Abstract
Application of monosulfuron, a new sulfonylurea herbicide, produced a simulative effect on heterocyst formation and nitrogenase activity but an inhibitory effect on photosynthesis, i.e., a lower net photosynthetic rate, fewer photosynthetic pigments, and a smaller Fv/Fm ratio at increasingly higher monosulfuron concentrations (0.001–10 mg/l) for three nonspecific filamentous nitrogen-fixing cyanobacteria: Anabaena azollae, A. flos-aquae, and A. azotica. The decrease in biliprotein of algal cells was less than that of carotenoid and chlorophyll-a. Monosulfuron was more readily degraded and less accumulated in A. azotica compared with A. azollae and A. flos-aquae. The three algae exhibited varying degrees of sensitivity to monosulfuron: Calculated 50% inhibition concentrations (IC50s) of algal growth and no observed-effect concentration (NOEC) values after 4 days of treatment were 0.014 and 0.005, 0.029 and 0.019, and 0.22 and 0.075 mg/l for A. flos-aquae, A. azollae, and A. azotica, respectively. Normal agricultural use of monosulfuron at postemergence rates of 0.3–0.8 mg/l in rice fields will likely be toxic to these three ubiquitous nitrogen-fixing cyanobacteria. Low-dose monosulfuron application (<0.1 mg/l) enables growth of the more tolerant A. azotica as biofertilizer, and the use of photosynthetic efficiency and growth rates as sensitive-indicator indexes of toxicity to nitrogen-fixing cyanobacteria are recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Soil nitrogen is the main source of nitrogen for crop growth, providing >50% of nitrogen consumed by rice plants (Fernández-Valiente et al. 2000). Cyanobacteria are one of the largest and most important groups of algae on the earth (Fay and Van Baalen 1987). In particular, nitrogen-fixing cyanobacteria are vital photosynthetic microorganisms that contribute to soil fertility by fixing atmospheric nitrogen; they are also important for maintaining the ecosystem’s stability (Irisarri et al. 2001). The agronomic importance of free-living symbiotic cyanobacteria as biofertilizers has been recognized (Habte and Alexander 1980); some strains that thrive in fields release small quantities of ammonia and other small nitrogenous polypeptides during the active growth period. Recent research by Shen et al. (2005) demonstrated that the application of a mixture of 12 species of nitrogen-fixing cyanobacteria (cultured for 90 days; 1 g dry algae weight/l) after sowing a rice crop increased the values means of nitrogen contents of rice stems and leaves by 27% and 39%, respectively. Compared with urea fertilizer and control treatments, cyanobacteria treatment improved rice yields (by 9% and 12%, respectively), increased soil’s organic contents (by 10% and 16%, respectively), and increased available nitrogen levels (by 13% and 67%, respectively). However, excessive doses of herbicides caused inhibition of the nitrogen-fixing capabilities of soil microbial communities in a paddy field (Patnaik et al. 1995). The extensive application of herbicides (e.g., bensulfuron-methyl, propanil, benthiocarb, and butachlor) has been responsible for decreased cultivation of nitrogen-fixing cyanobacteria in fields as well as the absence of several beneficial nitrogen-fixing cyanobacteria (e.g., A. azollae, A. azotica), which were commonly found in rice fields of the Shanghai region of China (Shen et al. 2005). Numerous side effects of herbicides on nonspecific cyanobacteria have been reported (Miquel and Ivo 2006); studies have been focused primarily on the effects of relatively old herbicides (e.g., atrazine, benthiocarb, bipyridylium, butachlor, molinate, metribuzin, and 2,4-dichlorophenoxyacetic acid [2,4-D]) on algal growth and metabolism. Few studies of the effects of newer classes of herbicides (e.g., sulfonylureas) on nitrogen-fixing cyanobacteria found in fields and the resulting damage to the structures of vegetative cells and heterocysts have been reported.
Because they have relatively low mammalian toxicity, high efficacy of control, and environmental safety, the use of sulfonylurea herbicides has increased rapidly since their introduction. Currently, >30 sulfonylurea herbicides are applied worldwide for selective control of weeds in a variety of crops, including corn (Zea mays L.), rice (Oryza sativa L.), wheat (Triticum aestivum L.), and potatoes (Solanum muricatum Oit.) (Tomlin 2003). Monosulfuron (N-[(4′-methyl) pyrimidin-2′-yl]-2-nitrophenylsulfonyl urea) is a relatively new sulfonylurea herbicide developed by the National Pesticide Engineering Research Center in Tianjin, China (Li et al. 1994). This herbicide exhibits low mammalian toxicity and is effective at postemergence rates of 15–30 g active ingredient/ha in a wide range of crops, including corn, wheat, rice, and millet (Panicum miliaceum L.) (Shen et al. 2008). Sulfonylureas inhibit the enzyme acetolactate synthase, which is present in all plants, bacteria, fungi, yeasts, and algae, including cyanobacteria (Shen et al. 2009). However, the effect of monosulfuron on nitrogen-fixing cyanobacteria that are capable of enhancing the fertility of agricultural soils has not been investigated.
