Abstract
This study investigated the occurrence of 16 polycyclic aromatic hydrocarbons (PAHs) and 6 phthalic acid esters (PAEs) in 11 vegetable species collected from nine farms of the Pearl River Delta, South China. Twelve PAH compounds and all PAE compounds were detected by gas chromatography coupled with mass spectrometry (GC-MS) in vegetables. The total concentrations of PAHs (ΣPAHs) and PAEs (ΣPAEs) ranged from 7.0 to 5353 μg kg−1 dry weight (d.w.), with a mean value of 1173 μg kg−1 d.w., and from 0.073 to 11.2 mg kg−1 d.w., with a mean value of 3.2 mg kg−1 (d.w.), respectively. The highest levels of ΣPAHs and ΣPAEs were found in Brassica juncea and Brassica parachinensis, respectively. For the same vegetable, the bioconcentration factors (BCFs; the ratio of contaminant concentration in plant tissue to the soil concentration) of PAHs (between 0.0037 and 5.5) are generally higher than those of PAEs (between <0.0001 and 0.61). It was also noted that there were great variations of organic contaminant levels, BCFs, and benzo[a]pyrene equivalent concentrations, which depend on the various contaminants, sampling locations, and vegetable species. The occurrences of PAHs and PAEs in this study are compared with those in other studies and their sources are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Polycyclic aromatic hydrocarbons (PAHs) and phthalic acid esters (PAEs) are two large groups of organic contaminants with a relatively low solubility in water and a high lipophilicity. Because of their toxic, mutagenic, and/or carcinogenic properties, 16 PAHs and 6 PAEs were classed as priority pollutants by both the Chinese Environmental Agency and the United States Environmental Protection Agency (USEPA). Both classes of organic compounds are of worldwide concern due to their hazards and global distribution (Thomas et al. 1986; Ma et al. 2003).
A lot of work has been conducted on the occurrences of PAHs and PAEs in soils and different food categories such as vegetables outside China (Voutsa and Samara 1998; Kipopoulou et al. 1999; Kazerouni et al. 2001; Wennrich et al. 2002; Camargo and Toledo 2003; Nadal et al. 2004; Bishnoi et al. 2006; Zohair et al. 2006). It was found that the levels of PAHs and PAEs were dependent on vegetable species as well as sampling sites, exhibiting a site-specific or species-specific feature. Vegetables taken from highly industrialized areas or roadsides or contaminated areas showed higher concentrations of contaminants than those from rural areas in general (Kipopoulou et al. 1999). So-called organically farmed vegetables were frequently found to accumulate organic contaminants, such as PAHs, polychlorinated biphenyls, and organochlorine pesticides (Zohair et al. 2006). Some work has been conducted within China focusing on the occurrences of PAHs and PAEs in soils and different food categories such as vegetables (Yin and Su 1996; Ma et al. 2003; Tao et al. 2004; Zhang et al. 2004; Maskaoui et al. 2006; Shen and Zhu 2007; Li et al. 2008). However, all this work was conducted in comparatively underdeveloped areas, which might be less disturbed by anthropogenic activities. More attention needs to be paid on economically developed areas in China to better understand the occurrence of PAHs and PAEs in vegetables, to evaluate their accumulation, distribution, and possible sources, and to assess their potential risk to human health.
The Pearl River Delta (PRD) region is one of the most important and financially developed regions in China, covering southern Guangdong Province (Canton), Hong Kong, and Macao. The natural conditions of sunshine and heat enable the PRD to be an important production base of vegetables aiming at supply to North China, Hong Kong, and Southeast Asia. Nevertheless, rapid industrialization, urbanization, growing agricultural activities, and increasing usage of chemicals during the last two decades have severely deteriorated the environmental quality of this region. One of the indicators for deteriorating environments is the elevated concentrations of organic contaminants identified in the environments (Fu et al. 2003; Cai et al. 2005, 2007a, 2008a; Li et al. 2006). Although there are a few data sets in the literature concerning the occurrence of organic contaminants in vegetables of China (Tao et al. 2004; Zhang et al. 2004; Maskaoui et al. 2006; Shen and Zhu 2007; Li et al. 2008), more work needs to be done in this area, especially on the vegetables from various farms, since vegetables are considered to be basic foods in the Chinese diet, accounting for 27%−45% of the total dietary fiber (Zhong-Guang Web 2008). Such information could be helpful for elucidating the health risk of exposure to PAHs and PAEs.
