilis (Mauchline and Gordon 1983) suggests that small-eyed rabbitfish consume small fishes and invertebrates nearer the ocean bottom and occupy a lower trophic position of 3.5.
Portuguese dogfish is harvested commercially for liver oil and flesh (Stevens and Correia 2003; Compagno et al. 2005; Kyne and Simpendorfer 2007). Great lanternshark is taken as by-catch (Kyne and Simpendorfer 2007) and sold for human consumption in the European market. Although rarely harvested as seafood, small-eyed rabbitfish was included in this survey because it has a contrasting trophic ecology, i.e., a lower trophic position and heavier reliance on benthic food sources than the two sharks.
Species Collection
Specimens were taken opportunistically from stations near the Azores (N42°40′, W29°00′), Charlie Gibbs Fracture Zone (N52°40′, W32°35′), and Bear Seamount (N39°55′, W67°25′) (Fig. 1). E. princeps was taken at all locations and H. affinis was taken at the Azores and Charlie Gibbs Fracture Zone sites, but C. coelolepis was taken only at the Azores site. Each Azores and Charlie Gibbs Fracture Zone specimen was collected during the Mid-Atlantic Ridge Ecology (MAR-ECO) expedition (Bergstad and Godo 2003), measured and determined on board for total length and sex, and processed as described by Fossen et al. (2008). Generally, the sex ratio for individuals taken from the locations was 1:1. Slightly more female than male C. coelolepis (female-to-male [F:M] ratio = 7:6) were analyzed from the Azores, and H. affinis analyzed from the Azores (F:M ratio = 7:9) and Bear Seamount (F:M ratio = 5:4) also deviated slightly from the general 1:1 F:M ratio.
Dorsal fin spines, collected to age individuals (Cotton 2010), and attached small wedges of muscle were frozen on the vessel and remained frozen until processed. Additional specimens of E. princeps were taken from Bear Seamount by bottom trawl during the March 2008 Research Vessel Delaware II cruise, according to the methods outlined by Moore et al. (2003). Frozen whole E. princeps were thawed in the laboratory, measured for total length, and sexed before dissection. Bear Seamount E. princeps tissue samples were taken from the liver, kidney, cartilage, gill, brain, and muscle adjacent to the dorsal fin spine.
Sample Preparation
Muscle tissue was taken from the right and left sides of the first and second dorsal fin spines of the sharks and from both sides of the single dorsal fin spine of H. affinis. All samples were dried on a Freezone 4.5 freeze-dryer (Labconco, Kansas, MO). The dry samples were then split for mercury and stable isotope ratios (δ13C and δ15N) analyses. The question about whether a single sample from any spine was sufficiently representative of axial muscle mercury concentration (wet weight [ww]) of a specimen was answered with variance components analysis (SAS PROC NESTED, version 9.2; SAS, Cary, NC) of the large MAR-ECO E. princeps data set (n = 162 individual muscle samples). Of the total variance in the data set, only minimal amounts of variance were associated with differences between two spines (2%) or between two samples taken on either side of a spine (<1%), indicating that taking one muscle sample from either spine was adequate to represent muscle concentration for an individual fish.
Total Mercury Analysis
Total mercury was measured in ground, freeze-dried samples with a Direct Mercury Analyzer-80 (Milestone, Shelton, CT), which required only a few milligrams of sample. Total mercury concentration was estimated on a dry-weight basis and then converted to ww values (mg/kg ww) using measured wet/dry quotients. Standard curves were made with the standard reference material, DORM-3 (fish protein; National Research Council of Canada). Precision and accuracy for the analytic system were checked with a second standard reference material, TORT-2 (lobster hepatopancreas; National Research Council of Canada). Analytic accuracy and precision were 101.7% recovery (SD = 1.6%, n = 80). Tissue sampling precision, estimated with mean differences between measured concentrations for laboratory splits of randomly selected samples, was 8.9% (SD = 6.8%, n = 50).