Three of the most common cyanobacteria in agricultural fields in China are the filamentous nitrogen-fixing species A. azotica Ley (a free-living soil cyanobacterium with a high nitrogen-fixing capability), A. flos-aquae (Lyngb) Breb. (a free-living soil cyanobacterium that may be toxic to some organisms), and A. azollae Strasb (a cyanobacterium that forms a symbiotic relation with the water fern, Azolla spp.) (Wu and Zhou 2004). This article presents experimental results to illustrate the effects of monosulfuron concentration on growth, photosynthesis, and nitrogen-fixation ability of the three cyanobacteria, the formation and structure of vegetative cells and heterocysts, and the toxic mode and toxicity of monosulfuron.
Materials and Methods
Source of Chemicals
Monosulfuron of 99.4% purity was synthesized at the National Pesticide Engineering Research Center in Tianjin, China. Monosulfuron was dissolved in a mixture of organic solvent dimethylformamide (DMF; N,N-dimethylformamide; Jianshan Chemicals, Jianshu province, China) and surfactant TritonX-100 (4-(1,1,3,3-tetramethylbutyl)phenyl-polyethylene glycol (Jibishi Gene Technology, Shanghai, China) to form a herbicide concentrate lower than the first-effect concentration, i.e., DMF 0.5% (v/v) and TritonX-100 0.005% (v/v). The concentrate was freshly prepared and sterilized before adding it to the culture medium to prepare test solutions having the desired monosulfuron concentrations.
Algal Cultures
Cultures of A. azotica, A. flos-aquae, and A. azollae, three nitrogen-fixing algal species in the Nostocaceae family, were obtained from the Institute of Hydrobiology of the Chinese Academy of Sciences in Wuhan, China. Axenic cultures were grown in a liquid sterilized medium (pH 7.2) as described by Shen et al. (2005) at 30°C ± 2°C under constant fluorescent light at an intensity of 36.2 μmol/m2/s. The experimental cultures were first grown in 250-ml flasks containing 100 ml medium with 0.5–1 million cells/ml under the same conditions. At the exponential growth phase of the algal cultures, small aliquots of the concentrate were added to the culture medium flasks to result in 0.001, 0.01, 0.1, 1, 10, and 100 mg/l monosulfuron in the test samples. The high concentrations tested were well below culture media solubility. Sterilized water was added to some of the culture media instead to serve as control samples.
Algal Growth
Growth of algae was measured by recording light absorbance of the culture medium at 448 nm using a spectrophotometer. Standard curves relating Abs448 with cell numbers were developed for continuous cultures of. A. azotica, A. flos-aquae, and A. azollae. These curves were used to determine cell numbers in continuous-culture samples used as inoculum for the screening bioassay. Data used to produce the standard curves were obtained from absorbance measurements. The controls (without the herbicide) and each concentration were replicated three times, and all experiments were conducted twice. During the test period, samples were removed after herbicide treatment at 0, 1, 2, 3, 4, 5, and 6 days after treatment to obtain growth measurements.
Algal growth rate (μ) was calculated by the following equation:
where X 1 is the number of algal cells (estimated from Abs448 nm using the respective standard curve) at time T 1, and X 0 is the number of algal cells at time T 0. To determine the dry weight of cells, corresponding cultures in triplicate were pelletized, and the pellets were washed three times using distilled water before drying to constant weight at 105°C for 8 h. Test runs were also conducted to estimate growth rate no observed-effect concentration (NOEC) and 50% inhibition concentrations (IC50) values after 1 day of treatment.
Analysis of Monosulfuron in Liquid and Solid Samples
To measure monosulfuron concentrations in the medium and in algal cells, a high-pressure liquid chromatography (HPLC) analysis procedure was developed employing a Vista 5500 high-pressure liquid chromatograph (Varian lnc., USA) equipped with a UV-200 detector; an octadecyl silane (ODS) Hypersil (5 μm) 250 × 4.6-mm column (Discovery C18); and a 500-mg ODS C18 precolumn. Operating conditions for the HPLC analysis were as follows: Mobile phase- methanol/water/glacial acetic acid (430/562/8 v/v/v); mobile phase flow rate 0.8 ml/min; sensitivity 0.02 Au/mV; sample injection volume 20 μl; wavelength 254 nm; and column temperature 40°C. Under these conditions, the resulting calibration curve correlating monosulfuron concentration to the apex area was y = 23.065x − 121.37 (R 2 = 0.9965), and the average recovery was 95% to 104%.