Food consumption is by far the main exposure route to PAHs and PAEs for the population (Phillips 1999; Falcó et al. 2005), and vegetables seem to be one of the main contributors to the total dietary intake of these pollutants except where there is high consumption of meat cooked over an open flame (Phillips 1999). This study investigated the occurrence of PAHs and PAEs in vegetables collected from nine representative farms within the PRD, evaluated their accumulation, distribution, and possible sources in vegetables, and assessed the toxicity of PAHs in vegetables based on benzo[a]pyrene equivalent concentration.
Materials and Methods
Chemicals
A composite stock standard solution (1000 μg mL−1 in dichloromethane; 99.8% purity) was used, containing 16 USEPA PAHs and 6 USEPA PAEs, namely, naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Fl), phenanthrene (Phe), anthracene (Ant), fluoranthene (Fla), pyrene (Pyr), benzo[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), dibenzo[a,h]anthracene (DahA), indeno[1,2,3-cd]pyrene (InP), benzo[ghi]perylene (BghiP), dimethyl phthalate (DMP), diethyl phthalate (DEP), di-n-butyl phthalate (DBP), butylbenzyl phthalate (BBP), di-n-octyl phthalate (DOP), and di(2-ethylhexyl) phthalate (DEHP). Working standard solutions in dichloromethane were prepared by diluting appropriate volumes of the stock standard solution. Surrogate standard mixture contained [2H8]naphthalene (naphthalene-d8), [2H10]acenaphthylene (acenaphthylene-d10), [2H10]phenanthrene (phenanthrene-d10), [2H12]chrysene (chrysene-d12), and [2H12]perylene (perylene-d12) in a mixture solution of 4000 μg mL−1. These standards were purchased from Ultra Scientific (North Kingstown, RI, US).
Analytical-grade dichloromethane (DCM) and ether were redistilled before use. Silica gel (80–200 mesh) and neutral alumina (80–100 mesh) were Soxhlet-extracted with DCM and n-hexane for 24 h, respectively, activated at 180° and 250°C, respectively, for 12 h, deactivated with 3% redistilled water, and kept in n-hexane prior to use. Anhydrous sodium sulfate was dried at 450°C for 6 h and stored in a sealed desiccator. These materials were purchased from Guangzhou Chemical Reagent Co., China. Special precautions were taken to avoid contamination as described by Cai et al. (2007b) during sampling and further processing of the samples. Briefly, detergents were not used during sample processing. No plastic equipment was used during sampling and processing. All glass apparatus was washed with a K2CrO4-H2SO4 solution, with tap water and redistilled water, and then dried at 120°C. Glassware was furnaced at 300°C for 4 h before use.
Sampling Locality, Description of Area, and Vegetable Sampling
Nine representative farms located in different cities including Guangzhou and Shenzhen within the PRD were selected according to their geographic location, cultivated areas, history and model of operation, and environment. A detailed description of the PRD and representative farms and sampling locations has been presented elsewhere (Cai et al. 2007a; also presented in Supplementary Fig. 1S). A description of the vegetable sampling sites and numbers of samples are reported in Table 1.
Eleven vegetable species (including 18 varieties and 45 samples; Table 2) were collected from nine farms. The vegetable sample was randomly collected from five different sites on the same farm, then homogenized, and 2 kg fresh weight taken as a sample. Vegetable samples were carefully washed with tap water to remove any attached soil particles, then rinsed twice with redistilled water. The samples were dried, ground in a solvent-cleaned stainless-steel mill, and refrigerated until analysis.
