Abstract
The heavy metal contamination in soils and cultivated corn plants affected by zinc smelting activities in the vicinity of a zinc smelting factory in Korea was studied. Soils and corn plants were sampled at the harvesting stage and analyzed for cadmium (Cd) and zinc (Zn) concentration, as well as Cd and Zn fraction and other chemical properties of soils. Cd and Zn were highly accumulated in the surface soils (0–20 cm), at levels higher than the Korean warning criteria (Cd, 1.5; Zn, 300 mg kg−1), with corresponding mean values of 1.7 and 407 mg kg−1, respectively, but these metals decreased significantly with increasing soil depth and distance from the factory, implying that contaminants may come from the factory through aerosol dynamics (Hong et al., Kor J Environ Agr 26(3):204–209, 2007a; Environ Contam Toxicol 52:496–502, 2007b) and not from geological sources. The leaf part had higher Cd and Zn concentrations, with values of 9.5 and 1733 mg kg−1, compared to the stem (1.6 and 547 mg kg−1) and grain (0.18 and 61 mg kg−1) parts, respectively. Cd and Zn were higher in the oxidizable fraction, at 38.5% and 46.9% of the total Cd (2.6 mg kg−1) and Zn (407 mg kg−1), but the exchangeable + acidic fraction of Cd and Zn as the bioavailable phases was low, 0.2 and 50 mg kg−1, respectively. To study the reduction of plant Cd and Zn uptake by liming, radish (Raphanus sativa L.) was cultivated in one representative field among the sites investigated, and Ca(OH)2 was applied at rates of 0, 2, 4, and 8 mg ha−1. Plant Cd and Zn concentrations and NH4OAc extractable Cd and Zn concentrations of soil decreased significantly with increasing Ca(OH)2 rate, since it markedly increases the cation exchange capacity of soil induced by increased pH. As a result, liming in this kind of soil could be an effective countermeasure in reducing the phytoextractability of Cd and Zn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contamination of soils through anthropogenic activities is a widespread and serious problem currently confronting scientists and regulators throughout the world (Liu et al. 2005). South Korea, which is known to have a long history of metalliferous mining and metal smelting process development, is reported to have 21% of arable soil near mining and industrial areas which were severely contaminated by heavy metals from 1995 to 1997 (NIAST 1997). A number of studies have been done on trace element contamination derived from mining activities in soils, plants, waters, and sediments in Korea (Jung and Thornton 1996; Chon et al. 1997; Jung 2001; Lee 2006), but only a few studies of heavy metal contamination in land caused by smelting activities have been undertaken.
Among the metal smelting factories in Korea is the zinc smelting factory, located in the mideastern part of the Korean peninsula; this is the third largest zinc smelting factory in the world. This factory was founded in the 1970 s and now produces 280,000 tons of Zn, 450,000 tons of sulfuric acid, 1700 tons of cupric sulfate, and 900 tons of Cd per year. However, there have been issues reported previously concerning the ill-health effects to heavy-metal-exposed workers, living communities, and the environment. Specifically, about 20 ha of arable land near the factory cultivated with different crops was reported to be contaminated by heavy metals.
Heavy metals are present in various forms in soil. Different forms of heavy metals have different mobilities and phytoextractabilities (Chlopecka and Adriano 1996). Knowledge of heavy metal speciation in contaminated soil is important for understanding the bioavailability and mobility of heavy metals in soils. Generally, plant uptake of heavy metals is correlated with extractable forms of the metals rather than with the total metal content in the soil (Xian 1989). The distribution of heavy metals among the water-soluble, exchangeable + acidic, reducible, organic, and residual fractions using sequential extraction analysis could help in assessing the potential phytoavailability of heavy metals in soil.