Twenty milliliters of algal samples having different initial monosulfuron concentrations were taken at various times after the start of treatment (i.e., at 12, 24, 48, 72, 96, 120, 144, and 168 h) and centrifuged (Sorvall Centrifuge; DJB Labcare) at 3000g for 5 min. Ten milliliters of the liquid phase was extracted three times with 5 ml dichloromethane by shaking for 1 min. After drying with anhydrous sodium sulfate, the dichloromethane phase was evaporated to near dryness at <40°C. Seventy percent methanol was added to dissolve the concentrated extract, and the monosulfuron containing methanol was finally cleaned up using a C18 cartridge before measurement. For measuring monosulfuron concentrations on and in the algal cells, the solid phase of algal cells was washed twice with 10 ml water; suspended in 10 ml water for sonication using an ultrasonic cell disruptor (250 mv/3 s/3 s); and centrifuged at 3000g for 5 min. The resulting aqueous solution was extracted three times with 5 ml dichloromethane for 10 min, and the monosulfuron containing dichloromethane was further processed and analyzed in a similar manner as described for the liquid-phase sample. Monosulfuron concentrations of control samples at the same initial concentration (i.e., no algal cells) were measured, and the concentration differences of the two sets of liquid samples were the estimated amounts of monosulfuron degraded by the algae during the treatment period.
Extraction and Analysis of Photosynthetic Pigments
Ten milliliters of algal samples having different initial monosulfuron concentrations were taken at various treatment times and then filtered using cellulose acetate membrane (0.8 μm). The membrane with retained algal mass was cut into pieces after vacuum filtration and placed in the test tube for 24 h in dark, after which extraction of the photosynthetic pigments using 5 ml 80% acetone was performed. The solution was centrifuged at 4000g for 5 min, and the light absorption spectra (350–750 nm) of the supernatant was monitored using a spectrophotometer (UV-1601; Shimadzu) against the reference cell of 80% acetone.
Chlorophyll-a was extracted with 90% methanol in the dark for 2 h and centrifuged at 3000g for 3 min, and its concentration was estimated from the Abs665 nm according to the method of Mackinney (1941). The algal biliproteins were extracted by repeatedly freezing and thawing the pellet in the presence of 0.05 M phosphate buffer (pH 6.7). The solution was centrifuged at 3000g for 15 min, and the absorbance at 618 nm was measured (Glazer and Hixson 1975). The amount of total carotenoid was calculated from the absorbance at 447 nm according to Jenssen (1978).
Estimates of Photochemical Efficiency and Photosynthetic Efficiency
The Fv/Fm ratio, an indicator of photochemical efficiency of photosynthesis PSII, was measured using a plant-efficiency analyzer (England Hansatech Instrument, Norfolk, England) according to Adams and Bate (1999). The culture was adapted in the dark for 10 min before measurement at room temperature. The dark-adapted culture was exposed to modulated light with an intensity of 3–4 μmol/m2/s to measure initial fluorescence (Fo). This was followed by exposure to a saturation pulse of white light of 4000 μmol/m2/s to provide the maximum fluorescence (Fm). Variable fluorescence (Fv) was determined by subtracting Fo from Fm, and the Fv/Fm ratio was then calculated.
Algal photosynthetic efficiency was obtained by measuring the dissolved oxygen (DO) concentration using modified Winkler’s method (Jin and Chu 1990). The sterilized liquid medium of algal culture was added into 10-ml algal samples to achieve the desired monosulfuron concentration in a 250-ml iodometric bottle, which was sealed with water after taking a sample for DO analysis. The remaining triplicate samples were incubated in the dark under constant fluorescent light (intensity 36.2 μmol/m2/s) at 30°C ± 2°C. After 24 h, residual DO was measured, and the mean value was computed. Algal photosynthetic oxygen was estimated by the difference in DO between the bottles under light and dark conditions. The photosynthesis inhibition rate (I) of the algae was calculated by the following equation:
where Po is the photosynthetic oxygen of the control, and Pn is the photosynthetic oxygen of the herbicide treatments.