Stable Isotope Analysis
Nitrogen and carbon isotopes allow quantification of trophic position for omnivorous species and suggest relative contributions of different carbon sources to the diet. Nitrogen-isotope ratios are commonly applied in trophic analyses, such as those examining the general structure of the Georges Bank food web (Fry 1988), or biomagnification of mercury (Atwell et al. 1998) or persistent organic pollutants (Fisk et al. 2001; Ruus et al. 2002) in marine food webs. δ15N increases 3.4 ‰ on average for every increase of one trophic level (Minagawa and Wada 1984). Although δ13C increases approximately 1‰/trophic level, it is used most often to reflect the source of carbon at the base of food webs (Takai et al. 2002). As a relevant example, carbon incorporated from methane into the biomass of geothermal vent producers had a recognizable abiogenic carbon and thermally derived sediment carbon δ13C signature (Van Dover and Fry 1994, Proskurowski et al. 2008). In studies such as those of the Georges Banks (Fry 1988) and Seta Inland Sea (Takai et al. 2002), δ13C also tended to be more negative (i.e., more 13C depleted) in individuals that obtained more carbon from pelagic than benthic sources.
To facilitate trophic analyses of these deep-water chondrichthyans with nitrogen and carbon isotopes, 1-mg portions of ground, freeze-dried samples were weighed and encapsulated in 5 × 9–mm tin capsules. δ13C and δ15N were determined at the Stable Isotope Facility of the University of California (Davis, CA) using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer. Results were presented as ratios relative to those of standards of Pee Dee belemnite limestone (δ13C) or atmospheric N2 (δ15N) and expressed as per mil (‰). Quality control and accuracy for the analyses were checked with the two mixtures of ammonia and sulfate generated at the Stable Isotope Facility. The mean % recoveries (SD, n) for δ15N in these mixtures were 101.3% (13.5%, 34) and 96.1% (48.5%, 16), and those for δ13C were 100.0% (27%, 34) and 100.0% (0.3%, 17).
Data Analysis
Conventional statistical significance testing was not used to make inferences because that general approach has come under increasing criticism (McCloskey 1995), most notably in health sciences (Sterne and Davey Smith 2001), socioeconomics (Ioannidis 2005; Ziliak and McCloskey 2004), psychology (Cohen 1994), ecology (Fidler et al. 2004, 2006), and, recently, environmental toxicology and chemistry (Newman 2008). Confidence interval analysis, as advocated by Altman et al. (2000), Di Stefano (2004), Fidler et al. (2004), and Cummin and Finch (2005), was applied instead to avoid the problems with conventional significance testing. Confidence interval analysis has the advantage of simultaneously presenting the statistical significance, precision, and importantly, magnitude of any differences (Altman et al. 2000). p-values for one set of F tests were reported for descriptive purposes, not to infer statistical significance.
Results
General Mercury Concentrations
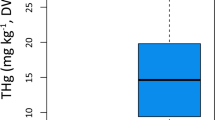
Axial muscle mercury concentrations for all species exceeded the current United States human health screening value of 0.3 mg/kg ww for fish (Table 1). The mean concentrations for E. princeps (1.72 mg/kg ww) and H. affinis (2.38 mg/kg ww) were similar to those reported by Martins et al. (2006) for specimens collected near mid–Atlantic Ridge hydrothermal vents. However, mercury concentrations for C. coelolepis (4.96 mg/kg ww) were higher than those reported for this species collected off southeast Australia by Turoczy et al. (2000) (3.08 mg/kg ww) and Pethybridge et al. (2010) (2.3 to 2.5 mg/kg ww).
