Abstract
The purpose of this study was to characterize the individual effect of different cations (Ca2+, Mg2+, Na+, K+, and H+) on the acute toxicity of Cd to the earthworm Eisenia fetida. Higher activities of the considered cations increased the 48-h LC50 (expressed as Cd2+ activities) linearly to different extents in simulated soil solution. The conditional constants for the binding of H+ (log K = 5.41), Cd2+ (log K = 4.0), Ca2+ (log K = 3.35), Mg2+ (log K = 2.82), Na+ (log K = 1.57), and K+ (log K = 2.31) to the biotic ligand (BL) of E. fetida were derived from the toxicity data based on the biotic ligand model (BLM). Furthermore, it was calculated that on average 72% of the BL sites needed to be occupied by Cd2+ to induce 50% lethal effect. Autovalidation of the model with the results of the test sets showed that 48-h LC50 could be predicted within a factor of two.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
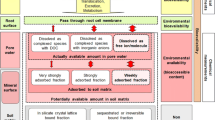
The widespread use of Cd, together with its toxicity potential, underlines the need for a correct risk assessment and appropriate environmental quality criteria for this heavy metal. There is extensive evidence that neither total nor dissolved metal concentrations are good predictors of metal bioavailability and toxicity (Lock and Janssen 2001; Peijnenburg and Jager 2003). It is now well accepted that chemical speciation of trace metals greatly influences their biological effect. Many researches (Sauvé et al. 1998; Slaveykova and Wilkinson 2002) have explained part of the observed variability in toxicity by attributing the toxic effect of metal to the differences of the free metal ion activity in the aqueous phase, and thus support the free-ion activity model (FIAM) (Morel 1983). However, metal toxicity does not seem to be determined by the free-ion activity alone. The presence of cations such as H+, Ca2+, and Na+, which are hypothesized to compete with metal ion for binding sites at the organism–water interface, can also decrease metal toxicity (Playle et al. 1993a; 1993b). This has led to the development of gill site interaction model (GSIM) proposed by both Pagenkopf (1983) and Morel (1983), which has since been extended and is increasingly recognized as the biotic ligand model (BLM) (Paquin et al. 2000).
The BLM was originally developed to predict Cu toxicity to fish (Paquin et al. 2002). To date the aquatic biotic ligand models (a-BLM) have been developed for several vertebrate species to predict acute as well as chronic toxicity of metal (De Schamphelaere et al. 2003). In analogy with the a-BLM, metal toxicity to terrestrial organisms and plants can also be predicted using the concept of BLM. A fundamental assumption of the BLM is that toxicity is driven by exposure to dissolved metal alone, rather than combined toxic effects from dissolved and dietary exposures (Niyogi and Wood 2004). Thus, development of a terrestrial biotic ligand model (t-BLM) that predicts site-specific metal toxicity in soil is only possible if the uptake and toxicity is caused by exposure to metals in the aqueous phase. To date, the BLM concept has been successfully applied to predict metal toxicity to soil organisms in a few studies. Steenbergen et al. (2005) developed a t-BLM using a quartz sand–nutrient solution system to predict acute toxicity of Cu to the earthworm Aporectodea caliginosa. Lock et al. (2006) developed a t-BLM to predict acute toxicity of Co to the potworm Enchytraeus albidus. Recently, Lock et al. (2007a, b) developed a Ni t-BLM and Co t-BLM for barley Hordeum vulgare in nutrient solutions.
The objective of this study is to fully assess the effects of the simulated soil solution chemistry to the acute toxicity of Cd for E. fetida and to estimate conditional constants for cations binding to biotic ligand (BL) based on toxicity data. Earthworms may be exposed to soil Cd at least in two ways: absorption through the skin and oral ingestion of contaminated soil particles. Nevertheless, many studies provided evidence that chemicals were bioavailable to earthworms in a dissolved state, and clearly demonstrated a predominance of dermal over oral uptake (Belfroid et al. 1993; Spurgeon and Hopkin 1996; Peijnenburg et al. 1999; Saxe et al. 2001). To allow for some control in the experimental set-up, research with earthworms has sometimes been performed in aqueous environments (Kiewiet and Ma 1991, Janssen et al. 1996). In our study, tests were conducted using a semistatic (24-h static solution renewal) method to determine the concentrations causing lethality to 50% of the organisms (LC50) in simulated soil solution.
