Abstract
4-chlorophenol (4-CP) is a well-known hazardous chlorinated compound and a precursor for the synthesis of the herbicide 2,4-dichlorophenoxyacetate. The relation between uptake, accumulation, toxicity, and removal of 4-CP in willow trees (Salix viminalis) was determined. In addition, the feasibility of implementing phytoremediation as a treatment method for 4-CP contamination was investigated. Willows were exposed to 4-CP levels ≤79.9 mg/L in hydroponic solution. The transpiration of the trees was used to determine toxic effects. Almost no inhibition of transpiration was detected at concentrations ≥15 mg/L. For concentrations ≥37.3 mg/L, transpiration decreased to ≤50%, and the trees wilted. Trees exposed to 79.9 mg/L wilted and eventually died. For concentrations of 79.9 mg/L, a significantly higher amount of 4-CP remained at the end of experiments in the test system compared with the amount remaining at all other concentrations. The loss of chemical from the system in experiments with trees was high, ≤99.5%. In treeless experiments, the mass loss of 4-CP was only 6% to 10%. The results indicated that degradation in the root zone is the main reason for the removal of 4-CP from the media. Phytoremediation of 4-CP in willow trees seems to be a remediation option, especially at concentrations <37.3 mg/L, at which point degradation of 4-CP is rapid and efficient, and the toxic effects on trees are not lethal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Chlorinated phenols are common environmental contaminants originating from wood-pulp bleaching, water chlorination, textile dyes, oil refineries, coking plants, and polymeric resins as well as chemical, agrochemical, and pharmaceutical industries. They are also used widely as biocides, fungicides, wood preservatives, and organic precursors of pesticides, such as 2,4-dichlorophenoxyacetate (2,4-D), 2,4,5-trichlorophenoxyacetate, and 2,4,6-trichlorophenol derivative (prochloraz) (Baggi et al. 2004; Clarkson et al. 1993). Chlorophenols are a threat to terrestrial and aquatic ecosystems because of their high toxicity, strong odor emission, high persistence in the environment, and suspected carcinogenesis and mutagenesis (Valli & Gold 1991; Mehmood et al. 1997; Quan et al. 2004; Tsuji et al. 2003; Oliver et al. 2003; Scraagg et al. 2003; Fahr et al. 1999; Kishino & Kobayashi 1994; Da Silva et al. 2003). Therefore, chlorophenols constitute a particular group of priority toxic pollutants listed by the United States Environmental Protection Agency in the Clean Water Act and by the European Commission 2455/2001/EC. One of the most important members of this chemical family is 4-chlorophenol (4-CP), a well known hazardous chlorinated compound and a precursor for the synthesis of the herbicide 2,4-D. 4-CP is released to the environment during the anaerobic degradation, followed by dechlorination, of polychlorinated phenols (Cole et al. 1994; Mikesell & Boyd 1986). Because 4-CP cannot be easily degraded under anaerobic conditions (Madsen & Aamand 1992; Woods et al. 1989; Häggblom & Valo 1995), it accumulates in the environment. Nevertheless, the main release of 4-CP is found at application sites of phenoxy acids and in wastewaters of the above-mentioned industries.
Different physicochemical and biologic methods have been proposed to treat chlorophenols, including 4-CP―such as activated carbon adsorption, chemical oxidation, and aerobic and anaerobic biologic degradation―in soil and water (Goswami et al. 2002; Kim et al. 2002; Wu & Kosaric 1991; Minero et al. 1995; Leonard & Lindley 1999; Jung et al. 2001; Hao et al. 2002; Danis et al. 1998; Loh & Wang 1998; Carvalho et al. 2001; Jianlong & Yi 1999; Bae et al. 1996). Available physicochemical techniques are expensive; do not yield full purification; and require a posttreatment process to remove the pollutant and undesirable byproducts from the contaminated environment (Goswami et al. 202; Pera-Titus et al. 2004). However, biodegradation by conventional activated sludge systems is usually slow because of the inhibitory effect of chlorophenols on microbial metabolism (Pera-Titus et al. 2004; Toscano et al. 2003) and often fails to achieve high efficiency in removing chlorophenols from wastewater (Quan et al. 2004). Biodegradation by way of the direct application of adapted microorganisms capable of degrading chlorophenols can be another method for the practical remediation of pollutants. However, because bacteria are heterotrophs and need organic nutrients for growth and for degrading pollutants, the addition of nutrients to the polluted area is inevitable. This makes it difficult to apply a bacterial method for the practical remediation of pollutants at low concentrations (Tsuji et al. 2003).