Differences Among Species
Interspecies comparisons were performed with the Azores location samples (Table 2) because all three species were collected there in sufficient numbers. The δ15N and δ13C data indicated that the species differed relative to trophic position, and pelagic and benthic contribution to the diet (Fig. 2). Differences were consistent with the literature (Mauchline and Gordon 1983; Marques and Porteiro 2000; Jakobsdottir 2001). The shark, E. princeps, is a micronekton consumer of cephalopods, teleosts (especially lanternfish), and crustaceans, and according to Mauchline and Gordon (1983) and Cortés (1999), C. coelolepis is more of a benthopelagic consumer of cephalopods, teleosts, decapod crustaceans, and mammal blubber. The less negative δ13C of C. coelolepis relative to E. princeps reflected the slightly higher dependence of C. coelolepis on the benthic food web. The higher δ15N for C. coelolepis (13.77‰; 95% confidence interval [CI] 13.57–13.97) than E. princeps (11.71‰; 95% CI 11.39–12.02) was also consistent with their respective trophic rankings by Cortés (1999) of 4.2 and 4.1. Chimaerids, such as H. affinis, are epibenthic feeders, primarily of invertebrates, although larger individuals do consume some fish (Mauchline and Gordon 1983). H. affinis sampled by Marques and Porteiro (2000) near hydrothermal vents consumed vent mussels. Consistent with the literature, mean δ13C values for Azores H. affinis (−15.49‰; CI −14.93 to −16.06) suggested the higher reliance of this species on benthic food sources than either E. princeps (−17.88‰; CI −18.02 to −17.73) or C. coelolepsis (−16.22‰; CI −16.43 to −16.00). The δ15N values for H. affinis were high relative to those of the two sharks; however, this likely reflected the δ15N signature of the benthos more than a higher trophic position of this species relative to the sampled shark species. Using the equation given by Cortés (1999) and diet data from a congeneric species (Mauchline and Gordon 1983), we estimated a trophic level of 3.5 for H. affinis, which is less than those of the two sharks (4.1 and 4.2, given in Cortés (1999)).
The mean mercury concentration in C. coelolepis muscle was significantly higher than those of E. princeps and H. affinis based on nonoverlapping CIs (Table 2 and Fig. 3). In contrast, the 95% CI for the difference between mean mercury concentrations for E. princeps and H. affinis included zero, suggesting the lack of statistically significant difference between these species. The highest mercury concentrations in chondrichthyans taken from the Azores location were associated with C. coelolepis, the shark species with the highest reliance on the benthic food web and the chondrichthyan with the highest trophic position.
Difference Among Locations
Because only E. princeps was collected at all three locations, differences in mercury concentration among locations were examined for this species only (Table 3). The mean mercury concentration at the geothermally least active Bear Seamount was lower than those of the other two locations but still exceeded the United States Food and Drug Administration human health screening value. This location was nearest to North American anthropogenic mercury sources. The 95% CIs for mean mercury concentration in E. princeps taken from the Bear Seamount location did not overlap with those for the other locations, but the CIs for samples from the Azores and Charlie Gibbs Fracture Zone did overlap. Mean mercury concentration in E. princeps from the location most distant from terrestrial anthropogenic sources of mercury (Charlie Gibbs Fracture Zone) was not the lowest of the three locations. The nonoverlapping CIs for mean δ13C of the Bear Seamount location and those of the other locations indicated 13C depletion at Bear Seamount relative to the other locations. However, differences in δ13C among locations for E. princeps were much smaller in magnitude (Table 3) than the differences among species just described. 13C depletion can be associated with organisms that obtain proportionally more carbon from the pelagic than the benthic trophic web (Fry 1988, Takai et al. 2002), but the small differences could also reflect interlocation differences in carbon flux into the food web from geothermal sources (see Van Dover and Fry 1994; Proskurowski et al. 2008).
General linear modeling (SAS PROC GLM; SAS, Cary, NC) of the E. princeps mercury data produced the following F test p-values: location (<0.0001), shark length (0.0009), δ15N (0.0266), and δ13C (0.0570). SAS PROC GLMSELECT (SAS, Cary, NC) was used to select the best multivariate model with location, shark length, δ15N, and δ13C as candidate explanatory variables. Minimum Akaike’s information criterion (AIC) estimation indicated that the best model (i.e., that with the most explanatory power per estimated parameter) included all candidate variables. Inclusion of location and δ13C had the most influence on AIC, and shark length had the least influence. Several qualities appeared to be correlated with mercury concentration for this species, including those related to trophic ecology. Analysis of variance (SAS PROC NESTED; SAS, Cary, NC) with location as the only class variable indicated that most (60%) of the total variance among E. princeps samples could be attributed to location. The distinct δ13C signature and lowest mercury concentrations from the least geothermally active location (Bear Seamount) suggested that proximity to natural, but not anthropogenic, mercury sources might influence mercury concentrations in this shark species.