Analytical Methods
Sample extraction and cleanup were performed according to USEPA methods 3550B and 3630C with modification, respectively. Twenty grams of dry samples spiked with surrogate standards was extracted in triplicate with 60 mL ether in a sonicator (SK5200H; China) for 30 min. The extracts were concentrated, cleaned up, and further processed as described in detail by Cai et al. (2007a, b).
Analysis of individual PAHs and PAEs was performed using gas chromatography coupled with mass spectrometry (GC-MS) by a modification of USEPA method 8270C. The temperature program for GC-MS analysis, the quantification method, and the details of quality assurance/quality control (QA/QC) have been presented elsewhere (Cai et al. 2007a). Briefly, analysis of PAHs and PAEs employed a Thermo Finnigan TRACE gas chromatograph, equipped with a Thermo Finnigan TRACE MS plus and HP-5 silica fused capillary column (30 m × 0.25-mm i.d., with 0.25-μm membrane thickness; Agilent Technology). Splitless surge injection of 1 μL of extract was conducted manually. Column temperature was programmed from 45°C (held for 1 min) to 110°C at 35 deg min−1, followed by an increase to 275°C at 15 deg min−1, then increased to 280°C at 25.0 deg min−1 (held for 5 min). The injection port and the interface line temperature were maintained at 230° and 250°C, respectively. Helium was used as the carrier gas, with split flow 50 mL min−1 for 2 min, surge pressure 300 kPa for 2 min, and gas saver flow 20 mL min−1 for 2 min. Column pressure was programmed from 80 to 120 kPa at 8 kPa min−1, followed by an increase to 150 kPa at 60 kPa min−1 and a hold for 5 min at 150 kPa. Mass spectra were acquired in the electron ionization (EI) mode. Identification of PAH and PAE components was based on comparison of retention time data between samples and the standard solution containing 16 PAHs and 6 PAEs. The external calibration method was applied based on a five-point calibration curve for individual components (1–10 μg L−1). The concentration of each PAH or PAE was determined by an individual calibration database set up from the certified standards. The detection limits of individual PAHs and PAEs ranged from 0.20 μg kg−1 (for Ace) to 1.4 μg kg−1 (for Pyr) and from 0.39 μg kg−1 (for BBP) to 1.4 μg kg−1 (for DMP), respectively.
Results
Polycyclic Aromatic Hydrocarbons
The occurrence of PAHs in vegetable samples is reported in Table 2 (all results of this study are expressed on a dry weight basis). Twelve PAH compounds of interest were detected, and other PAHs including BkF, BaP, InP, and BghiP were below the detection limits. The total concentrations (ΣPAHs) ranged from 7.0 μg kg−1 (Ipomoea aquatica) to 5353 μg kg−1 (Brassica juncea), with an average value of 1173 μg kg−1 (n = 45). The ΣPAHs were >1000 and <100 μg kg−1 in 36.7% and 26.5% of samples, respectively. The ΣPAHs in Brassica juncea was about 790 times higher than that in Ipomoea aquatica, indicating large variations for ΣPAHs in various plant species.
Comparable ΣPAHs in vegetables collected within China were found in the literature. Tao et al. (2004) reported ΣPAHs in vegetables collected from Tianjin, northern China, ranging from 280 to 690 μg kg−1. Vegetables grown near an iron and steel industrial area of Hangzhou, Zhejiang Province, had ΣPAHs of 227 to 1533 μg kg−1 (Shen and Zhu 2007). Even higher ΣPAHs were found in vegetables collected from Minjiang River Estuary (8600–111,000 μg kg−1) (Zhang et al. 2004) and from some locations in Xiamen (8236–58873 μg kg−1), Fujian Province (Maskaoui et al. 2006). However, comparatively low ΣPAHs were reported for vegetables collected in other countries, e.g., Greece (25–294 μg kg−1) (Voutsa and Samara 1998; Kipopoulou et al. 1999), Germany (1–120 μg kg−1) (Wennrich et al. 2002), Brazilian (4.4–48 μg kg−1) (Camargo and Toledo 2003), India (60 to 194 μg kg−1) (Bishnoi et al. 2006), and England (from 8.4 ± 0.9 to 40.1 ± 4.9 μg kg−1) (Zohair et al. 2006). The remarkably higher ΣPAHs in vegetables from China than in those from other countries might suggest heavier contamination by PAHs than in the other, aforementioned countries. Further investigation of PAH levels in vegetables from different regions is necessary in the future.