High cost, incomplete purification, hazardous byproduct formation, and limited concentration-range applicability to traditional physicochemical and biologic treatments of contaminants have spurred the development of new remediation technologies. Advanced oxidation processes have been reported as one of the effective new remediation technologies for the degradation of chlorophenols from waters and soils, but they also involve great cost and require large amounts of reactants (Pera-Titus et al. 2004). Enzymes, such as peroxidases, have recently been used in many remediation processes to target specific pollutants for treatment. This treatment has many advantages with respect to other biologic or physicochemical methods: Handling and storage of isolated enzymes are easier than microorganism manipulation, and enzyme concentration is not simply related to bacterial growth. Moreover, conventional methods are not very selective, whereas the specificity of isolated enzymes is greater compared with other catalysts (Laurenti et al. 2003). In addition, insoluble polymers, which are formed during enzymatic removal, precipitate and can be separated by simple filtration or flocculation (Tong et al. 1997). However, enzymatic treatment has not been applied on a large scale, mainly because of (1) the high cost of enzymatic treatment and (2) the losses in enzymatic activity caused by adsorption of enzyme molecules on end-product polymers (Dec & Bollag 1994; Song et al. 2003). Finally, plant materials were found to be useful for the decontamination of phenolic compounds in water, during which the detoxification effect was caused by peroxidases contained in the plant tissue (Dec & Bollag 1994; Durán & Esposito 2000). Roper et al. (1996) illustrated that the process of using horseradish roots as plant material to treat waters contaminated with phenols and anilines had broad substrate specificity and, therefore, a wide array of potential waste-treatment applications. Although plant materials, especially from agricultural wastes, can be used as an inexpensive enzyme source, no toxicologic test has been performed to ascertain whether or not plant materials release potentially hazardous compounds into the water. Moreover, significant amounts of plant material are required to treat the contamination, and their handling might be another environmental problem.
Plants and actinomyces can modify chlorophenols, often making them more soluble and easier to degrade by other microorganisms (Webb et al. 2001). Therefore, phytoremediation, which has been shown to be an effective and economic way of treating recalcitrant contaminants (Trapp & Karlson 2001; Singh & Jain 2003; McCutcheon & Schnoor 2003), might also be used to treat chlorophenols, especially at low concentrations, when bacterial degradation is not feasible. In this respect, the main questions are how 4-CP is removed and whether it is taken up into trees and causes toxic effects. The objectives of this study were therefore (1) to determine the toxicity of 4-CP to willow trees, (2) to estimate the removal velocity and mechanism of 4-CP, (3) to illustrate the relation between external and internal 4-CP exposure with respect to its toxicity to trees, and (4) to test the feasibility of implementing phytoremediation using willow trees as a treatment method for 4-CP in wastewater and soil.
Materials and Methods
Line of Experiments
Genetically identical willow cuttings (Salix viminalis) of similar size were grown in 500-mL Erlenmeyer flasks filled with modified International Organization for Standardization (ISO) 8692 nutrient (hydroponic) solution (ISO 1997) and exposed to 4-CP at varying levels. Transpiration of the trees was determined by weighing the flasks once a day for several days and used as the toxicologic end point. The possible role of 4-CP in root-zone degradation was also determined by using trees with detached leaves (stem with roots [leafless trees]) or detached roots (stem with leaves [rootless trees]). At the end of the experiment, the remaining concentrations of 4-CP in the nutrient solution―as well as its concentration in roots, leaves, and stems―were measured to relate external to internal exposures and effects. To determine the bacterial degradation and volatilization of 4-CP in the system, additional experiments without trees were also performed.
Phytotoxicity Tests
The willow tree transpiration test for acute toxicity (Trapp et al. 2000) was applied to determine the short-term toxicity of 2,4-dichlorophenol (2,4-DCP) to willow trees. Reduction of transpiration was used as the end point to express toxicity.