Differences Among Tissues
Mercury concentrations measured in all but the cartilagenous and brain tissues of E. princeps exceeded current United States health-screening concentrations (Fig. 4). The liver, from which squalene oil is produced, had mercury concentrations as high as muscle tissue. Liver oil and meat of C. coelolepis and other deep-water elasmobranch species are harvested for human consumption (Kyne and Simpendorfer 2007). Cartilagenous tissues from other shark species are used in the Asian seafood and United States health-supplements markets. Mercury in this tissue of E. princeps was below United States health-screening concentrations.
Discussion
Our evidence indicated that trophic ecology had a more consistent influence than proximity to anthropogenic sources on axial muscle mercury concentrations for these deep-water chondrichthyans. Our conclusion about the relative importance of trophic position was supported by the intraspecies positive relation between mercury and δ15N of the rabbitfish (“H” in Fig. 3, top panel: slope = 0.66; 95% CI = 0.12–1.21), and by the higher mercury concentrations and δ15N values in C. coelolepis relative to E. princeps (Fig. 3). However, the largest differences in mercury concentration among the three chondrichthyans were not associated with trophic position alone (as indicated by δ15N values) (Fig. 3). Comparing E. princeps with C. coelolepis, those sharks feeding more from the benthos and higher in the food web tended to have higher muscle mercury concentrations. H. affinis mercury concentrations would be judged anonymously low based on δ15N-assigned trophic level alone; however, the distinct δ15N and δ13C signatures relative to those of the sharks appeared to result from the higher dependence of H. affinis on the benthic food web. Regardless, only by understanding trophic ecology could these trends be explained.
Although mercury concentration in E. princeps from the three locations increased slightly with change in δ13C, it was not possible to attribute these small differences to any particular feature of the species’ diet, e.g., relative dependence on pelagic versus benthic food webs. Because the geothermal activity at the three locations differed, the slight 13C depletion could reflect differences in geothermal carbon movement (Van Dover and Fry 1994, Polz et al. 1998, Proskurowski et al. 2008) into the diet of E. princeps at these locations. The differences in E. princeps mercury concentrations could have been influenced by proximity to natural, geothermal sources.
Deep-water chondrichthyans at all locations had axial muscle mercury concentrations exceeding those protective of human health. Differences in species trophic ecology best explained the observed differences in mercury concentrations among species. Material influence of proximity to anthropogenic mercury sources was not evident.
References
Altman D, Machin D, Bryant TN, Gardner MJ (2000) Statistics with confidence: confidence intervals and statistical guidelines, 2nd edn. British Medical Journal Books, London, UK
Atwell L, Hobson KA, Welch HE (1998) Biomagnfication and bioaccumulation of mercury in an arctic marine food web: Insights from stable nitrogen isotope analysis. Can J Fish Aquat Sci 55:1114–1121
Bergstad OA, Godo OR (2003) The pilot project “Patterns and processes of the ecosystems of the northern Mid-Atlantic”: aims, strategy and status. Oceanol Acta 25:219–226
Cabana G, Rasmussen JB (1994) Modeling food chain structure in Ontario lakes: a determinant of mercury in lake trout (Salvelinus namaycush). Can J Fish Aquat Sci 51:381–389
Cohen J (1994) The earth is round (p < 0.05). Am Psychol 49:997–1003
Compagno LJV, Dando M, Fowler S (2005) Sharks of the World. Princeton University Press, Princeton, NJ