Mobilization of heavy metals in soils for plant uptake and leaching to groundwater can be minimized by reducing their bioavailability through chemical and biological immobilization (Adriano 2001). Recently there has been keen interest in the immobilization of heavy metals using a range of inorganic compounds, such as lime, phosphate fertilizers, and alkaline biosolid (Basta et al. 2001). However, the feasibility of phosphate utilization would be very low in the field, since phosphate should be applied at a very high rate to immobilize heavy metals and this results in new environmental problems (Hong et al. 2008). Organic matter is well-known to reduce Cd extractability in soil, due to soil negativity increase (Bolan et al. 2003b). However, there were many cases where compost application increased the dissolved organic carbon concentration in soil and then resulted in stimulating plant Cd uptake (Hamon et al. 1995; Antoniadis and Alloway 2002). In contrast, liming of contaminated soils is considered a more realistic soil management practice to reduce the bioavailability of heavy metals within a short term and is most widely used as a remediation treatment (Andersson and Siman 1991; Miller et al. 1995; Basta and Sloan 1999; Chen et al. 2000).
The objectives of this study were mainly to investigate the extent of heavy metal contamination and Cd and Zn dynamics in soils located near the smelting factory and to examine the possibility of lime treatment for decreasing the phytoextractability of Cd and Zn.
Materials and Methods
Sampling and Analysis
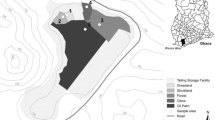
Sampling of soils and plants was carried out on 7 August 2006 to examine the heavy metal contamination (Fig. 1). About 20 ha of arable land is located in a sloping area in the northeastern part of the vicinity of a zinc smelting factory in Bonghwa-gun, Kyeongbuk (37 02′N and 129 03′E). Soils were randomly sampled by auger (diameter, 5 cm) at three depths (0–20, 40–60, and 80–100 cm) at 20 sites of arable land near the smelting factory, air-dried for 5 days, and ground to the desired soil size (<2 mm).
The sieved soils were analyzed for pH (1:5 with H2O) and organic matter content (Walkley and Black method [Allison 1965]). The available P content was determined using the Lancaster method ([RDA 1988] 5 g soil was extracted with 20 ml of 0.33 M CH3CHOOH, 0.15 M lactic acid, 0.03 M NH4F, 0.05 M (NH4)2SO4, and 0.2 M NaOH at pH 4.25). Exchangeable Ca2+, Mg2+, and K+ were extracted with 1 M NH4OAc (pH 7.0) at a soil:solution ratio of 1:5 for 1 h. Cation exchange capacity (CEC) of soil was measured using 0.1 M NaCl following the ion retention method of Schofield (1949). The size distribution of soils was measured according to the Bouyoucos (1936) hydrometer method.
In a previous test (Hong et al. 2007a), we found that Cd and Zn were highly accumulated in this arable soil over the Korean standard (ME 2005). In order to analyze the extent of contamination in detail, soil samples were extracted with a 0.1 N HCl solution for Cd and digested in 3:1 concentrated HCl and HNO3 (aqua regia) for Zn following the Korean standard method (ME 2005).
In order to characterize the Cd and Zn fraction in soil samples, 1 g of dry soil in a 50-ml PTFE centrifuge tube was extracted with 10 ml of 0.11 mol l−1 HOAc shaken overnight for 16 h using end-over-end shaking at 24 ± 2°C for the exchangeable + acidic fraction (fraction 1; F1). The above residue was then resuspended with 10 ml of 0.1 mol L−1 NH2OH HCl (pH 2 with HNO3) for the reducible fraction (fraction 2; F2). The soil residue from F2 was then rinsed with 5 ml of 30% H2O2 (adjusted to pH 2–3 with HNO3). The tube was left at room temperature for 1 h with periodical manual shaking (every 15 min) and thereafter placed for 1 h at 85°C in a water bath to decompose the organic matter. The volume was reduced to a few milliliters by further heating in an uncovered tube and then an aliquot of 5 ml of 30% H2O2 was added to the residue. The tube was covered again, heated at 85°C for 1 h. The cover was then removed and the volume was reduced almost to dryness. After cooling at room temperature, it was resuspended with 10 ml of 1 mol L−1 NH4OAc (adjusted to pH 2 with HNO3) for the oxidizable fraction (fraction 3; F3). Dried soil was digested by aqua regia (3:1, concentrated HCl:HNO3) for total concentration (fraction 5; F5). The residual fraction (fraction 4; F4) was calculated as the difference between the total (F5) and the sum of F1, F2, and F3. Herein, between each successive extraction, separation was effected by centrifugation at 6,000 rpm for 15 min. The supernatant was filtered through a 0.2-μm cellulose acetate membrane filter (Ure et al. 1993).