The experiments were carried out in a fully climatized room with constant conditions (26.8°C ± 0.5°C and 30% ± 5% relative humidity), under a rack of 36 W fluorescent lights, with a continuous light intensity of 54 μmol/m2/s. The racks were sequenced at intervals of 20 cm. Basket-willow cuttings with a length of approximately 40 cm were provided from Aage Bach (Tylstrup, Denmark) and pregrown for up to 5 weeks in buckets using tap water. Once the cuttings had well-developed leaves and roots, they were weighed and transferred into 500-mL aluminum foil–wrapped Erlenmeyer flasks with 150 mL ISO 8692 nutrient solution (pH 7.5), which were sealed with a cork stopper to avoid evaporation from the media. Further sealing of the flasks was done around the cuttings using plasticine (Modello Schulknete; Rudolf Reiser KG, Nürnberg, Germany). The trees were left for 2 days to adapt to the new environment.
After adaptation of the willows to the test conditions, transpiration was measured by weighing the flasks once a day to rank them. The trees were grouped based on their average transpiration, so that high- and low-transpiring willows occurred in every group containing five willow trees. Different 4-CP solutions (150 mL), which varied between 0 (control) and 80 mg/L, were added to these almost-identical groups based on transpiration. In other words, five replicates for each concentration were used in the toxicity tests. All concentrations were prepared with modified ISO 8692 nutrient solution to prevent nutrient deficiency from occurring during the test.
An additional set of experiments, in which roots were removed from the trees, was performed to determine the influence of 4-CP microbial degradation by associated bacteria located on and inside the roots. In another set of experiments, the leaves were detached to determine the effect of transpiration on the removal of 4-CP. A solution (150 mL) containing 7 mg/L 4-CP was added to the trees without roots and leaves.
Removal and Accumulation of 4-CP
At the end of the phytotoxicity tests, leaves, roots, and stems were rinsed with deionized water, sealed in separate bottles with gas-dense closures, and stored at –18°C until the extraction to determine the accumulation of 4-CP in willows. The final 4-CP concentration in nutrient solution was determined immediately after the termination of the phytotoxicity tests.
To determine biotic and abiotic degradation losses of the compound, two sets of flasks with 4-CP solution were prepared without willow cuttings. The initial 4-CP concentration was 11 mg/L for both sets. The first set of samples was placed without willow trees in Erlenmeyer flasks sealed with plasticine as in the phytotoxicity experiments, whereas the second set of samples was put into glass bottles closed with gas-dense blue caps. Four replicates were used for each set of controls.
Calculation of Results
To compare the toxic effect of 4-CP on tree cuttings with different initial transpiration (before the addition of toxicant), transpiration was normalized with respect to initial transpiration and transpiration of the control cuttings. This was necessary because individual trees have different transpiration rates, and, concurrently, (healthy) trees grew during the test. Normalized relative transpiration (NRT) was calculated by:
where C is concentration of the compound in hydroponic solution (mg L–1); t is time period (hours); T is absolute transpiration (g h–1); and n and m are the number of replicates for exposed trees and control trees, respectively. The NRT of the controls was always 100%. Values of the treated trees <100% indicate inhibition of transpiration.
Effective concentrations (ECs) were also calculated based on the NRT at 72 hours. For calculation of EC values and confidence limits, a statistical programme using weighted nonlinear regression was employed. The programme assumes a logarithmic normal distribution of data, and for calculation of confidence limits it uses inverse estimation, taking into account the covariance within the control response (Andersen 1994).
The loss of 4-CP from the flasks was determined based on the difference between the initial and final 4-CP mass. The final 4-CP mass was the sum of remaining mass in the hydroponic solution at the end of test plus the mass recovered from the plant material.
Chemicals and Chemical Analysis
4-CP with a purity of 98% was purchased from Merck-Schuchardt (Munich, Germany). The concentration of 4-CP in plant material and hydroponic solution was measured by a gas chromatographer–flame ionization detector (model GC-2010; Shimadzu, Kyoto, Japan) equipped with a nonpolar capillary column of polyethylene glycol (model ZB5MS; (Phenomenox, Torrance, CA) with a respective length and inner diameter of 30 m and 0.25 mm, respectively. The temperature in the column varied between 60°C and 240°C. The carrier gas in the column was hydrogen with a flow of 47 mL/min. A volume (2 μL) of sample was injected by Shimadzu autoinjector AC-20i at 300°C. Peaks were analyzed with GCsolution software (Shimadzu). Standards for the calibration curve were prepared directly from the stock solution (150 mg/L). The method used in this study was validated as previously described in the literature (Harris 2003). The detection limit was found to be 0.72 mg l–1. Aqueous samples were extracted within 2 minutes by a solvent mixture of 49.8% (v/v) diethyl ether and 49.8% (v/v) pentane with 0.4% undecane-11 as internal standard. Plant materials were extracted at room temperature, in Pyrex gas-dense bottles with stoppers containing the same solvent mixture, for 48 hours using an autoshaker (Struers LS5 Shaker; Gerhardt, Berlin, Germany).