Great variations were observed not only in different vegetable species, but also in different vegetable cultivars or growth periods (Voutsa and Samara 1998; Zohair et al. 2006). For example, white Vigna sesquipedalis Fruvvirth and Vigna sesquipedalis Fruvvirth both were collected from the same farm, but they showed big differences in ΣPAHs values (2930 vs 990 μg kg−1; data not shown). Similarly, filiform-leaf Ipomoea aquatica and Ensiform green Ipomoea aquatica had ΣPAHs of 3610 and 1039 μg kg−1, respectively (data not shown). Shen and Zhu (2007) reported the highest ΣPAHs in leafy vegetables, while Wennrich et al. (2002) found the highest one in parsley and kale. The present study did not find such a big difference of ΣPAHs in different vegetable species.
Table 2 shows that the total concentrations of the seven carcinogenic PAHs (ΣPAHs carc; BaA, Chr, BbF, BkF, BaP, InP, and DahA) in the investigated vegetables ranged from not detectable (ND) to 2361 μg kg−1, with an average value of 155 μg kg−1. These ΣPAHs carc values were comparable to or even higher than those in vegetables grown near an iron and steel industrial area of Hangzhou (ranging from 7.1 to 231 μg kg−1, with a mean value of 71 μg kg−1) (Shen and Zhu 2007) but markedly higher than those reported in vegetables of Greece (Voutsa and Samara 1998; Kipopoulou et al. 1999).
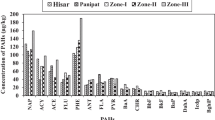
It is worth noting that the concentrations of individual PAHs in the vegetable samples, when detected, varied widely. Only Nap was detected in all samples, at concentrations ranging from 5.6 μg kg−1 (Amaranthus tricolor) to 739 μg kg−1 (Momordica charantia) and accounting for 1.4–78% of the ΣPAHs (Fig. 1a). Fla was the most abundant among 12 detectable PAHs, accounting for 35–87% of the ΣPAHs in seven vegetable species (Brassica parachinensis, Brassica chinensis, Ipomoea aquatica, Brassica juncea, Loctuca satira, Vigna sesquipedalis, Momordica charantia), while Pyr was the dominant compound, accounting for 57% and 71% of the ΣPAHs in Amaranthus tricolor and Cucumis sativus, respectively. Phe and Ant were the predominant compounds, accounting for 92% of the ΣPAHs only in Allium ascalonicum. Fla and BbF accounted for 37% and 27% of the ΣPAHs in Brassica juncea (Fig. 1a). These results indicated the different distribution profiles of PAHs in various vegetables and implied different accumulation of various PAH compounds in vegetables. Maskaoui et al. (2006) reported that InP was the most abundant (accounting for >80% of the ΣPAHs) in all vegetables in some locations in Xiamen, Fujian Province, which is different from the results of the present study. The concentrations of Ant, Fla, BaA, and BaP, when detected, were comparable to or higher than those in vegetables collected from northern Chinese wholesale markets (between 3.3 and 6.5 μg kg−1 fresh weight) (Zhong et al. 2002). The concentrations of BaP in vegetable samples in this study were below the detection limit and also lower than the limit (1.0 μg kg−1) set for vegetable food in Germany (Wennrich et al. 2002).