Materials and Methods
Test Organisms
The organism used for the toxicity tests was the earthworm E. fetida. The earthworms were purchased from the Dachang Earthworm Cultivation Farm in Nanjing and maintained in a climate-controlled room. The culture was kept in darkness at a constant temperature of 20 ± 2°C, with moisture content of 63–68%, and pH of 6–7. Cattle manure was provided as food every week. This species was chosen as it can be used as a bio-indicator for soil quality and has been recommended as a terrestrial invertebrate test species by the Organization for Economic Cooperation and Development in Europe (OECD 1984) as well as the Environmental Protection Agency in the USA (EPA 1996). Moreover, E. fetida can survive in an aquatic environment, which is interesting in view of the development of the BLM in simulated soil solution.
Experimental Design
Tests with various concentrations of Ca2+, Mg2+, Na+, K+, and H+ were performed. To distinguish the effects of the different parameters, each one was changed univariately, keeping all other concentrations (except cadmium) as constant as possible. Five sets of Cd bioassays were performed for: Ca, Mg, Na, K, and pH (Table 1). The pH was 6.0 ± 0.1 in all the sets (except for the pH set). For each test solution, six treatments including one control and five previous decided values of Cd concentrations (2.0 × 10−4–4.0 × 10−3 mol/L) were tested.
Preparation of Simulated Soil Solution
For the assessment of the acute effects of Cd with the exclusion of the effects of soil, simulated soil solution based on the assumption of Kiewiet and Ma (1991) was designed. All chemicals were analytical reagent (AR) or higher grade. The containers used were cleaned with HNO3 and thoroughly rinsed with deionized water. The simulated soil solution was prepared by adding different volumes of stock solutions of Ca(NO3)2, MgSO4, NaNO3, and KNO3 to deionized water. In the Ca, Mg, Na, and K sets the test media was adjusted to pH 6.0 controlled using 0.75 g/L 3-[N-morpholino] propane sulfonic acid (MOPS) buffering and the addition of diluted NaOH. In the pH set, the pH was controlled by different buffers. At pH 6–7, MOPS was used as a buffer, while at pH 5–6, pH was buffered by 2 mmol/L 2-[N-morpholino] ethane sulfonic acid (MES) adjusted with NaOH. After equilibration at atmospheric conditions, the prepared test medium was subsequently used as the dilution water to make a concentration series of Cd2+ (added as CdCl2) for each toxicity assay.
Acute Toxicity Tests
Prior to the acute toxicity tests, adult earthworms weighing approximately 200–400 mg were removed from the cultures, rinsed with deionized water, and placed on moist filter paper for 24 h to depurate most of the gut content from their intestinal tracts. To reach near-equilibrium situations, all media were stored at 20 ± 1°C for at least one day before being used in the toxicity tests. Each treatment was performed by exposing eight randomly chosen earthworms singly into a plastic cup containing 100 mL test media. If the earthworms are not exposed singly, they are often affected by dead specimens, which may lead to false results in the evaluation of lethal concentrations of Cd. A lid with small holes was employed to prevent moisture loss and to prevent the worms from escaping from the containers. Tests were carried out in an environmental chamber (20 ± 1°C) in darkness, and after 48 h of exposure the number of surviving earthworms was counted. The simulated soil solution was renewed daily. All toxicity assays of the same set were conducted simultaneously to minimize variability. Previous studies revealed that E. fetida survived well for 48 h without feeding (100% survival), thus the potential of dissolved organics from added food to complex Cd was excluded.
The dissolved Cd concentration was determined at the end of the media renewal periods using a flame atomic absorption spectrophotometer (Hitachi 180–80 atomic absorption spectrophotometer). In all cases, the measured solution Cd concentrations were above 95% of the nominal values. Since a previous study had shown that the concentrations of other cations (Ca, Mg, Na, and K) in the simulated soil solution does not significantly vary from their initial values, these ions were not analyzed at the end of the test. The pH values were measured daily with an Orion 868 pH meter.