Results
Phytotoxicity of 4-CP to Willows
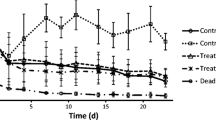
Figure 1 shows the NRT of willows grown in spiked hydroponic solution. Willows exposed to 6.8 mg/L 4-CP showed almost no sign of inhibition. At a 4-CP concentration of 14.5 mg/L, transpiration of the willow decreases only slightly lower than that of controls and totally recovered by 92 hours. The trees exposed to 37.3 mg/L 4-CP showed decreased transpiration of 45% after 92 hours. Finally, at a 4-CP concentration of 79.9 mg/L, the trees showed signs of wilting and almost stopped transpiring after 92 hours. The EC10, EC20, and EC50 values were calculated based on the NRT of willows and are listed in Table 1. The EC50 for willow trees in the toxicity test was 32.20 mg/L.
Mass Balance
4-CP was detected neither in plant nor in the remaining solution for the controls (0 mg/L). The mass balance of 4-CP for the willows exposed to the chemical is listed in Table 2. No accumulation in leaves or stems was detected for the given concentrations. Although it was clearly observed that 4-CP accumulated in roots, no significant differences in accumulation were found in the roots of trees exposed to different 4-CP concentrations, as shown in Figure 2 (one-tailed Student t test; α = 5%). Average concentrations in exposed roots were in the range of 10 to 15 mg/kg. At the end of the experimental period, all of the final 4-CP concentrations were <1 mg/L, except for the tests performed with trees exposed to 79.9 mg/L initial 4-CP solutions. No significant difference in the losses was detected during the experiments (α = 5%). Almost all of the supplied 4-CP was removed.
The losses from the treeless samples in flasks closed with cork stoppers and those in flasks with gas-tight caps were 10% and 6%, respectively, for the samples spiked with 11.4 mg/L 4-CP. This shows that sorption to roots and microbiologic degradation might be responsible 6% of the loss and volatilization for additional 4% because volatilization may occur in the bottles closed with cork stoppers but not in bottles closed with gas-tight caps.
Loss of 4-CP from the media between the experiments performed with and without trees was significantly different (one-tailed Student t test; α = 5%). Willow trees significantly increased the removal of 4-CP compared with treeless experiments because almost all 4-CP in the hydroponic solution was removed for all examined initial concentrations (see Table 2), whereas only 10% loss was detected for the treeless experiments.
Root Concentration Factor
The root concentration factor (RCF; in L/kg) describes the ratio between concentration in roots (mg/kg) and concentration in external solution (mg/L). Organic compounds may dissolve in the aqueous root sap or sorb to root lipids (Trapp 2002). Often, there is a linear relation, i.e., the root concentration increases when the concentration in solution increases, and the RCF increases with increasing lipophilicity of the compound. This is described by the RCF regression from Briggs et al. (1982):
Given the KOW of 4-CP as 2.42 (Rippen 1991), an RCF of 3.01 L/kg results. This theoretical value, based on phase equilibrium between external solution and roots, can be compared with the experimentally determined RCF values (Table 3). Measured RCF values ranged from 0.2 to 1.55 for intact trees and decreased with increasing 4-CP concentrations in the solution. Throughout the experiments, actual RCF values were lower than the calculated RCF value. In the experiment with trees having detached leaves, an RCF value of 3.0 was found, which was close to the predicted RCF value.
Determination of Root-Zone Degradation
In the experiments using rootless trees, loss of 4-CP from the system was drastically decreased; the final 4-CP mass remaining in the nutrient solution at the end of experiments with rootless trees was 43.8% of the initial mass (Table 4). However, leafless willows showed no significant difference in remaining 4-CP mass in hydroponic solution from that of intact plants (Student t test, α = 5%). As expected, transpiration was lower when roots or leaves were detached (Fig. 3).
Almost all of the initial supplied volume (150 mL 4-CP) to rootless trees and leafless trees was still present at the end of experiment. The remaining volume was 138 (±4) and 137 (±2) mL for trees with detached leaves and roots, respectively.