Measurements of Heterocyst and Nitrogenase Activity
Heterocyst numbers, resulting from exposure to different monosulfuron concentrations, were measured by counting frame using a microscope (50× magnification) after 6 days of herbicide treatment, and damage to heterocyst structure was analyzed using a scanning electron microscope.
A study of the nitrogen-fixing capability of the three cyanobacteria subjected to 0.001, 0.1, and 10 nmol/l monosulfuron treatment was performed by measuring the amounts of acetylene reduction according to the method of Burris (1975). Thirty milliliters of nitrogen-fixing cyanobacteria samples were added into a 100-ml serum bottle, which was immediately sealed with a rubber septum. Ten percent of air in the bottle was then replaced with the equivalent amount of acetylene (C2H2) gas using a sterilized syringe; the bottled content was incubated at 30°C ± 2°C under constant fluorescent light (36.2 μmol/m2/s) for 24 h. After incubation, 0.05 ml headspace gas in the serum bottle was removed using a syringe through the rubber septum and injected into a GDX502 column-equipped gas chromatograph with flame ionization detector (China Chromatography 9800) to determine the amount of C2H4. Nitrogen at 0.057 MPa was used as carrier gas, and air and H2 were regulated at 0.15 and 0.098 MPa, respectively. The temperature for the injector, column, and detector were 140°C, 60°C, and 120°C, respectively. The dry weight of cells was measured as described previously. Nitrogenase activity was expressed as nMC2H4/mg dry weight/h.
Statistical Analyses
A completely randomized design with three replications was used in all duplicated experiments. Analyses of variance were performed on the nontransformed data. Significant differences were determined using Duncan’s test at a significance level of p = 0.05 (PROC GLM; SAS, Cary, NC [2001]). NOEC and IC50 values were calculated using the software program OriginPro7.5SR1 (OriginLab).
Results
Effect of Treatment Time on Algal Growth
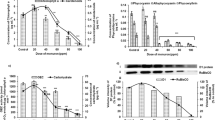
The growth rates of A. flos-aquae, A. azollae, and A. azotica in the control group decreased gradually with culturing time; the corresponding growth rates of the treated groups exposed to 0.001–100 mg/l monosulfuron exhibited wave patterns of different magnitudes (Fig. 1). Compared with controls, the growth rates of A. flos-aquae, A. azollae, and A. azotica decreased, respectively, by 36% to 81%, by 43% to 76%, and by 25% to 67% after 1 day of treatment; however, because monosulfuron became stimulative at 1–2 days of treatment, the growth rates increased before decreasing again after 2 days. The increase in growth rate was higher for the more heavily dosed group, with growth rates being 29% to 109% greater compared with controls after 2 days of treatment. The opposite effect prevailed as culturing time was further extended; monosulfuron became inhibitory again. The decrease in growth rates was more pronounced at high (1.0–100 mg/l) than at low treatment levels (0.001–1.0 mg/l). Calculated IC50 and NOEC values for A. flos-aquae, A. azollae, and A. azotica were 0.014 and 0.005, 0.029 and 0.019, and 0.22 and 0.075 mg/l, respectively, after 4 days of treatment. The three algae had different degrees of sensitivity to monosulfuron; A. flos-aquae was the most sensitive, followed by A. azollae and A. azotica.
SEM results showed that low concentrations (<1 mg/l) did not affect cell structures of the three algal species (Fig. 2A). In contrast, at concentrations of ≥10 mg/l, monosulfuron caused notable damage, such as parted algal filaments, abnormal cells, damaged gelatious sheaths, broken cellular walls, and even death (Fig. 2B).