Cortés E (1999) Standardized diet compositions and trophic levels of sharks. ICES J Mar Sci 56:707–717
Cotton CF (2010) Age, growth and reproductive biology of deep-water chondrichthyans. Dissertation, Virginia Institute of Marine Science, College of William & Mary, Gloucester Point, VA
Crump KS, Van Landingham C, Shamlaye C, Cox C, Davidson PW, Myers GJ et al (2000) Benchmark concentrations for methylmercury obtained from the Seychelles Child Development Study. Environ Health Perspect 108:257–263
Cummin G, Finch S (2005) Inference by eye. Am Psychol 60:170–180
Denton GRW, Breck WG (1981) Mercury in tropical marine organisms from North Queensland. Mar Pollut Bull 12:116–121
Di Stefano J (2004) A confidence interval approach to data analysis. For Ecol Manag 187:173–183
Eaton RDP, Farant JP (1982) The polar bear as a biological indicator of environmental mercury burden. Arctic 35:422–425
Ebinghaus R, Tripathi RM, Wallshlager D, Lindberg SE (1999) Natural and anthropogenic mercury sources and their impact on the air-surface exchange of mercury on regional and global scales. In: Ebinghaus R, Turner RR, de Lacerda LD, Vasiliev O, Salomons W (eds) Mercury contaminated sites. Springer, Berlin, Germany, p 3
Fidler F, Cumming G, Burgman M, Thomason N (2004) Statistical reform in medicine psychology, and ecology. J Socioeconomics 33:615–630
Fidler F, Burgman MA, Cumming G, Buttrose R, Thomason N (2006) Impact of criticism of null-hypothesis significance testing on statistical reporting practices in conservation biology. Conserv Biol 20:1539–1544
Fisk AT, Hobson KA, Norstrom RJ (2001) Influence of chemical and biological factors on trophic transfer of persistent organic pollutants in the northwater polynya marine food web. Environ Sci Technol 35:732–738
Fossen I, Cotton CF, Bergstad OA, Dyb JE (2008) Species composition and distribution patterns of fishes captured by long lines on the Mid-Atlantic Ridge. Deep Sea Res Part II: Top Stud Oceanogr 55:203–217
Fry B (1988) Food web structure on Georges Bank from stable C, N, and S isotopic compositions. Limnol Oceanogr 33:1182–1190
Fujino T (1994) Clinical and epidemiological studies on chronic Minamata disease. Part I. Study on Katsurajima Island. Kumamoto Med 44:139–155
Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K et al (1997) Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 19:417–428
Hornung H, Krom MD, Cohen Y, Bernhard M (1993) Trace metal content in deep-water sharks from the eastern Mediterranean Sea. Mar Biol 115:331–338
Ioannidis JPA (2005) Why most published research findings are false. Publ Library Sci Med 2:e124–e126
Jakobsdottir KB (2001) Biological aspects of two deep-water squalid sharks: Centroscyllium fabricii (Reinhardt, 1825) and Etmopterus princeps (Collett, 1904) in Icelandic waters. Fish Res 51:247–265
Kraepiel AM, Keller K, Chin HB, Malcolm EC, Morel FMM (2003) Sources and variations of mercury in tuna. Environ Sci Technol 37:5551–5558
Kyne PM, Simpendorfer AC (2007) A collation and summarization of available data on deepwater chondrichthyans: biodiversity, life history and fisheries. A report prepared by the IUCN SSC Shark Specialist Group for the Marine Conservation Biology Institute, pp 1–137
Lyle JM (1986) Mercury and selenium concentrations in sharks from northern Australian waters. Aust J Mar Freshw Res 37:309–321
Manohar DM, Krishnan KA, Anirudhan TS (2002) Removal of mercury(II) from aqueous solutions and chlor-alkali industry wastewater using 2-mercaptobenzimidazole-clay. Water Res 36:1609–1619
Marques A, Porteiro F (2000) Hydrothermal vent mussel Bathymodiolus sp (Mollusca: Mytildae): Diet item of Hydrolaus affinis (Pisces: Chimaeridae). Copeia 2000:806–807