Data Statistics
The activities of cations were used for the development of the model. All activity calculations were conducted using the chemical equilibrium model Visual MINTEQ (Windows version of MINTEQA2) (Allison et al. 1991), based on the chemical composition of the test solution and the nominal Cd concentrations. Inorganic carbon was assumed to be in equilibrium with atmospheric CO2.
LC50{Cd2+} values (expressed as Cd2+ activity) were then calculated based on the activities and the associated mortality after 48 h exposure, using the trimmed Spearman–Karber method (Hamilton et al. 1977). This method gives the 95% confidence intervals. The logit transformation of the mortality data was used in the regression analysis according to the formula, logit P = log P/(1–P), where P was the proportion of dead earthworms. LC50{Cd2+} was plotted against the activities of Ca2+, Mg2+, Na+, K+, and H+, and linear regressions were calculated using Origin 6.0.
Mathematical Description of the BLM and Derivation of Parameters
Various methodologies have been proposed to derive the conditional binding constants of metals and competing cations to the biotic ligand (BL) (Bury et al. 1999; Galvez and Wood 1997; Playle et al. 1992; Janes and Playle 1995). In this study, the cation ligand equilibrium constants will be derived from univariate toxicity test results, using the method developed by De Schamphelaere and Janssen (2002). The obtained constants with this method were consistent with previously reported values that were derived with other approaches (De Schamphelaere and Janssen 2004; Heijerick et al. 2002). A short mathematical description of the BLM is given here to clarify the calculation of constants.
The fraction of Cd binding sites on the biotic ligand ([CdBL +]/CC BL ), abbreviated to f CdBL , which was assumed to dominate the magnitude of toxic effect (Paquin et al. 2002), was expressed as:
where CC BL is the complexation capacity of the biotic ligand (mol/L); [CdBL +], [CaBL +], [MgBL +], [KBL], [NaBL], and [HBL] are the concentrations of the cation-biotic ligand complexes (mol/L), and [BL −] is the concentration of unoccupied biotic ligand sites (mol/L).
The fraction of Cd binding sites at 50% lethal effect was termed \( {f_{CdBL}^{50\% }} \). Thus Eq. (1) can be rewritten as,
where LC50{Cd2+} is the free Cd activity (mol/L) resulting in 50% mortality of the E. fetida after 48 h exposure.
Equation (2) shows that a linear relationship can be observed between LC50{Cd2+} and the activity of one cation, when the activities of other cations are kept constant. So we can obtain the corresponding Intercept H and Slope H for the pH tests when other cations were constant. Certainly, similar expressions can also be derived for the Ca, Mg, Na, and K sets:
Thus, the relationships between 48-h LC50{Cd2+} and competing cations (i.e., Ca2+) which have been found from observed mortality data, can then be used to derive their stability constants. The value of f CdBL was calculated with Eq. (1) for varying K CdBL . According to the BLM hypothesis, the best approximation of K CdBL would result in the best correlation between the calculated f CdBL and the logit of the percentage mortality (except for mortality = 0% or 100%).
Results
Effects of Cations and pH on Cd Toxicity
As predicted by Eq. (2), the 48-h LC50{Cd2+} significantly increased from 0.35 mmol/L to 0.59 mmol/L with increasing Ca2+ activity in a linear fashion (1.8-fold increase, R 2 = 0.99) (Fig. 1a). Similar to Ca, increasing Mg concentrations also had an ameliorating effect on Cd toxicity to E. fetida but to a lesser extent than Ca (1.5-fold increase, R 2 = 0.98) (Fig. 1b). With the lowest Na concentration (1.0 mmol/L), a mortality of 37.5% was observed at a total Cd concentration of 7.0 × 10−4 mol/L. In comparison, all the earthworms survived at the same Cd concentration with the greatest solution Na concentration (7.0 mol/L). Like Ca and Mg, increases in Na+ activity affected the 48-h LC50{Cd2+} in a linear fashion within the tested Na concentration range (R = 0.98) (Fig. 1c). In the K set, increasing K+ activity (0.1–2.0 mmol/L) linearly increased the 48-h LC50 value expressed as free Cd ion activity from 0.37 mmol/L to 0.45 mmol/L (R = 0.96) (Fig. 1d). In the pH test, a similar protective proton effect was also evident. The observed 48-h LC50{Cd2+} values ranged from 0.34 to 0.51 mmol/L as the pH decreased from 7.1 to 5.5. The analysis of the best fit linear regression gave Eqs. (5–9).