Phthalic Acid Esters
Descriptive statistics for PAEs are reported in Table 3. Six PAEs were detected in the investigated vegetable samples and their total concentrations (ΣPAEs) ranged from 0.073 mg kg−1 (Ipomoea aquatica) to 11.2 mg kg−1 (Brassica parachinensis), with an average value of 3.2 mg kg−1. These values are quite high, even close to those observed in the radish (Raphanus sativus) planted in sewage sludge-amended soils (Cai et al. 2008b). The high concentrations of PAEs in the vegetables might result from extensive utilization of agricultural chemicals such as film and plastics in recent years (Agriculture Department of Guangdong Province 2005).
The concentrations of individual PAEs varied, with different detection frequencies. DOP was detected only in 22%, DMP and BBP in 45%, and DBP and DEHP in 67 and 80% of the samples (n = 45), respectively. DMP, DEP, and DOP had concentrations lower than 1.0 mg kg−1, and DBP lower than 3.0 mg kg−1. Concentrations of BBP and DEHP reached 7.7 and 9.3 mg kg−1, respectively (Table 3). BBP and/or DEHP were the most abundant in most vegetable samples (except Brassica juncea), accounting for 67%–90% of the ΣPAEs (Fig. 1b). The concentrations of DEHP, when detected, were remarkably higher than those in vegetables (with a geometric mean of 41.8 μg kg−1) from the Netherlands (Peijnenburg and Struijs 2006) but far lower than those (2.6–75.5 mg kg−1 fresh weight) in the edible fruit flesh of Benincasa hispida (wax gourd) collected from southern and northern provinces in China (Du et al. 2006).
Discussion
Bioconcentration Factors of PAHs and PAEs
Bioconcentration factors (BCFs) are expressed as the ratio of PAH (or PAE) concentration in the vegetable on a dry weight basis to the respective concentration in the soils. The concentrations of PAHs and PAEs in the corresponding soils have been presented in detail elsewhere (Cai et al. 2005, 2007a; also presented in Supplementary Tables 1S and 2S). From those values as well as Tables 2 and 3 here, the BCFs are calculated and presented in Table 4. Because the concentrations of some individual PAHs or PAEs in some vegetable samples were not detectable in the present study, BCFs are reported in Table 3 only for ΣPAHs, ΣPAEs, and three typical PAE compounds.
BCFs for PAHs in different species varied from 0.0037 (Ipomoea aquatica) to 5.5 (Loctuca satira Linn); 68.2% and 25% of the investigated samples (n = 45) had BCFs lower than 1.0 and 0.1. Loctuca satira Linn showed a higher BCF, which is due to its large surface area in comparison to other vegetable species (Kipopoulou et al. 1999; Camargo et al. 2003), because atmospheric deposition is an important input of PAHs (Kipopoulou et al. 1999). BCFs in the present study were comparable to those recorded in Shunde, China (Li et al. 2008), and substantially higher than those reported in Tianjin, China (Tao et al. 2004), England (Zohair et al. 2006), and Greece (Kipopoulou et al. 1999), but far lower than those in the Xiamen region and Minjiang River Estuary, when calculated using the values reported by Zhang et al. (2004) and Maskaoui et al. (2006).
As for the PAEs, the BCF values for DBP, DEHP, and the ΣPAEs were lower than 1.0 (except for the maximum value in Brassica parachinensis) (Table 4). Fifty percent of the samples had BCFs for the ΣPAEs lower than 0.1. The highest BCF value for the ΣPAEs was observed in Ipomoea aquatica. Our previous study showed that, after growth in sewage sludge-amended soil, Ipomoea aquatica accumulated PAEs with BCF values in the range of 0.09–0.34 (Cai et al. 2006).