About 20 ha of arable lands was mostly planted with corn, and a little portion was devoted to vegetables like red pepper, radish, and sesame. Corn plants were collected randomly from 20 sites and separated into three main parts (stem, leaf, and grain). The samples were washed with deionized water, oven-dried at 70°C for 72 h, ground, and digested using a solution of HNO3:H2SO4:HClO4 (10:1:4, vol/vol) for quantifying heavy metal concentration.
To qualify the sequential extraction and digestion method used in this study, Cd and Zn concentrations of BCR 701 and SRM 1573a used as reference materials in soil and plant samples were analyzed following the above methods, respectively. Our observed values for total and fractionized Cd and Zn concentration were over 90% of the recovery rate of the values of the certified reference materials (Table 1). The F3 residue was then digested with aqua regia to obtain the residual fraction (F4) to compare with the above F4 concentration calculated from the difference between the sum of fractions (F1 + F2 + F3) and the total fraction (F5). Cd and Zn recovery rates of the sum of fractions to the total concentration were 103% and 105%, respectively, in the reference material (BCR 701). As a result, we are confident of the accuracy of Cd and Zn fraction analysis in this study.
Heavy metals and exchangeable cations in the solutions were quantified by ICP-OES (inductively coupled plasma-optical emission spectrophotometer; Perkin Elmer Model Optima 4300 DV; Shelton, CT, USA). This ICP-OES maintained a very high accuracy, with detection limits of <0.004 and 0.015 mg kg−1 Cd and Zn, respectively, for soil blank extracts and 0.006 and 0.021 mg kg−1 for blank digest solutions.
Field Experiment
To determine the effect of liming on reducing plant Cd and Zn uptake, one representative field (S3) among the surveyed sites, which was highly contaminated with Cd (2.83 mg kg−1) and Zn (599 mg kg−1), was selected for the field test. Since the corn cultivation period was over, radish (Raphanus sativa L.) was selected as the test crop. In the previous incubation test (Hong et al. 2007b), Ca(OH)2 was found to be the most effective among the liming materials in reducing the plant-available Cd concentration in soil. Calcium hydroxide was therefore selected as the liming material in this study and applied at levels of 0, 2, 4, and 8 Mg ha−1. Experimental plots were arranged in a completely randomized design with three replicates. Radish seeds were seeded by hand at intervals of 20 × 15 cm on 11 September 2006 and harvested on 19 November. Fertilization included N (234 kg ha−1), P2O5 (51 kg ha−1), and K2O (81 kg ha−1) applied together with compost (10 Mg ha−1) before seedling (RDA 1999).
The harvested radish plants were separated into shoot and root parts, oven-dried at 70°C for 72 h, ground, and digested using a solution of HNO3:H2SO4:HClO4 (10:1:4, vol/vol) for Cd and Zn analysis. Soil samples were collected at the harvesting stage for analysis of chemical properties.
Statistics
Statistical analysis was performed with the SAS package, version 8.2 (SAS Institute Inc. 2001). One-way ANOVA was carried out to compare the means of the different treatments where significant F values were detected.