Discussion
Comparison of 4-CP Toxicity to Willow Trees with Toxicity to Other Species
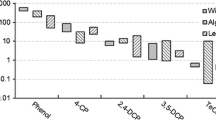
In the present study, 4-CP was found to be less toxic to willows than to aquatic plants, such as duckweed (Lemna gibba) and Selenastrum capricornutum, which have been reported to have EC50s of 23.5 and 29 mg/L, respectively (Sharma et al. 1997; Shigeoka et al. 1988). However, willow trees were at least as sensitive to 4-CP as green algae, Chlorella vulgaris, which was reported to have an EC50 of 38 mg/L (Shigeoka et al. 1988). 4-CP also seemed to be more toxic to willows than to most of the other tested terrestrial plants, such as cabbage and millet (Panicum milliaceum). The EC50 values for these species in root-elongation tests were reported to be 47.4 and 130 mg/L, respectively (Feng et al. 1996; Wang 1985).
Comparison of 4-CP, Phenol, and 2,4-DCP Toxicities to Willow Trees
The toxicity from phenol compared with monochlorophenols and dichlorophenols increased with the number of chloride atoms attached to the phenol ring. The respective approximate EC50 values for phenol, 4-CP, and 2,4-DCP were 500, 32.2, and 10 mg/L based on the 3-day willow tree transpiration tests (Ucisik & Trapp 2006; Ucisik et al. 2007). The higher toxicity of the higher chlorinated phenols might be explained by increased lipophilicity, which leads to a greater potential for uptake into the plant. Furthermore, the toxicity of substituted phenols was contributed to their uncoupling activity of electron transfer in membranes (Escher et al. 1999). The concentration ratio between biomembranes and external solution can be described partially by the partition coefficient between n-octanol and water, i.e., KOW (Trapp 2004). Therefore, a higher KOW leads to a higher concentration in the membrane and subsequently to higher toxicity (i.e., lower EC50). The KOW values for phenol, 4-CP, and 2,4-DCP were 30.2, 263, and 1,288 L/L–1, respectively (Rippen 1991), and, in fact, the product of the EC50 in the willow tree toxicity test and the KOW for the three compounds was relatively constant―15,100, 8,469, and 12,882 for phenol, 4-CP, and 2,4-DCP, respectively―suggesting that the internal concentration in the membrane is the relevant concentration for the toxic effects observed in the test.
4-CP Loss and Accumulation
Two major mechanisms exist by which there is chemical loss from the system. The first mechanism is volatilization to air, either directly from the flask or after uptake into trees and translocation to leaves. The second mechanism is removal by metabolism, either by microorganisms or by the trees. A closer look at the results obtained in the experiments may help evaluate the relevance of these processes.
Loss from treeless flasks was on average 8%, which indicates some loss by volatilization. Accumulation in leaves was not observed (lower than detection limit) in any of the treatments with willows. This indicates that if there was a translocation of 4-CP to leaves, it was followed by volatilization or metabolization. Translocation of the chemical to leaves cannot be faster than the flow of the transport-medium water. For neutral organic compounds, the concentration ratio between transpiration stream and external solution, the so-called transpiration stream concentration factor, is maximally 1 but usually lower (Briggs et al. 1982; Trapp 2000). Therefore, if 4-CP was taken up by trees together with transpiration water, the concentration in the remaining solution would not decrease by this process (but rather eventually increase); however, the mass of 4-CP would decrease because the volume of water also decreases. However, in all experiments, the final concentrations in the remaining solution decreased to values far lower than the initial concentrations (Fig. 2). In addition, when leaves were detached, loss from the system was as high as the loss observed for the intact plants (Table 4). Therefore, loss of chemical by direct volatilization and by translocation to leaves with subsequent volatilization can not be the dominating fate process. However, loss from the system was drastically decreased in the experiment using rootless trees. This provides evidence that the major reason for the loss of 4-CP from the media was degradation in the root zone, either by microbes located in or on roots or by root enzymes.
The accumulation of 4-CP differed among the individuals of willow cuttings in the roots exposed to the same 4-CP concentrations (replicates; Table 2). The high variation of the results was not surprising. In recent studies with phenol, 2,4-DCP, and cyanide, a nonlinear relation between uptake, metabolism, and toxicity was found, and small changes in the properties of the trees resulted in large changes in accumulation (Ucisik et al. 2007; Ucisik & Trapp 2006; Larsen et al. 2005).