SEM inspection of algal cell structure. A Normal algal cell without herbicide (43,000×) and B after 6 days of exposure to 10 mg/l monosulfuron (26500×). a = thylakoides; b = cell wall; c = cell central body; d = gelatinous sheath; e = cytoplasm wall; f = transverse wall II section; g = transverse wall I section; h = vacuole
Effect on Photosynthesis
Effect on Photosynthetic Pigments
The effect of monosulfuron concentration on the production of photosynthetic pigments by the three algal species is listed in Table 1. Photosynthetic pigments found in cells of A. flos-aquae were clearly inhibited by monosulfuron at all treatment levels (0.001–10 mg/l), and the decrease showed the characteristic concentration-dependent response, i.e., greater inhibition was observed at higher monosulfuron concentrations. Compared with controls and dependent on treatment concentration, carotenoid, biliprotein, and chlorophyll-a contents of A. flos-aquae cells decreased by 18% to 88%, by 17% to 51%, and by 25% to 69%, respectively, after 1 day of treatment. Their levels in A. azollae cells were not changed in the 0.001 mg/l treatment but significantly decreased in other treatments; decreases of 33% to 39% for carotenoid, 18% to 26% for biliprotein, and 17% to 53% for chlorophyll-a were noted in A. azollae exposed to 0.1–10 mg/l monosulfuron. Of particular interest is the stimulative effect of monosulfuron, at low concentrations (0.001–0.1 mg/l), on the production of carotenoid, biliprotein, and chlorophyll-a in A. azotica. These pigments, however, decreased as monosulfuron concentration increased, and statistically significant differences were observed at concentrations at treatment levels of 1–10 mg/l. The calculated IC50 values for carotenoid, biliprotein, and chlorophyll-a were, respectively, 0.064, 2.53, and 0.52 mg/l for A. flos-aquae; 25.12, 138.99, and 8.37 mg/l for A. azollae; and 7.62, 409.26, and 338.06 mg/l for A. azotica (Table 2). The photosynthetic pigment end points of A. azotica were less sensitive to monosulfuron than those of A. azollae and A. flos-aquae. The end points for carotenoid and chlorophyll-a were more sensitive than that for biliprotein.
The effect of monosulfuron on the 350- to 750-nm light spectra (UV-1601 spectrophotometer) of the pigment extracts was the same. Monosulfuron did not totally inhibit any photosynthetic pigments in cyanobecteria cells; however, monosulfuron concentration did affect their amounts in agal cells.
Effect on Photosynthetic Efficiency
Figure 3 presents the effect of monosulfuron on photosynthetic inhibition of three cyanobacteria. The herbicide monosulfuron inhibited photosynthetic efficiency in a dose-dependent manner, i.e., the inhibitory effect increased with increasing monosulfuron concentration. The degrees of photosynthetic inhibition corresponding to 0.001, 0.01, and 0.1 mg/l monosulfuron were 21%, 53%, and 57%, 19%, 76%, and 79%, and 67%, 71%, and 82% for A. flos-aquae, A. azotica, and A. azollae, respectively. Calculated IC50 values for net photosynthetic rate were, respectively, 0.000039, 0.0027, and 0.0048 mg/l.
Effect on Photochemical Efficiency of PSII
The effect of monosulfuron on PSII photochemical efficiency of the three cyanobacteria is shown in Fig. 4. The results showed that monosulfuron had different effects on PSII photochemical efficiency of the three cyanobacteria. The chlorophyll fluorescence ratio (Fv/Fm) of A. azotica increased 3%, 10%, 31%, and 88% at 0.01, 0.1, 1.0, and 10 mg/l monosulfuron concentrations, respectively, after 1 day of treatment; it then decreased by 14% to 28% (p < 0.05) as culture time increased from 2 to 3 days. In contrast, the chlorophyll fluorescence ratio (Fv/Fm) of A. azollae at low monosulfuron concentrations (0.01–0.1 mg/l) did not differ significantly from that of controls but rather decreased by 11% to 21% at higher concentrations monosulfuron (1–10 mg/l, p < 0.05). The chlorophyll fluorescence ratio (Fv/Fm) in A. flos-aquae showed a concentration-response relation. Decreases in Fv/Fm ratio increased to 11%, 26%, 43%, and 54% as monosulfuron concentration increased to 0.01, 0.1, 1.0, and 10 mg/l, respectively. There was a significant difference in Fv/Fm between each concentration (p < 0.05).
Effects of monosulfuron nominal concentrations on PSII photochemical efficiency during the first 3 days. The Fv/Fm ration of the three nitrogen-fixing cyanobacteria was well correlated to its Fv/Fo according to the following equation \( \begin{gathered} (A.\,azotica) y = 21.755x^{2} - 18.42x + 4.6877, \; R^{2} = 0.9949; \hfill \\ (A.\,azollae) y = 4.0561x - 1.0007, \; R^{2} = 0.9751; {\rm and} \hfill \\ \left( {\it A.\,flos}{\text{-}}{\it aquae} \right) y = 8.8702x^{2} - 4.1399x + 0.8511, R^{2} = 0.9954 \hfill \\ \end{gathered} \)
Effect on Nitrogen Fixing
Effect on Heterocyst
Heterocyst development was first observed 24 h after growing the vegetative cells. The effect of monosulfuron concentration on the formation of heterocysts in the three algal species is illustrated in Fig. 5. In general, monosulfuron applied at low concentrations (0.01–1 mg/l) stimulated heterocyst formation in the three algal species, whereas it was inhibitory at higher concentrations (>10 mg/l). After 144 h of culturing in media containing 0.01, 0.1, and 1.0 mg/l monosulfuron, heterocyst numbers of A. azotica increased, respectively, by 162%, 217%, and 51% compared with controls (75%, 134%, and 108% in A. azollea and 164%, 139%, and 44% in A. flos-aquae). However, at 10 mg/l monosulfuron, heterocyst formation decreased notably compared with controls (by 10%, 20%, and 38% [p < 0.05] in A. azotica, A. azollea, and A. flos-aquae, respectively).