Derivation of the Conditional Constants
Linear regression analysis between the 48-h LC50{Cd2+} (mol/L) and the activities of the cations (mol/L) gave the following expressions.
Thus, the slopes and intercepts of these relationships (Eqs. 5–9) can then be used to derive the stability constants as described by De Schamphelaere and Janssen (2002). This calculation resulted in log K H = 5.41, log K Ca = 3.35, log K Mg = 2.82, log K Na = 1.57 and log K K = 2.31.
For the final development of BLM, the only constant that needs to be found is \( \frac{{f^{{50\% }}_{{CdBL}} }} {{(1 - f^{{50\% }}_{{CdBL}} ) \cdot K_{{CdBL}} }} \). Values for log K CdBL = 4.0 and the associated \( {f_{CdBL} ^{50\% }} \) = 72% resulted in the best fit (R 2 = 0.45) (Fig. 3). Incorporation of the BLM constants derived the following expression for the 48-h LC50{Cd2+}:
Model Validation
With the developed Cd-BLM, the 48-h LC50{Cd2+} was predicted for all the test sets used to develop the model (i.e., autovalidation) and compared to the observed LC50{Cd2+}. The accuracy of the model toxicity predictions is shown in Fig. 2. Fig. 3 The solid line indicates a perfect match between the observed and the predicted 48-h LC50{Cd2+}, and the dashed lines indicate a factor of two difference between the observed and the predicted values. The 48-h LC50{Cd2+} values predicted with the BLM match rather well the observed values, with an error of less than a factor two, indicating that the BLM might be used to predict Cd toxicity to terrestrial organisms. Further validation with soils with a range of soil solution characteristics is needed to test this model’s applicability in the field soils.
Relationship between the calculated fraction of the biotic ligand sites occupied by Cd (f CdBL ) and the logit of the observed percentage mortality of E. fetida after 48 h exposure. The value of f CdBL was calculated for very treatment with Eq. (1) using the stability constants obtained in this study
Discussion
In our bioassay sets, the observed 48-h LC50s for dissolved Cd varied from 0.48 to 1.86 mmol/L. This support the result of Fourie et al. (2007) that 50% of E. fetida earthworms died at 0.45 mmol/L Cd after 48-h exposure to an artificial soil solution. The observed 48-h LC50{Cd2+} ranged from 0.34 to 0.75 mmol/L, which is a 2.2-fold difference. These observations clearly showed the limitations of using free-ion activity to predict Cd toxicity. If complexation was the primary mechanism for the reduction of Cd toxicity, the LC50 values calculated on a free Cd2+ basis should be relatively constant in the different solution conditions, whereas if competition for uptake by cations weer the primary mechanism for reduction of toxicity, then LC50 values calculated on a free Cd2+ basis should increase as the solution cation concentration increase.
Due to their similar ionic radius, it has been suggested that Ca2+ and Cd2+ share a common uptake pathway (Verbost et al. 1987; 1989). Ca2+ will thus compete with Cd2+ for binding sites (BL) on the Cd transporters and less Cd will be taken up. In tests with the earthworm Lumbricus rubellus (Kiewiet and Ma 1991), the presence of Ca2+ decreased the uptake of Cd2+, which supports the hypothesis that Cd2+ and Ca2+ compete for the same binding sites (BL). In the present study, increasing Mg concentrations also had an ameliorating effect on Cd toxicity to E. fetida but to a lesser extent than Ca (Fig. 1). This is in contrast with the findings where Mg2+ appeared to have a greater protective effect than Ca2+ in the development of a t-BLM to predict acute toxicity of Co to the potworm Enchytraeus albidus (Lock et al. 2006). To date, this has been explained by the physiological mechanism of Ca (but not Mg) in modifying membrane permeability to metal ions and by the evidence that the binding affinity of Ca to metal receptors at the cell membrane surface is superior to that of Mg. The protected effect of Na and K to Cd toxicity for E. fetida was also found, although many other authors did not find their influence on Cu or Co toxicity to earthworms (Lock et al. 2006, Steenbergen et al. 2005). In the validation of the developed Cd-BLM, the 48-h LC50{Cd2+} values (except the Ca set) were slightly underestimated (Fig. 2). The observed differences may result from the extra addition of Na (added as NaOH) to maintain constant solution pH.