Final 4-CP-concentrations in roots were similar for all exposure levels and, for intact trees, were all lower than the equilibrium concentration (i.e., the RCF). A plausible explanation for this is that the loss of 4-CP occurred by way of metabolism by bacteria in the vicinity of the roots. Bacterial metabolism increases with increasing substrate availability (Monod kinetics). Therefore, the loss is faster at higher initial solution concentrations, leading to similar 4-CP levels in the roots at the end of the experiment despite different initial concentrations.
Rhizodegradation as a Treatment Option for 4-CP
Chlorophenols are frequently found in raw wastewater and sewage sludge. Concentrations of 4-CP in raw wastewater ranged from 200 ng/L to 2 mg/L (Rippen 1991). Concentrations in sewage sludge ranged from 0.028 to 90 mg/kg dry weight (mean 17) (Rippen 1991). The freely dissolved concentration in porewater can be estimated from the adsorption to organic carbon and is approximately half of that.
Subsequently, the concentrations of 4-CP in contaminated environments are usually lower than the EC10 (i.e., 12 mg/L) for willows determined in this study, and the occurrence of 4-CP does not inhibit the growth of willows. Therefore, root-zone degradation using willows is an option to treat wastewater or sludge that is contaminated with 4-CP, if concentrations are in the ranges given previously. It was shown in this study that 4-CP is rapidly degraded in the root zone and does not accumulate in stem or leaves at any nontoxic concentration. The amount volatilizing to air was also low. Therefore, phytoremediation is feasible without the risk of spreading the pollutant or contaminating the wildlife food chain.
Conclusion
The transpiration of willow trees (S. viminalis) was either not inhibited at all, or was only inhibited to a minor extent, by 4-CP levels ≤14.5 mg/L. The EC50 was 32.2 mg/L, whereas the respective EC50s for phenol and 2,4-DCP were 500 and 10 mg/L, respectively. The trees did not survive at 4-CP concentrations ≥79.9 mg/L. Degradation in the root zone was the main reason for the loss of 4-CP. Phytoremediation of 4-CP using willow trees can be considered an efficient remediation option because of both its high removal efficiency and negligible accumulation inside plant tissue.
References
Andersen H (1994) Statistical methods for evaluation of toxicity of wastewater [in Danish]. Masters thesis. Institute of Mathematical Statistic and Operation Analysis, Technical University of Denmark, Lyngby, Denmark
Bae HS, Lee JM, Kim YB, Lee ST (1996) Biodegradation of the mixtures of 4-chlorophenol and phenol by Comamonas testosteroni CPW301. Biodegradation 7:463–469
Baggi G, Cavalca L, Francia P, Zangrossi M (2004) Chlorophenol removal from soil suspensions: effects of a specialized microbial inoculum and a degradable analogue. Biodegradation 15:153-160
Briggs G, Bromilow R, Evans A (1982) Relations between lipophilicity and root uptake and translocation of nonionised chemicals by barley. Pestic Sci 13:495–504
Carvalho MF, Vasconcelos I, Bull AT, Castro PML (2001) A GAC biofilm reactor for the continuous degradation of 4-chlorophenol: Treatment efficiency and microbial analysis. Appl Microbiol Biotechnol 57:419–426
Clarkson WW, Yang CP, Harker AR (1993) 2,4-D degradation in monoculture biofilm reactors. Water Res 27:1275–1284
Cole JR, Cascarelli AL, Mohn WW, Tiedje JM (1994) Isolation and characterization of a novel bacterium growing by way of reductive dehalogenation of 2-chlorophenol. Appl Environ Microbiol 60:3536–3542
Danis TG, Albanis TA, Petrakis DE, Pomonis PJ (1998) Removal of chlorinated phenols from aqueous solutions by adsorption on alumina pillared clays and mesoporous alumina aluminum phosphates. Water Res 32:295-302
Da Silva JP, Ferreira LFV, Da Silva AM, Oliveira AS (2003) Phytochemistry of 4-chlorophenol on cellulose and silica. Environ Sci Technol 37:4798–4803