Effect on Nitrogenase Activity
Figure 6 shows that monosulfuron had a different effect on nitrogenase activity of the three cyanobacteria. Compared with controls, monosulfuron applied at 0.001–10 mg/l stimulated nitrogenase activity in A. azotica; maximum stimulation occurred at 0.001 mg/l). There was a significant difference in activity. For example, the nitrogenase activity of A. azotica cell cultures treated for 24 h with 0.001, 0.1, and 10 mg/l monosulfuron increased by 85%, 44%, and 22%, respectively, compared with controls. There was a significant difference in activity between each concentration (p < 0.05). In contrast, nitrogenase activity of A. azollae was stimulated (by 185%) at 1 mg/l monosulfuron. In the 0.001 and 10 mg/l treatments, monosulfuron did not produce a notable effect on nitrogenase activity. For A. flos-aquae, the 0.001 mg/l monosulfuron treatment only stimulated nitrogenase activity by 17% compared with controls [p < 0.05]). Decreased activity increased to 27% and 78% as monosulfuron concentrations increased to 0.1 and 10 mg/l (p < 0.05), respectively, indicating that A. flos-aquae was more sensitive to monosulfuron than the two other cyanobacteria.
Discussion
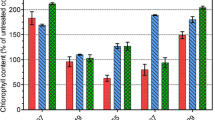
The transient stimulative effect of monosulfuron on algal growth observed on treatment day 2 of this study is similar to that reported by Shen et al. (2005) for butachlor and acetochlor on several Anabaena species. Hormesis, the stimulative effect of a toxin at subtoxic concentrations has been documented after application of other herbicides and allelochemicals (Prithiviraj et al. 2007). Although inhibition of algal growth increased with increasing monosulfuron concentration, inhibition at low concentrations (0.001–0.1 mg/l) gradually decreased as the experiment progressed. This phenomenon was more notable in A. azotica than in A. flos-aquae and A. azollae. Such results may have been caused in part by their different monosulfuron-degradation capabilities. Intracellular monosulfuron concentration profiles showed that A. azotica is most capable of degrading monosulfuron, followed by A. azollae and then A. flos-aquae, as evidenced by respective maximum intracellular monosulfuron concentrations of 5%, 10%, and 11% of the initial liquid-phase concentration after 3–4 days of treatment. Intracellular degradation of monosulfuron was responsible for the subsequent decreases noted in the observed concentrations (Fig. 7).
The herbicide monsulfuron can also markedly impact the photosynthetic efficiency of cyanobacteria (Fig. 3). Monosulfuron treatments decreased Fo and Fm. Lower Fo is an indicator of less excitation energy reaching the photosynthetic II (PS II) reaction center, whereas lower Fm is an indicator of damage to the PS II reaction center itself. The Fv/Fm ratio indicates the highest photochemical yield of PSII, i.e., the number of electron transfers or charges separates in the PS II reaction center when it absorbs a light quantum. The Fv/Fm ratio in our study shows that the cells were more photoinhibited at higher monosulfuron concentrations and/or longer treatment periods. The observed greater decrease in photosynthetic pigments content due to monosulfuron in three cyanobacteria may also be related to the greater decrease in Fv/Fm ratio of these species. Bester et al. (1995) observed that the herbicide triazine resulted in lower pigment content, highly truncated chlorophyll antenna size, and accumulation of photo-damaged PS II centers in chloroplast thylakoids in marine phytoplankton cells. Mattoo (1984) also considered that a truncated electron pathway may be a consequence of the generation of reactive oxygen species (ROS), which affects fatty acid membrane composition. This again results in damage to cellular membranes and ultimately in crashed cells. Low photosynthetic efficiency may also be related to truncated electron transport, resulting in the action of ROS with enzymes that regulate fatty acid metabolism and composition of membranes, finally causing cell damage (Fig. 2B).