As we know, there is no formal published terrestrial Cd-BLM until now, although a Cd gill-surface binding model was developed by Playle et al. (1993a; 1993b) for fathead minnows in soft water. It appears that the conditional binding constants of competing cations for E. fetida in our study were slightly lower than that for the published Co-BLM for potworm Enchytraeus albidus (Lock et al. 2006) and a Cu-BLM for earthworm Aporectodea caliginosa (Steenbergen et al. 2005) (Table 2). A possible explanation for this apparent discrepancy may be due to the difference in experimental conditions between these studies, including the water hardness and ionic concentrations of the simulated solution. The high ionic concentration probably produced more competitive effect on Cd2+ for the binding at the biotic ligand (BL), and therefore lowered the affinity and thus the log K value. In addition, the nature and dynamic properties of the biotic ligand (BL) needs to be considered, as the BL (biological membrane) is a part of a living organism that is very likely to change in response to environmental perturbations such as ionic strength and pH.
Central to the BLM is the notion that the chemistry of the system is at equilibrium, thus allowing the use of thermodynamic and conditional binding constants to calculate the concentrations of metal species in the system. Recent studies have, however, indicated that this may not always be the case (Tran et al. 2001; 2002). A further improvement of BLM predictions may be achieved by taking this phenomenon (i.e., nonequilibrium conditions) into consideration. Many studies have indicated that the dermal route was the most important route for metal accumulation (Belfroid et al. 1994, Vijver et al. 2003). However, not in all cases is pore water the sole contributor of pollutant accumulation (Marinussen et al. 1997). Earthworms in aqueous systems are not representative of that in a soil matrix. Thus the relative contribution of the oral and dermal uptake route need to be examined. It should also be noted that metals do not occur in isolation in the environment but are present as a mixture. Mixture toxicity is not yet part of BLM research but should be developed in the future. Thus the BLM approach needs to be refined, not only to deal with acute toxicity but also with chronic toxicity and ultimately with metal mixtures.
Conclusions
From this study we can conclude that, with the present methodology, acute Cd-BLM constants can be estimated from toxicity data alone, provided that valid metal ion activities are used as a starting point for the calculations. The characterization of the effects of simulated soil solution chemistry on acute Cd toxicity to E. fetida is the first important step towards the effort to develop a terrestrial Cd-BLM for earthworms, although only limited data were available for testing the ability of the acute Cd-BLM to predict Cd toxicity to E. fetida. However, there are many other organisms such as animals, plants and microorganisms that may react differently from earthworms. Further terrestrial Cd-BLM development for other organisms may have great prospects as a new tool for the assessment of soil Cd risk.
References
Allison JD, Brown DS, Novo-Gradac KJ (1991) MINTEQA/PRODEFA2, a geochemical assessment model for environmental systems: Version 3.0. EPA/600/3091/021, U.S. Environmental Protection Agency, Athens, GA
Belfroid AC, Van Wezel A, Sikkenk M, Van Gestel K, Seinen W, Hermens J (1993) The toxicokinetic behavior of chlorobenzenes in earthworms (Eisenia andrei): experiments in water. Ecotoxicol Environ Saf 25:154–165
Belfroid AC, Meiling J, Sijm D, Hermens J, Seinen W, Van Gestel K (1994) Uptake of hydrophobic halogenated aromatic hydrocarbons from food by earthworms (Eisenia andrei). Arch Environ Contam Toxicol 27:260–265
Bury NR, McGeer JC, Wood CM (1999) The effects of altering freshwater chemistry on the physiological responses of rainbow trout to silver exposure. Environ Toxicol Chem 18:49–55