Dec J, Bollag JM (1994) Use of plant material for the decontamination of water polluted with phenols. Biotechnol Bioeng 44:1132–1139
Durán N, Esposito E (2000) Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: A review. Appl Catal B Environ 28:83–99
Escher BI, Hunziker R, Schwarzenbach RP (1999) Kinetic model to describe the intrinsic uncoupling activity of substituted phenols in energy transducing membranes. Environ Sci Technol 33:560–570
Fahr K, Wetzstein HG, Grey R, Schlosser D (1999) Degradation of 2,4-dichlorophenol and pentachlorophenol by two brown rot fungi. FEMS Microbiol Lett 175:127-132
Feng L, Wang LS, Zhao YH, Song B (1996) Effects of substituted anilines and phenols on root elongation of cabbage seed. Chemosphere 32:1575–1583
Goswami M, Shivaraman N, Singh RP (2002) Kinetics of chlorophenol degradation by benzoate-induced culture of Rhodococcus erythropolis M1. J Microbiol Biotechnol 18:779-783
Häggblom MM, Valo RJ (1995) Bioremediation of chlorophenol wastes. In: Young LY, Cerniglia CE (eds) Microbial transformation and degradation of toxic organic chemicals. Wiley-Liss, New York, NY, pp. 389–434
Hao OJ, Kim MH, Seagren EA, Kim H (2002) Kinetics of phenol and chlorophenols use by Acinetobacter species. Chemosphere 46:797-807
Harris DC (2003) Quantitative chemical analysis. WH Freeman, New York, NY
International Organization for Standardization (1997) Water quality―Freshwater algal growth test with Scenedesmus subspicatus and Raphidocelis subcapitata. ISO Standard 8692. Geneve, Switzerland
Jianlong W, Yi Q (1999) Microbial degradation of 4-chlorophenol by microorganisms entrapped in carrageenan-chitosan gels. Chemosphere 38:3109–3117
Jung MW, Ahn KH, Lee Y, Kim KP, Rhee JS, Park JT, et al. (2001) Adsorption characteristics of phenol and chlorophenols on granular activated carbons (GAC). Microchem J 70:123-131
Kim JH, Oh KK, Lee ST, Kim SW, Hong SI (2002) Biodegradation of phenol and chlorophenols with defined mixed culture in shake-flasks and a packed bed reactor. Process Biochemistry 37:1367–1373
Kishino T, Kobayashi K (1994) Relation between the chemical structures of chlorophenols and their dissociation constants and partition constants in several solvent-water systems. Water Res 28:1547–1552
Larsen M, Ucisik AS, Trapp S (2005) Uptake, metabolism, accumulation and toxicity of cyanide in willow trees. Environ Sci Technol 39:2135–2142
Laurenti E, Ghibaudi E, Ardissone S, Ferrari RP (2003) Oxidation of 2,4-dichlorophenol catalyzed by horseradish peroxidase: Characterization of the reaction mechanism by UV-visible spectroscopy and mass spectrometry. J Inorg Biochem 95: 171-176
Leonard D, Lindley ND (1999) Growth of Ralstonia eutropha on inhibitory concentrations of phenol: Decreased growth can be attributed to hydrophobic perturbation of phenol hydroxylase activity. Enzyme Microb Technol 25:271-277
Loh KC, Wang SJ (1998) Enhancement of biodegradation of phenol and a nongrowth substrate 4-chlorophenol by medium augmentation with conventional carbon sources. Biodegradation 8:329–338
Madsen T, Aamand J (1992) Anaerobic transformation and toxicity of trichlorophenols in a stable enrichment culture. Appl Environ Microbiol 58:557–561
McCutcheon SC, Schnoor JL (2003) Phytoremediation: Transformation and control of contaminants. John Wiley, Hoboken, NJ
Mehmood Z, Kelly DE, Kelly SL (1997) Cytochrome P450 3A4 mediated metabolism of 2,4-dichlorophenol. Chemosphere 34:2281-2291
Mikesell MD, Boyd SA (1986) Complete reductive dechlorination and mineralization of pentachlorophenol by anaerobic microorganisms. Appl Environ Microbiol 52:861–865
Minero C, Pelizzetti E, Pichat P, Sega M, Vincenti M (1995) Formation of condensation products in advanced oxidation technologies: The photocatalytic degradation of dichlorophenols on TiO2. Environ Sci Technol 29:2226-2234
Oliver S, Scragg AH, Morrison J (2003) The effect of chlorophenols on the growth of Chlorella VT-1. Enzyme Microb Technol 32:837-842
Pera-Titus M, Garcia-Molina V, Banos MA, Gimenez J, Esplugas S (2004) Degradation of chlorophenols by means of advanced oxidation process: A general review. Appl Catal 47:219-256