The study results demonstrated that the effects of monosulfuron on nitrogen fixation were different from that on photosynthesis in three cyanobacteria. Heterocysts have a photosystem I, which they use to generate adenosine triphosphate (ATP) and are also sites of N2 fixation of cyanobacteria; nitrogenase is distributed throughout the center of the heterocyst. Ernst et al. (1983) observed that ATP levels (i.e., energy charge) have no relation to nitrogen fixation. The formation of heterocysts in three cyaobacteria was stimulated by monosulfuron at concentrations of 0.01–1 mg/l, consistent with the results of Mishra et al. (1989). Monosulfuron also exhibited a generally stimulative effect on nitrogenase activity of the three cyaobacteria, except at the higher concentrations of 0.1–10 mg/l for A. flos-aquae. This is in agreement with the results of Chen et al. (2008), who found that soil nitrogen-fixing ability significantly increased throughout rice growth stages in paddy soil treated with the herbicide butachlor. Das and Debnath (2006) also reported that compared with controls, application of butachlor stimulated populations of free-living nitrogen-fixing bacteria by 32% and nitrogen-fixing ability by 10%. Moreover, in this study, heterocyst numbers in A. azotic were decreased by 12%, but nitrogenase activity increased by 22% at monosulfuron concentrations of 10 mg/l compared with controls. This result is similar to those for carbamate herbicides on cyanobacteria. Padhy (1985) reported that carbamate herbicides can affect nitrogenase activity of cyanobacteria but did not report a change in the frequency of heterocysts. This may be related to heterocysts only as sites of N2 fixation for nitrogenase under anaerobic conditions. Nitrogenase has higher activity when Fe-protein is complexed with MoFe-protein (Jian 1981). We thereby may assume that monsulfuron cannot act on both proteins of nitrogenase activity. The decrease in heterocyst formation and nitrogenase activity of the three cyanobacteria exposed to a higher monosulfuron concentration (10 mg/l) was probably related to damaged cellular membrane integrity (see Fig. 3).
Based on IC50 inhibition indexes for growth, photosynthesis, and nitrogen-fixing ability, we clearly observed that the effect of monosulfuron on photosynthesis in three cyanobacteria was more sensitive than that on fixation (Table 2), and photosynthetic efficiency may be an indicator index by which to determine or monitor monosulfuron toxicity to cyanobacteria in fields. Shen et al. (2008) reported that this herbicide exhibited effective weed control at application postemergence rates of 0.3–0.8 mg/l in rice fields. However, by all appearances, monosulfuron was shown to exhibit algicidal or algistatic effects on three ubiquitous nitrogen-fixing cyanobacteria at greater-than-exposure concentrations than would normally be found with normal agricultural use of the herbicide.
Conclusion
The herbicide monosulfuron affected photosynthetic functions, which may have inhibited the growth of three nitrogen-fixing cyanobacteria by affecting the production of photosynthetic pigments in the antenna complex, electronic transfer, and membrane integrity. It generally had a stimulative effect on the nitrogen-fixing capability of the three cyanobacteria due to increased heterocyst formation and enhanced nitrogenase activity, except at high concentrations (>10 mg/l), which may damage heterocyst structure. Three common but distinct nitrogen-fixing cyanobacteria responded differently to monosulfuron at low concentrations; this may be related to homeostatic response and chemoheterotrophical ability. Therefore, in rice-cropping systems where the herbicide monosulfuron is applied at low concentrations (<0.1 mg/l) and the more monosulfuron-tolerant A. azotica is used as a biofertilizer, the use of photosynthetic efficiency and growth rate as sensitive-indicator indexes of toxicity to nitrogen-fixing cyanobacteria are recommended.