Davies PH, Gorman WC, Carlson CA, Brinkman SF (1993) Effect of hardness on bioavailability and toxicity of cadmium to rainbow trout. Chem Speciation Bioavailability 5:67–77
De Schamphelaere KAC, Janssen CR (2002) A biotic ligand model predicting acute copper toxicity to Daphnia magna: the effects of calcium, magnesium, sodium, potassium and pH. Environ Sci Technol 36:48–84
De Schamphelaere KAC, Janssen CR (2004) Development and field validation of a biotic ligand model predicting chronic copper toxicity to Daphnia magna. Environ Toxicol Chem 3:1365–1375
De Schamphelaere KAC, Vasconcelos FM, Heijerick DG, Tack FMG, Delbeke K, Allen HE, Janssen CR (2003) Development and field validation of a predictive copper toxicity model for the green alga Pseudokirchneriella subcapita. Environ Toxicol Chem 22:2454–2465
EPA United States Environmental Protection Agency. 1996. Ecological Effects Test Guidelines. Earthworm subchronic toxicity test
Fourie F, Reinecke SA, Reinecke AJ (2007) The determination of earthworm species sensitivity differences to cadmium genotoxicity using the comet assay. Ecotoxicol Environ Saf 67:361–368
Galvez F, Wood CM (1997) The relative importance of water hardness and chloride levels in modifying the acute toxicity of silver to rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 16:2363–2368
Hamilton MA, Russo RC, Thurston RV (1977) Trimmed Spearman–Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol 11:714–719
Heijerick DG, De Schamphelaere KAC, Janssen CR (2002) Predicting acute zinc toxicity for Daphnia magna as a function of key water chemistry characteristics: development and validation of a biotic ligand model. Environ Toxicol Chem 21:1309–1315
Janes N, Playle RC (1995) Modeling silver binding to gills of rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 14:1847–1858
Janssen MPM, Glastra P, Lembrechts JFMM (1996) Uptake of cesium-134 by the earthworm species Eisenia foetida and Lumbricus rubellus. Environ Toxicol Chem 15:873–877
Kiewiet AT, Ma WC (1991) Effect of pH and calcium on lead and cadmium uptake by earthworms in water. Ecotoxicol Environ Saf 21:32–37
Lock K, De Schamphelaere KA, Becaus S, Criel P, Van Eeckhout H, Janssen CR (2006) Development and validation of an acute biotic ligand model (BLM) predicting cobalt toxicity in soil to the potworm Enchytraeus albidus. Soil Biol Biochem 38:1924–1932
Lock K, De Schamphelaere KA, Becaus S, Criel P, Van Eeckhout H, Janssen CR (2007b) Development and validation of a terrestrial biotic ligand model predicting the effect of cobalt on root growth of barley (Hordeum vulgare). Environ Poll 147:626–633
Lock K, Janssen CR (2001) Cadmium toxicity for terrestrial invertebrates: Taking soil parameters affecting bioavailability into account. Ecotoxicology 10:315–322
Lock K, Van Eeckhout H, De Schamphelaere KA, Criel P, Janssen CR (2007a) Development of a biotic ligand model (BLM) predicting nickel toxicity to barley Hordeum vulgare. Chemosphere 66:1346–1352
Marinussen MPJC, Van der Zee SEATM, De Haan FAM, Bouwman LM, Hefting MM (1997) Heavy metal (copper, lead, and zinc) accumulation and excretion by the earthworm Dendrobaena veneta. J Environ Qual 26:278–284
Morel FMM (1983) Principles of Aquatic Chemistry. Wiley, New York
Niyogi S, Wood CM (2004) Biotic ligand model, a flexible tool for developing site-specific water quality guidelines for metals. Environ Sci Technol 38:6177–6192
OECD 1984. Guideline for Testing Chemicals vol. 207, Paris, Earthworm, acute toxicity tests [S]
Pagenkopf GK (1983) Gill surface interaction model for trace metal toxicity to fishes: Role of complexation, pH, and water hardness. Environ Sci Technol 17:42–347
Paquin PR, Santore RC, Wu KB, Kavvadas CD, DiToro DM (2000) The biotic ligand model: A model of the acute toxicity of metals to aquatic life. Environ Sci Pol 3S:175–182