Quan X, Shi H, Zhang Y, Wang J, Qian Y (2004) Biodegradation of 2,4-dichlorophenol and phenol in an airlift inner-loop bioreactor immobilized with Achromobacter sp. Sep Purif Technol 34:97-103
Rippen G (1991) Handbuch umweltchemikalien. Ecomed, Landsberg, Germany
Roper JC, Dec J, Bollag JM (1996) Using minced horseradish roots for the treatment of polluted waters. J Environ Qual 25:1242–1247
Scraagg AH, Spiller L, Morrison J (2003) The effect of 2,4-dichlorophenol on the microalga Chlorella VT-1. Enzyme Microb Technol 32:616-622
Sharma HA, Barber JT, Ensley HE, Polito MA (1997) A comparison of the toxicity and metabolism of phenol and chlorinated phenols by Lemna gibba, with special reference to 2,4,5-trichlorophenol. Environ Toxicol Chem 16:346–350
Shigeoka T, Sato Y, Takeda Y, Yoshida K, Yamauchi F (1988) Acute toxicity of chlorophenols to green algae, Selenastrum capricornutum and Chlorella vulgaris, and quantitative structure-activity relations. Environ Toxicol Chem 7:847–854
Singh OV, Jain RK (2003) Phytoremediation of toxic aromatic pollutants from soil. Appl Microbiol Biotechnol 63:128–135
Song HY, Liu JZ, Xiong YH, Weng LP, Ji LN (2003) Treatment of aqueous chlorophenols by phthalic anhydride-modified horseradish peroxidase. J Mol Catal B Enzym 22:37–44
Tong Z, Qingxiang Z, Hui H, Qin L, Yi Z (1997) Removal of toxic phenol and 4-chlorophenol from wastewater by horseradish peroxide. Chemosphere 34:893–903
Toscano G, Colarieti ML, Greco G (2003) Oxidative polymerisation of phenols by a phenol oxidase from green olives. Enzyme Microb Technol 33:47–54
Trapp S (2000) Modeling uptake into roots and subsequent translocation of neutral and ionisable organic compounds. Pest Manage Sci 56:767–778
Trapp S (2002) Dynamic root uptake model for neutral lipophilic organics. Environ Toxicol Chem 21:203–206
Trapp S (2004) Plant uptake and transport models for neutral and ionic compounds. Environ Sci Pollut Res 11:33–39
Trapp S, Karlson U (2001) Aspects of phytoremediation of organic pollutants. J Soils Sediments 1:37–43
Trapp S, Zambrano KC, Kusk KO, Karlson U (2000) A phytotoxicity test using willow. Arch Environ Contam Toxicol 39:154–160
Tsuji N, Hirooka T, Nagase H, Hirata K, Miyamoto K (2003) Photosynthesis-dependent removal of 2,4-dichlorophenol by Chlorella fusca var. vacuolata. Biotechnol Lett 25:241-244
Ucisik AS, Kusk KO, Trapp S (2007) Uptake, accumulation, phytotoxicity and removal of 2,4-dichlorophenol in willow trees (Salix viminalis). Environ Toxicol Chem 26:1165–1171
Ucisik AS, Trapp S (2006) Uptake, removal, accumulation, and phytotoxicity of phenol in willow trees (Salix viminalis). Environ Toxicol Chem 25:49–54
Valli K, Gold MH (1991) Degradation of 2,4-dichlorophenol by the lignin-degrading fungus Phanerochaete chrysosporium. J Bacteriol 173:345-352
Wang W (1985) Use of millet root elongation for toxicity tests of phenolic compounds. Environ Int 11:95–98
Webb MD, Ewbank G, Perkins J, McCarthy A (2001) Metabolism of pentachlorophenol by Saccharomomospora viridis strains isolated from mushroom compost. Soil Biol Biochem 33:1903-1914
Woods SL, Ferguson JF, Benjamin MM (1989) Characterization of chlorophenols and chloromethoxybenzene biodegradation during anaerobic treatment. Environ Sci Technol 23:62–68
Wu XF, Kosaric N (1991) Removal of organochlorine compounds in an upflow flocculated algae photobioreactor. Water Sci Technol 24:221-232
Acknowledgments
Support for this work was provided through a doctoral grant from the Technical University of Denmark and the Research School of Environmental Chemistry and Ecotoxicology awarded to A. S. Ucisik.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ucisik, A.S., Trapp, S. Uptake, Removal, Accumulation, and Phytotoxicity of 4-Chlorophenol in Willow Trees. Arch Environ Contam Toxicol 54, 619–627 (2008). https://doi.org/10.1007/s00244-007-9065-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-007-9065-6