Results and Discussion
Heavy Metal Contamination in Soil
Surface soils of the selected sites were slightly acidic and contained very low concentrations of exchangeable cations. In comparison, organic matter and available phosphate levels were similar to the average Korean upland soil (RDA 1999) (Table 2). In the case of Cd and Zn, almost all the surface soil (0- to 20-cm) samples exceeded the warning criteria (Cd, 1.5 mg kg−1; Zn, 300 mg kg−1) as described in the Soil Environmental Conservation Act of Korea (ME 2005). The warning criterion is the permissible level to prevent the proliferation of heavy metal contamination in soil. The average values for Cd and Zn concentration were 1.7 ± 0.7 and 407 ± 143 mg kg−1, respectively. About 65% (Cd) and 80% (Zn) of the total sampling sites gave values higher than the warning level (Table 3). Concentrations of Cd and Zn decreased significantly with increasing soil depth. The average values for Cd and Zn concentration at a soil depth of 40–60 cm (Cd, 0.53 mg kg−1; Zn, 149 mg kg−1) and 80–100 cm (Cd, 0.2 mg kg−1; Zn, 102 mg kg−1) were lower than the warning criterion values for these metals. The higher Cd and Zn concentrations in the surface soil than in the subsoil horizons mean that the heavy metal sources might have come from the external environment rather than from the parent materials. In addition, the farther the sampling site was from the zinc smelting factory, the lower the Cd and Zn contents obtained from the samples. Using the linear equation model, Cd and Zn concentrations were related to sampling distance (D) from the zinc smelting factory as Cd concentration in soil = −0.0043 D + 3.988, r = 0.382, and Zn concentration in soil = −0.973 D + 927, r = 0.443 (p < 0.05), where concentration is expressed as milligrams per kilogram soil, and distance as meters. The distance from the smelting factory to the sampling site significantly affected Cd and Zn concentration as dispersed by aerial deposition and wind action. In the same location, Hong et al. (2007a) detected Cd and Zn in the aerosol samples at trace level and 30.2 mg kg−1, respectively. In general, soils containing an excess of 1 mg Cd kg−1 soil are considered to be evidence of anthropogenic pollution (Uminska 1993). Numerous investigations have shown that pronounced amounts of Cd and Zn are often found in arable soils adjacent to nonferrous metal production bases (Pierzynski and Schwab 1993; Dahmain-Muller et al. 2000).
Metal fractionation using sequential extraction techniques has primarily been used to identify the fate of metals applied to sewage sludge and in soils contaminated by smelters and mine drainage wastes (Sposito et al. 1982). In this soil, Cd and Zn concentrations of individual fractions in the sequential extraction analysis are listed in Table 4. The order of the mean Cd concentration in fractions, from highest o lowest, was F3 (oxidizable fraction) > F2 (reducible fraction) > F4 (residual fraction) > F1 (exchangeable + acidic fraction), but that in Zn was F3 > F4 ≥ F2 > F1. Highest mean concentrations in Cd and Zn were obtained from the oxidizable fraction (F3), with values of 1.0 mg kg−1 (38.5% of the total Cd) and 191 mg kg−1 (46.9% of the total Zn), respectively. In comparison, the mean concentrations of Cd and Zn in bioavailable form (F1) were relatively low, at 0.2 mg kg−1 (7.7% of the total Cd) and 50 mg kg−1 (12.3% of the total Zn), respectively.
Heavy Metal Uptake by Corn Plants
Cd and Zn toxicity symptoms were not observed in corn plants in this study. However, the leaf part had a higher Cd concentration, with a corresponding value of 9.5 mg kg−1, than the stem and grain parts, with 1.6 and 0.18 mg kg−1, respectively (Table 5). A higher Zn concentration was also observed in the leaf part (mean, 1733 mg kg−1) than in the stem and grain parts, with means of 547 and 61 mg kg−1, respectively. These results were similar to the previous report by Dudka et al. (1994), who found that the concentrations of Cd and Zn in spring wheat plant were higher in straw than in grain, by factors of 1.5–2 and 3–7 for Cd and Zn, respectively, and the field experiment of Granato et al. (2004), who reported that the leaf part had a higher Cd concentration, with the corresponding value of 10 mg kg−1, compared to the grain part (0.2 mg kg−1). Hong et al. (2007b) reported that Cd was more concentrated (4.1–5.9 times) in shoots of radish than in roots under field conditions. In this study, 80% and 85% of the corn grain samples yielded concentrations higher than the maximum safe intake level for Cd (0.1 mg kg−1) and Zn (40 mg kg−1) respectively, established by Bowen (1979). Corn stover is generally consumed by animals as feed stuff or recycled as organic matter sources in Korea. This highly accumulated Cd and Zn in plants can easily reach the human body through the food chain, and therefore, a soil management strategy for reducing Cd and Zn phytoextractability urgently needs to be established in this area.