Paquin PR, Gorsuch JW, Apte S, Batley GE, Bowles KC, Campbell PG, Delos CG, Di Toro DM, Dwyer RL, Galvez F, Gensemer RW, Goss GG, Hostrand C, Janssen CR, McGeer JC, Naddy RB, Playle RC, Stantore RC, Schneider U, Stubblefield WA, Wood CM, Wu KB (2002) The biotic ligand model: a historical overview. Comp Biochem Physiol Part C 133:3–35
Pascoe D, Evans SA, Woodworth J (1986) Heavy metal toxicity to fish and the influence of water hardness. Arch Environ Contam Toxicol 15:481–487
Peijnenburg WJGM, Baerselman R, De Groot AC, Jager T, Posthuma L, Van Veen RPM (1999) Relating environmental availability to bioavailability: soil-type-dependent metal accumulation in the oligochaete Eisenia andrei. Ecotoxicol Environ Saf 44:294–310
Peijnenburg WJGM, Jager T (2003) Monitoring approaches to assess bioaccessibility and bioavailability of metals: matrix issues. Ecotoxicol Environ Saf 56:63–77
Playle RC, Gensemer RW, Dixon DG (1992) Copper accumulation on gills of fathead minnows: influence of water hardness, complexation and pH on the gill micro-environment. Environ Toxicol Chem 11:381–391
Playle RC, Dixon DG, Burnison K (1993a) Copper and cadmium binding to fish gills: Modification by dissolved organic carbon and synthetic ligands. Can J Fish Aquat Sci 50:2667–2677
Playle RC, Dixon DG, Burnison K (1993b) Copper and cadmium binding to fish gills: Estimates of metal-gill stability constants and modelling of metal accumulation. Can J Fish Aquat Sci 50:2678–2687
Sauvé S, Dumestre A, McBride MB, Hendershot WH (1998) Derivation of soil quality criteria using predicted chemical speciation of Pb2+ and Cu2+. Environ Toxicol Chem 17:1481–1489
Saxe JK, Impellitteri CA, Peijnenburg WJGM, Allen HE (2001) A novel model describing heavy metal concentrations in the earthworm Eisenia andrei. Environ Sci Technol 35:4522–4529
Slaveykova VI, Wilkinson KJ (2002) Physicochemical Aspects of Lead Bioaccumulation by Chlorella vulgaris. Environ Sci Technol 36:969–975
Spurgeon DJ, Hopkin SP (1996) Effects of variations of the organic matter content and pH of soils on the availability and toxicity of zinc to the earthworm Eisenia fetida. Pedobiologia 40:80–96
Steenbergen NT, Iaccino F, De Winkel M, Reijnders L, Peijnenburg WJ (2005) Development of a biotic ligand model and a regression model predicting acute copper toxicity to the earthworm Aporrectodea caliginosa. Environ Sci Technol 39:5694–5702
Tran D, Boudou A, Massabuau JC (2001) How water oxygenation level influences cadmium accumulation pattern in the Asiatic clam Corbicula fluminea: a laboratory and field study. Environ Toxicol Chem 20:2073–2080
Tran D, Boudou A, Massabuau JC (2002) Relationship between feeding-induced ventilatory activity and bioaccumulation of dissolved and algal-bound cadmium in the Asiatic clam Corbicula fluminea. Environ Toxicol Chem 21:327–333
Verbost PM, Flik G, Lock RAC, Wendelaar Bonga SE (1987) Cadmium inhibition of Ca2+ uptake in rainbow trout gills. Am J Physiol 253:216–221
Verbost PM, Rooij JV, Flik G, Lock RAC, Wendelaar Bonga SE (1989) The movement of cadmium through freshwater trout branchial epithelium and its interference with calcium transport. J Exp Biol 145:185–197
Vijver MG, Vink JPM, Miermans CJH, van Gestel CAM (2003) Oral sealing using glue: a new method to distinguish between intestinal and dermal uptake of metals in earthworms. Soil Biol Biochem 35:125–132
Acknowledgments
This work was financially supported by the CAS Research Program on Soil Biosystems and Agro-product Safety (No. CXTD-Z2005-4-1) and the National Natural Science Foundation of China (No. 40671095).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, LZ., Zhou, DM., Luo, XS. et al. Effect of Major Cations and pH on the Acute Toxicity of Cadmium to the Earthworm Eisenia fetida: Implications for the Biotic Ligand Model Approach. Arch Environ Contam Toxicol 55, 70–77 (2008). https://doi.org/10.1007/s00244-007-9100-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-007-9100-7