Martins I, Costa V, Porteiro FM, Colaço A, Santos RS (2006) Mercury concentrations in fish species caught at Mid-Atlantic Ridge hydrothermal vent fields. Mar Ecol Prog Ser 320:253–258
Mason RP, Heyes D, Sveinsdottir A (2006) Methylmercury concentrations in fish from tidal waters of the Chesapeake Bay. Arch Environ Contam Toxicol 51:425–437
Mauchline J, Gordon JDM (1983) Diets of the sharks and chimaeroids of the Rockall Trough, northeastern Atlantic Ocean. Mar Biol 75:269–278
McCloskey DN (1995) The insignificance of statistical significance. Am Sci 272:32–33
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: Further evidence and the relation between 15N and animal age. Geochim Cosmachim Acta 48:1135–1140
Moore JA, Vecchione M, Collette BB, Gibbons R, Hartel KE, Galbraith JK et al (2003) Biodiversity of Bear Seamount, New England seamount chain: results of exploratory trawling. J Northw Atl Fish Sci 31:363–372
Newman MC (2008) “What exactly are you inferring?” A closer look at hypothesis testing. Environ Toxicol Chem 27:1013–1019
Pethybridge H, Cossa D, Butler ECV (2010) Mercury in 16 demersal sharks from southeast Australia: biotic and abiotic sources of variation and consumer health implications. Mar Environ Res 69:18–26
Pirrone N, Costa P, Pacyna JM, Ferrara R (2001) Mercury emissions to the atmosphere from natural and anthropogenic sources in the Mediterranean region. Atmos Environ 35:2997–3006
Polz MR, Robinson JJ, Cavanaugh CM, Van Dover CL (1998) Trophic ecology of massive shrimp aggregations at a mid-Atlantic ridge hydrothermal vent site. Limnol Oceanogr 43:1631–1638
Proskurowski G, Lilley MD, Seewald JS, Fruh-Green GL, Olson EJ, Lupton JE et al (2008) Abiogenic hydrocarbon production at Lost City hydrothermal field. Science 319:604–607
Ruelas-Inzunza J, Páez-Osuna F (2005) Mercury in fish and shark tissues from two coastal lagoons in the Gulf of California, Mexico. Bull Environ Contam Toxicol 74:294–300
Ruus A, Ugland KI, Skaare JU (2002) Influence of trophic position on organochlorine concentrations and compositional patterns in a marine food web. Environ Toxicol Chem 21:2356–2364
Sterne JAC, Davey Smith G (2001) Sifting the evidence—what’s wrong with significance tests? Br Med J 322:226–230
Stevens J, Correia JPS (2003) Centroscymnus coelolepis. In: IUCN 2009. IUCN red list of threatened species. Version 2009.2
Storelli MM, Giacominelli Stuffler R, Marcotrigiano GO (2002) Mercury accumulation and speciation in muscle tissue of different species of sharks from Mediterranean Sea, Italy. Bull Environ Contam Toxicol 68:201–210
Sunderland EM (2007) Mercury exposure from domestic and imported estuarine and marine fish in the U.S. seafood market. Environ Health Perspect 115:235–242
Takai N, Mishima Y, Yorozu A, Hoshika A (2002) Carbon sources for demersal fish in the western Seta Inland Sea, Japan, examined by δ13C and δ15N analyses. Limnol Oceanogr 47:730–741
Turoczy NJ, Laurenson LJB, Allinson G, Nishikawa M, Lambert DF, Smith C et al (2000) Observations on metal concentrations in three species of shark (Deania calcea, Centroscymnus crepidater, and Centroscymnus owstoni) from southeastern Australian waters. J Agric Food Chem 48:4357–4364
Van Dover CL, Fry B (1994) Microorganisms as food resources at deep-sea hydrothermal vents. Limnol Oceanogr 39:51–57
Ziliak ST, McCloskey DN (2004) Significance redux. J Socioeconomics 33:665–675
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Newman, M.C., Xu, X., Cotton, C.F. et al. High Mercury Concentrations Reflect Trophic Ecology of Three Deep-Water Chondrichthyans. Arch Environ Contam Toxicol 60, 618–625 (2011). https://doi.org/10.1007/s00244-010-9584-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-010-9584-4