The plant uptake of heavy metals is correlated with extractable forms of the metals rather than with the total metal content in the soil (Xian 1989). In general, the soluble and exchangeable form of heavy metals is regarded as labile in plants (Adriano 2001). In this study, comparatively labile Cd and Zn fractions (F1 + F2) in soil showed a significantly positive correlation with these metal accumulations in corn leaf and grain, but this significant level was decreased upon shifting into more strongly bound fractions (F3 and F4) (Table 6). These results suggest that shifting the solid phases of Cd and Zn from mobile to immobile fractions can reduce the metal uptake of corn plants.
In the selected soils, the strongly bound Cd and Zn fractions (F3 and F4) in soils showed a highly positive correlation with soil pH, available P, exchangeable Ca and Mg, and CEC of the chemical properties investigated, corresponding with an increasing negative correlation with the weakly bound fractions (F1 and F2) (Table 7). These results imply that improved pH, phosphate concentration, and CEC by soil management can immobilize labile Cd and Zn and thus reduce plant uptake. Soil amendments, such as lime and phosphate compounds, have been found to be effective in immobilizing heavy metals, thereby reducing their bioavailability in soils (Basta et al. 2001; Knox et al. 2000). However, the feasibility of using phosphate fertilizer to immobilize Cd was reported to be very low in the field (Hong et al. 2008). In comparison, liming is considered a more realistic management practice to reduce the bioavailability of heavy metals within a short term by increasing the soil pH and CEC (Bolan et al. 2003a; Hong et al. 2007b).
Liming Effect
In this study, Ca(OH)2 was selected as the liming material to reduce plant Cd and Zn uptake at the selected sites. As shown in Fig. 2, Ca(OH)2 significantly decreased Cd and Zn concentrations in radish root and shoot (Fig. 2). Cd concentration in radish (Y; mg kg−1) was related to Ca(OH)2 (X; Mg ha−1) application rates as follows: shoot, Y = 4.636 − 0.44X (r = 0.839***); and root, Y = 0.824 – 0.071X (r = 0.829***). The Cd content of radish root and shoot was decreased by ca. 20% by 2 Mg ha−1 Ca(OH)2 application, which is the recommended level for radish cultivation in general upland soil in Korea (RDA 1999), and reduced by about 50% by ca. 5 Mg ha−1 Ca(OH)2 application. However, the effect of Ca(OH)2 application on decreasing radish Zn uptake was different from its effect on reducing Cd uptake. Zinc concentrations of radish shoot and root were reduced by only 10% and 3% by 5 Mg ha−1 Ca(OH)2 application, respectively.
Contrary to Cd, Zn is an essential element for plant growth and plays a functional and structural role in enzyme reactions for plant metabolism (Vallee and Auld 1990). The efficient homeostatic regulation by plants might prevent the severe reduction of radish Zn uptake in the limed soil. There was no investigation as to the optimum Zn level for radish plants. In general, it is known that a sufficient Zn level for plants is 20–100 mg kg−1 on a dry matter basis, and Zn toxicity is observed at >400 mg kg−1 (Bennett 1993). Zinc concentration in radish shoot and root was ca. 105 and 78 mg kg−1 in the control, respectively, but Zn uptake was not reduced markedly by Ca(OH)2 application compared with the radish Cd uptake response to the liming. The FAO/WHO suggested that the concentration of Zn in vegetables should not exceed 60 mg kg−1 (Codex Alimentarius Commission 1984), but the Zn concentration in radish shoot and root was reduced to just 90 and 75 mg kg−1 by a high-level Ca(OH)2 application (8 Mg ha−1). This might indicate a high Zn requirement of radish plants, and another soil management strategy would be required.