References
Adams JB, Bate GC (1999) Growth and photosynthetic performance of Phragmites australis in estuarine waters: a field and experimental evaluation. Aquat Bot 64:359–367
Bester K, Huhnerfuss H, Brockmann U, Rick HJ (1995) Biological effects of triazine herbicide contamination on marine phytoplankton. Arch Environ Contam Toxicol 29:277–283
Burris RM (1975) The acetylene-reduction technique. In: Stewart WDP (ed) Nitrogen fixation by free-living micro-organism. Cambridge University Press, London, England, pp 249–257
Chen WC, Yen JH, Chang CS, Wang YS (2008) Effects of herbicide butachlor on soil microorganisms and on nitrogen-fixing abilities in paddy soil. Ecotoxicol Environ Saf 25:1–7
Das AC, Debnath A (2006) Effect of systemic herbicides on N2-fixing and phosphate solubilizing microorganisms in relation to availability of nitrogen and phosphorus in paddy soils of west Bengal. Chemoshpere 65:1082–1086
Ernst A, Böhme H, Böger P (1983) Phosphorylation and nitrogenase activity in isolated heterocysts from Anabaena variabilis. Biochim Biophys Acta 723:83–90
Fay P, Van Baalen C (1987) The cyanobacteria. Elsevier, New York, NY, p 543
Fernández-Valiente E, Ucha A, Quesada A, Leganés F, Carreres R (2000) Contribution of N2 fixing cyanobacteria to rice production: availability of nitrogen from 15N-labelled cyanobacteria and ammonia sulphate to rice. Plant Soil 221:107–112
Glazer AN, Hixson CS (1975) Characterization of R-phycocyanin chromophore content of R-phycocyanin and C-phycoerythrin. J Biol Chem 250:5487–5495
Habte M, Alexander M (1980) Nitrogen fixation by photosynthetic bacteria in lowland rice culture. Appl Environ Microbiol 39:342–347
Irisarri P, Gonnet S, Monza J (2001) Cyanobacteria in Uruguayan rice fields: diversity, nitrogen fixing ability and tolerance to herbicides and combined nitrogen. J Biotechnol 91:95–103
Jenssen A (1978) Chlorophyll and carotenoids. In: Hellebust JA, Craigie JS (eds) Handbook of phycological methods: physiological and biochemical methods. Cambridge University Press, Cambridge, UK, pp 59–70
Jian YM (1981) The secret of biological nitrogen fixation. Sciences Press, Beijing, China, pp 212–330
Jin XC, Chu QY (1990) The rule of eutrophication investigation in lake. Environmental Sciences Press, Beijing, China, pp 30–150
Li ZM, Jia GF, Wang LX (1994) Sulfonylurea compounds and its herbicide usage. Chinese patent no. CNI 080 116A
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Mattoo HK (1984) Adaptive reorganization of protein and lipid components in chloroplast members as associated with herbicide binding. J Cell Biochem 24:145–163
Miquel LL, Ivo R (2006) On the way to cyanobacteria blooms: Impact of the herbicide metribuzin on the competition between a green alga (Scenedesmus) and a cyanobacterium (Microcystis). Chemosphere 65:618–626
Mishra AK, Pandey AB, Kumsr HD (1989) Effects of three pesticides on MSX-induced ammonia photopronuction by the cyanobacterium Nostoc linkia. Ecotoxicol Environ Saf 18:145–148
Padhy RN (1985) Cyanobacteria and pesticides. Residue Rev 95:1–4
Patnaik GK, Kanungo PK, Moorthy BTS, Mahana PK, Adhya TK, Rajaramamohan RV (1995) Effect of herbicides on nitrogen fixation (C2H2 reduction) associated with rice rhizosphere. Chemosphere 30:339–343
Prithiviraj B, Perry LG, Badri DV, Vivanco JM (2007) Chemical facilitation and induced pathogen resistance mediated by a root-secreted phytotoxin. New Phytol 173:852–860
Shen JY, Lu YT, Cheng GH (2005) Effects of chemical herbicides on toxicity of non-target nitrogen-fixing cyanobacteria in paddy fields. In: Proceedings of the 20th Asian-Pacific Weed Science Conference. Asian-Pacific Weed Science Society, Ho Chi Minh City, Vietnam, pp 665–670
Shen JY, Ding H, She Y, Li ZM (2008) Effects of monosulfuron and monosulfuron-ester on rice growth and efficacy control of weeds. Weed Sci 2:13–17
Shen JY, DiTommaso D, Lu W, Shen MQ, Li ZM (2009) Molecular basis for differential metabolic responses to monosulfuron in three nitrogen-fixing cyanobacteria. Weed Sci 57:133–141
Tomlin CDS (2003) The pesticide manual, 13th edn. The British Crop Protection Council, London, England, p 1037
Wu ZD, Zhou YQ (2004) An introduction to Anabaena flos-aquae toxins. Yunnan Environ Sci 23:8–11
Acknowledgments
Funding for this work was provided by the Medi-Agriculture Research Foundation (Grant No. YG2009MS24) and Nation Public Science Foundation (Grant No. 200903056). We also thank Weichi Ying of the Research Institute of Environmental Engineering, East China University of Science and Technology, for gracious advice and all the graduate students and technical support personnel, especially Zhengpei Zhong who worked on this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, J., Luo, W. Effects of Monosulfuron on Growth, Photosynthesis, and Nitrogenase Activity of Three Nitrogen-Fixing Cyanobacteria. Arch Environ Contam Toxicol 60, 34–43 (2011). https://doi.org/10.1007/s00244-010-9534-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-010-9534-1