It should be noted that the BCFs for ΣPAEs were generally lower than those for ΣPAHs in the same sample, despite most samples having higher ΣPAEs than ΣPAHs. This might be attributed to their concentrations in farm soils, their physicochemical properties, and uptake and translocation mechanism by vegetables. In the corresponding farm soils, the ΣPAEs (ranging from 3.0 to 46 mg kg−1) (Cai et al. 2005) were significantly higher than the ΣPAHs (ranging from 160 to 3700 μg kg−1) (Cai et al. 2007a). The accumulation of PAEs in vegetables mainly resulted from soil-to-root transfer and subsequently root-to-shoot translocation, while that of PAHs mainly derived from atmospheric deposition or foliar uptake from the air (Kipopoulou et al. 1999; Tao et al. 2006). In Guangzhou the ΣPAHs in the air (vapor + particulate phases) ranged from 60.9 to 602 ng m−3 (mean, 337 ± 137 ng m−3) and were higher than in many urban areas in the world (Li et al. 2006). Simonich and Hites (1995) suggested that vapor-phase absorption and dry particle deposition are the two most important pathways of contribution to vegetation concentrations of PAHs. On the other hand, PAHs can also be taken up from the soil by the vegetable roots and then transported to the shoots (Zohair et al. 2006; Cai et al. 2008b). However, no obvious relationship (R 2 < 0.2) was observed between BCF and logK ow (octanol-water partition coefficient) values of contaminants. This is likely attributable to the fact that their concentrations varied widely and even were not detectable, but it also implies that contaminant concentrations in vegetables might be influenced by processes (e.g, atmosphere deposition) other than uptake from the soil. The contribution of PAHs in the soil and atmospheric deposition to the vegetable PAHs in the field/farm is unidentified. This emphasizes that further research is necessary.
Toxicity Based on BaP Equivalent Concentration
Since BaP is one of the most toxic compounds and the only PAH for which toxicological data are sufficient for derivation of a carcinogenic potency factor. The USEPA (1993) suggested a first way to determine the toxicity of seven carcinogenic PAHs referring to BaP toxicity, being similar to the toxic equivalents applied for assessment of the toxicity of polychlorinated dibenzo-p-dioxins and dibenzofurans. Other values of individual PAH toxic equivalence factor (TEF) were established, as compiled by Tsai and Shih (2004) (Table 2). In the present study, in order to estimate the carcinogenic potencies associated with the total PAH exposures via vegetables, the BaP equivalent concentration (BaPeq) for each PAH in vegetables is calculated by multiplying its concentration by the corresponding TEF (Table 2). When calculating the BaPeq, “ND” for individual PAHs was substituted by one-half of the detection limit.
The sum of each individual BaPeq (total BaPeq) in vegetables is presented in Table 5. Apparently, the total BaPeq varied greatly among different vegetable species, ranging from 0.72 to 419 μg total BaPeq kg−1. The minimum total BaPeq values obtained for six vegetable species were similar. This is attributed to the comparatively high TEFs (=1.0) for BaP and DbahA, which have a concentration below the detection limits. Except for the maximum total BaPeq for Brassica juncea Linn and Loctuca satira Linn, all total BaPeq values were <10 μg kg−1, and total VaPeq values were <1.0 μg kg−1 in 31.8% of samples (n = 45). The total BaPeq values in vegetables from nine farms of PRD (except for the maximum value for Loctuca satira Linn) were noticeably lower than those for broiled vegetables (40 ± 50 μg kg−1) (Kuo et al. 2006), but significantly higher than those for vegetables from Greece (Kipopoulou et al. 1999) and England (Zohair et al. 2006), when calculating their total BaPeq using the values reported.
Conclusion
The detection frequencies and the total concentrations of PAHs in vegetables were both generally lower than those of PAEs, whereas the BCFs for ΣPAHs were higher than those for ΣPAEs. Large variations in PAH and PAE levels, BCFs, and total BaPeq values were observed among different farms as well as various vegetable species even within cultivars. Because there are seasonal changes of PAHs in the air (vapor and particulate) of Guangzhou, even the PRD, additional data are required to test seasonal variations of PAHs in vegetables as well as to test more classes of vegetables (e.g., root vegetables, tomato) and obtain more information.