The concentration of NH4OAc-extractable Cd and Zn in soil, which is the plant-available form (Gommy et al. 1998; Brun et al. 2001), changed with the same tendency as the metal uptake characteristics of radish (Fig. 3). They were significantly decreased by Ca(OH)2 application with soil pH and CEC increase (Fig. 4). Cadmium and Zn adsorption in soil increases with increasing CEC (Bolan et al. 1999; Naidu et al. 1994), therefore resulting in low Cd and Zn phytoavailability. Notably, an increase in pH increases the CEC of soil, which is attributed to dissociation of H+ from the soil surface due to the increased pH (Bolan et al. 2003a; Hong et al. 2007b). As a result, increased Cd and Zn adsorption caused by pH-induced increase in soil CEC might decrease NH4OAc extractable Cd and Zn concentration in soil and reduce metal uptake by the radish plant.
Conclusion
Surface soils and cultivated corn plants in the upland farming lands in the vicinity of the zinc smelting factory were heavily contaminated with Cd and Zn. The concentrations of these metals were significantly decreased with increasing soil depth and distance from the factory, which indicated that contaminant sources may come from the factory rather than from the parent materials. Cadmium was accumulated mainly within the reducible and oxidizable fraction (F2 and F3), and Zn in the oxidizable fraction (F3), but labile Cd and Zn phases (F1) were less than the mean 0.2 and 50 mg kg−1, respectively. The Cd and Zn content of the corn plants showed a highly positive correlation with the weakly bound fraction (F1 + F2). The strongly bound fraction (F3 and F4) also showed a high positive correlation with pH, available P, exchangeable Ca and Mg, and CEC of soils. Ca(OH)2, selected to decrease Cd and Zn phytoavailability, as a liming material significantly reduced radish Cd and Zn uptake and NH4OAc extractable Cd and Zn concentration in soil, due to increased soil pH and CEC. In conclusion, liming could be an effective countermeasure to decrease Cd and Zn bioavailability in these areas contaminated by heavy metals.
Acknowledgments
Mr. C. O. Hong was supported by a scholarship from the BK 21 Program, the Ministry of Education and Human Resources Development, Korea.
References
Adriano DC (2001) Trace elements in terrestrial environments; biogeochemistry, bioavailability and risks of metals, 2nd edn. Springer, New York
Allison LE (1965) Organic carbon. In: Black CA (ed) Methods of soil analysis, Part II. Am. Soc. Agron. Publ, Madison, WI, pp 1367–1378
Andersson A, Siman G (1991) Levels of Cd and some other trace elements in soils and crops as influenced by lime and fertilizer level. Acta Agr Scand 41:3–11
Antoniadis V, Alloway BJ (2002) The role of dissolved organic carbon in the mobility of Cd, Ni and Zn in sewage sludge-amended soils. Environ Pollut 117:515–521. doi:10.1016/S0269-7491(01)00172-5
Basta NT, Sloan JJ (1999) Bioavailability of heavy metals in strongly acidic soils treated with exceptional quality biosolids. J Environ Qual 28:633–638
Basta NT, Gradwohl R, Snethen KL, Schroder JL (2001) Chemical immobilisation of lead, zinc and cadmium in smelter contaminatedsoils using biosolids and rock phosphate. J Environ Qual 30:1222–1230
Bolan NS, Naidu R, Khan MAR, Tillman RW, Syers JK (1999) The effects of anion sorption on sorption and leaching of cadmium. Aust J Soil Res 37:445–460. doi:10.1071/S97046
Bolan NS, Adriano DC, Mani PA, Duraisamy A (2003a) Immobilization and phytoavailability of cadmium in variable charge soils. II. Effect of lime addition. Plant Soil 251:187–198. doi:10.1023/A:1023037706905
Bolan NS, Adriano DC, Duraisamy A, Mani PA (2003b) Immobilization and phytoavailability of cadmium in variable charge soils. III. Effect of biosolid compost addition. Plant Soil 256:231–241. doi:10.1023/A:1026288021059
Bouyoucos GJ (1936) Directions for making mechanical analysis of soils by the hydrometer method. Soil Sci 4:225–228
Bowen HJM (1979) Environmental chemistry of the elements. Academic Press, New York
Brun LA, Maillet J, Hinsinger P, Pepin M (2001) Evaluation of copper availability to plants in copper-contaminated vineyard soils. Environ Pollut 111:293–302. doi:10.1016/S0269-7491(00)00067-1
Chen HM, Zheng CR, Tu C, Shen ZG (2000) Chemical methods and phytoremediation of soil contaminated with heavy metals. Chemosphere 41:229–234. doi:10.1016/S0045-6535(99)00415-4
Chlopecka A, Adriano DC (1996) Mimicked in-situ stabilization of metals in cropped soil: bioavailability and chemical form of zinc. Environ Sci Technol 30:3294–3303. doi:10.1021/es960072j
Chon HT, Ahn JS, Jung MC (1997) Environmental contamination of toxic heavy metals in the vicinity of some Au–Ag mines in Korea. In: Proceedings of the 4th Biennial SGA Meeting, Turku, Finland, pp 891–894
Codex Alimentarius Commission (1984) Contaminants, Joint FAO/WHO Food Standards Program. Vol XVII. 1st edn. Codex Alimentarius
Dahmain-Muller H, Oort FV, Gelie B, Bababane M (2000) Strategies of heavy metal uptake by the three species growing in a new metal smelter. Environ Pollut 109:231–238. doi:10.1016/S0269-7491(99)00262-6
Dudka S, Piotrowska M, Chlopecka A (1994) Effect of evelvated concentratios of Cd and Zn in soil on spring wheat yield and the metal contents of the plants. Water Air Soil Pollut 76:33–341. doi:10.1007/BF00482710
Gommy C, Perdrix E, Galloo JC, Guillermo R (1998) Metal speciation in soil: extraction of exchangeable cations from a calcareous soil with a magnesium nitrate solution. Int J Environ Anal Chem 72:27–45. doi:10.1080/03067319808032642
Granato TC, Pietz RI, Knafl GJ, Carlson CR, Tata JP, Lue-Hing C (2004) Trace element concentrations in soil, corn leaves, and grain after cessation of biosolids applications. J Environ Qual 33:2078–2089
Hamon RE, Lorenz SE, Holm PE, Christensen TH, McGrath SP (1995) Changes in trace metal species and other components of the rhizosphere during growth of radish. Plant Environ 18:749–756. doi:10.1111/j.1365-3040.1995.tb00577.x
Hong CO, Gutierrez JM, Lee SB, Lee YB, Yu C, Kim PJ (2007a) Determination of cadmium and zinc contamination source in arable soil in the vicinity of a zinc smelting factory. Kor J Environ Agr 26(3):204–209
Hong CO, Lee DK, Chung DY, Kim PJ (2007b) Liming effects on cadmium stabilization in upland soil affected by gold mining activity. Environ Contam Toxicol 52:496–502. doi:10.1007/s00244-006-0097-0
Hong CO, Lee DK, Kim PJ (2008) Feasibility of phosphate fertilizer to immobilize cadmium in a field. Chemosphere 70:2009–2015. doi:10.1016/j.chemosphere.2007.09.025
Jung MC (2001) Heavy metal contamination of soils and waters in and around the Imcheon Au-Ag mine, Korea. Appl Geochem 16:1377–1386. doi:10.1016/S0883-2927(01)00040-3
Jung MC, Thornton I (1996) Heavy metal contamination of soils and plants in the vicinity of a lead-zinc mine, Korea. Appl Geochem 11:53–59. doi:10.1016/0883-2927(95)00075-5