References
Agriculture Department of Guangdong Province (2005) General introduction to the agricultural of Guangdong Province. Available at: http://www.gd.agri.gov.cn/zwb/nygk/nyzb/P020050812519247189922.doc
Bishnoi NR, Mehta U, Pandit GG (2006) Quantification of polycyclic aromatic hydrocarbons in fruits and vegetables using high performance liquid chromatography. Indian J Chem Technol 13:30–35
Cai QY, Mo CH, Li YH, Zeng QY, Wang BG, Xiao KE, Li HQ, Xu GS (2005) Preliminary study of PAEs in soils from typical vegetable fields in areas of Guangzhou and Shenzhen, South China. Acta Ecol Sinica 25:283–288 (in Chinese, with English abstract)
Cai QY, Mo CH, Wu QT, Zeng QY (2006) Accumulation of phthalic acid esters in water spinach (Ipomoea aquatica) and in paddy soil. Bull Environ Contam Toxicol 77:411–418
Cai QY, Mo CH, Li YH, Zeng QY, Katsoyiannis A, Wu QT, Férard JF (2007a) Occurrence and assessment of polycyclic aromatic hydrocarbons in soils from vegetable fields of the Pearl River Delta, South China. Chemosphere 68:159–168
Cai QY, Mo CH, Wu QT, Zeng QY, Katsoyiannis A (2007b) Quantitative determination of organic priority pollutants in the composts of sewage sludge with rice straw by gas chromatography coupled with mass spectrometry. J Chromatogr A 1143:207–214
Cai QY, Mo CH, Wu QT, Katsoyiannis A, Zeng QY (2008a) Contamination of semivolatile organic chemicals (SVOCs) in the soil of China: a review. Sci Total Environ 389:209–224
Cai QY, Mo CH, Wu QT, Zeng QY (2008b) Polycyclic aromatic hydrocarbons and phthalic acid esters in the soil-radish (Raphanus sativus) system with sewage sludge and compost application. Bioresour Technol 99:1830–1836
Camargo MCR, Toledo MCF (2003) Polycyclic aromatic hydrocarbons in Brazilian vegetables and fruits. Food Control 14:49–53
Du Q, Shen L, Xiu L, Jerz G, Winterhalter P (2006) Di−2-ethylhexyl phthalate in the fruits of Benincasa hispida. Food Addit Contam 23:552–555
Falcó G, Bocio A, Llobet JM, Domingo JL (2005) Health risks of dietary intake of environmental pollutants by elite sportsmen and sportswomen. Food Chem Toxicol 43:1713–1721
Kazerouni N, Sinha R, Hsu CH, Greenberg A, Rothman N (2001) Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem Toxicol 39:423–436
Kipopoulou AM, Manoli E, Samara C (1999) Bioconcentration of polycyclic aromatic hydrocarbons in vegetables grown in an industrial area. Environ Pollut 106:369–380
Kuo CY, Lee HS, Lai JH (2006) Emission of polycyclic aromatic hydrocarbons and lead during Chinese mid-autumn festival. Sci Total Environ 366:233–241
Li J, Zhang G, Li XD, Qi SH, Liu GQ, Peng XZ (2006) Source seasonality of polycyclic aromatic hydrocarbons (PAHs) in a subtropical city, Guangzhou, South China. Sci Total Environ 355:145–155
Li YT, Li FB, Chen JJ, Yang GY, Wan HF, Zhang TB, Zeng XD, Liu JM (2008) The concentrations, distribution and sources of PAHs in agricultural soils and vegetables from Shunde, Guangdong, China. Environ Monit Assess 139:61–76
Ma LL, Chu SG, Xu XB (2003) Organic contamination in the greenhouse soils from Beijing suburbs, China. J Environ Monit 5:786–790
Maskaoui K, Hu Z, Zhou JL, Han YL (2006) Levels of polycyclic aromatic hydrocarbons in some agricultural, industrial and urban areas along Xiamen coastal waters, China. J Environ Sci 18:318–322
Nadal M, Schuhmacher M, Domingo JL (2004) Levels of PAHs in soil and vegetation samples from Tarragona County, Spain. Environ Pollut 132:1–11
Phillips DH (1999) Polycyclic aromatic hydrocarbons in the diet. Mutat Res Gen Tox En 443:139–147
Shen F, Zhu LZ (2007) Concentration and distribution of PAHs in vegetables grown near an iron and steel industrial area. Environ Sci 28:699–702 (in Chinese with English abstract)
Simonich S, Hites R (1995) Organic pollutant accumulation in vegetation. Environ Sci Technol 29:2905–2914
Tao S, Cui YH, Xu FL, Li BG, Cao J, Liu WX, Schmitt G, Wang XJ, Shen WR, Qing BP Sun R (2004) Polycyclic aromatic hydrocarbons (PAHs) in agricultural soil and vegetables from Tianjin. Sci Total Environ 320:11–24
Tao S, Jiao XC, Chen SH, Xu FL, Li YJ, Liu FZ (2006) Uptake of vapor and particulate polycyclic aromatic hydrocarbons by cabbage. Environ Pollut 140:13–15
Thomas JA, Wienckowski DB, Gillies BA, Thomas MJ, Youkilis EJ (1986) Effects of phthalic acid esters (PAEs) on the neonate and aspects of teratogenic actions. Environ Health Perspect 65:243–248
Tsai PJ, Shih TS (2004) Assessing and predicting the exposure of PAHs and their carcinogenic potencies from vehicle engine exhausts to highway toll station workers. Atmos Environ 38:333–343
USEPA (1993) Provisional guidance for quantitative risk assessment of PAH. U.S. Environmental Protection Agency EPA/600/R-93/089
Voutsa D, Samara C (1998) Dietary intake of trace elements and polycyclic aromatic hydrocarbons via vegetables grown in an industrial Greek area. Sci Total Environ 218:203–216
Wennrich L, Popp P, Zeibig M (2002) Polycyclic aromatic hydrocarbon burden in fruit and vegetable species cultivated in allotments in an industrial area. Int J Environ Anal Chem 82:677–690
Yin MC, Su KH (1996) Investigation on risk of phthalate ester in drinking water and marketed foods. J Food Drug Anal 4:313–318
Zhang ZL, Hong HS, Zhou JL, Yu G (2004) Phase association of polycyclic aromatic hydrocarbons in the Minjiang River Estuary, China. Sci Total Environ 23:71–86
Zhong W, Wang M (2002) Some polycyclic aromatic hydrocarbons in vegetables from northern China. J Environ Sci Health A 37:287–296
Zhong-Guang Web (2008) Guideline establishment of meals for new residents—tower of balanced meals for Chinese residents. Available at: http://www.cnr.cn/nx/shtc/msjy/200803/t20080305_504725064.html. Accessed: 5 March 2008
Zohair A, Salim AB, Soyibo AA, Beck AJ (2006) Residues of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and organo-chlorine pesticides in organically-farmed vegetables. Chemosphere 63:541–553
Acknowledgments
This work was supported by the Natural Science Foundation of China (Nos. 40773062, 30671208, 30600372, and 30471007), Key Scientific Research Project of the Ministry of Education of China (No. 02112), the Natural Science Foundation of Guangdong Province (Nos. 021011, 036716, 043005970, and 07117909), and projects of the Department of Science & Technology of Guangdong (Nos. 01C21202, 03A20504, and 03C34505, 06B20601003), and the Research Foundation of the State Key Laboratory of Organic Geochemistry, Chinese Academy of Sciences. Thanks are also due to the personnel for supplying vegetable samples.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mo, CH., Cai, QY., Tang, SR. et al. Polycyclic Aromatic Hydrocarbons and Phthalic Acid Esters in Vegetables from Nine Farms of the Pearl River Delta, South China. Arch Environ Contam Toxicol 56, 181–189 (2009). https://doi.org/10.1007/s00244-008-9177-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-008-9177-7