Knox AS, Seaman JC, Mench MJ, Vangronsveld J (2000) Remediation of metal and radionuclides-contaminated soils by in situ stabilization techniques. In: Iskander IK (ed) Environmental restoration of metals-contaminated soils. Lewis, New York, pp 21–60
Lee SH (2006) Geochemistry and partitioning of trace metals in paddy soils affected by metal mine tailings in Korea. Geoderma 135:26–37. doi:10.1016/j.geoderma.2005.11.004
Liu H, Probst A, Liao B (2005) Metal contamination of soils and crops affected by the Chenzhon lead/zinc mine spill (Hunan, China). Sci Total Environ 339:153–166. doi:10.1016/j.scitotenv.2004.07.030
ME (Ministry of Environment, Republic of Korea) (2005) The Korean Soil Environmental Conservation Act. ME, Gwacheon (in Korean)
Miller RW, Al-Khazraji ML, Sisson DR, Gardiner DT (1995) Alfalfa growth and absorption of cadmium and zinc from soils amended with sewage sludge. Agr Ecosyst Environ 53:179–184. doi:10.1016/0167-8809(94)00559-W
Naidu R, Bolan NS, Kookana RS, Tiller KG (1994) Ionic strength and pH effects on the adsorption of cadmium and the surface charge of soils. Eur J Soil Sci 45:419–429. doi:10.1111/j.1365-2389.1994.tb00527.x
NIAST (National Institute of Agricultural Science and Technology, Korea) (1997) Survey of heavy metals contamination degree of arable soil located in mining area. Annual Report. Department of Agricultural Environment, pp 237–243 (in Korean)
Pierzynski GM, Schwab AP (1993) Bioavailability of zinc, cadmium and lead in a metal contaminated alluvial soil. J Environ Qual 22:247–254
Pueyo M, Rauret G, Luck D, Yli-Halla M, Muntau H, Ph Quevauviller, Lopez-Sanchez JF (2001) Certification of the extractable contents of Cd, Cr, Cu, Ni, Pb and Zn in a freshwater sediment following a collaboratively tested and optimized three-step sequential extraction procedure. J Environ Monit 3:243–250. doi:10.1039/b010235k
RDA (Rural Development Administration, Korea) (1988) Methods of soil chemical analysis. National Institute of Agricultural Science and Technology, RDA, Suwon (in Korean)
RDA (1999) Recommendation standard of fertilization for crops. National Institute of Agricultural Science and Technology, RDA, Suwon (in Korean)
SAS Institute Inc (2001) User’s guide: statistics SAS version 8.2. SAS Institute, Cary, NC
Schofield RK (1949) Effect of pH on electric charges carried by clay particles. J Soil Sci 1:1–8. doi:10.1111/j.1365-2389.1950.tb00713.x
Sposito F, Sund LJ, Chang AC (1982) Trace metal chemistry in arid-zone field soils amended with sewage sludge: fractionation of Ni, Cu, Zn, Cd and Pb in solid phases. Soil Sci Soc Am J 46:260–264
Uminska R (1993) Cadmium contents of cultivated soils exposed to contamination in Poland. Environ Geochem Health 15:15–19. doi:10.1007/BF00146288
Ure AM, Quevauviller PH, Muntau H, Griepink B (1993) Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. J Environ Anal Chem 51:135–151. doi:10.1080/03067319308027619
Vallee BL, Auld DS (1990) Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 29:5647–5659. doi:10.1021/bi00476a001
Xian X (1989) Effect of chemical forms of cadmium, zinc and lead in polluted soils on their uptake by cabbage plants. Plant Soil 113:257–264. doi:10.1007/BF02280189
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hong, C.O., Gutierrez, J., Yun, S.W. et al. Heavy Metal Contamination of Arable Soil and Corn Plant in the Vicinity of a Zinc Smelting Factory and Stabilization by Liming. Arch Environ Contam Toxicol 56, 190–200 (2009). https://doi.org/10.1007/s00244-008-9195-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-008-9195-5