Abstract
Sediment and American oyster (Crassostrea virginica) collected from nine selected marsh/estuarine ecosystems in Savannah, Georgia were analyzed for elements such as Al, As, B, Cd, Cr, Cu, Fe, Hg, Mn, Mo, Ni, Pb, Si, and Zn. Sediments were extracted by ammonium acetate (NH4OAc), Mehlich-3 (M-3), and water procedures, whereas an acid digestion procedure was adopted for oyster tissue. Concentrations of elements were higher in M-3 extractions followed by NH4OAc and water extraction procedures. Calcium and Mg was greater in sediments by any of the extractions, whereas other elements differed depending upon the extraction procedures. There were no significant spatial variations (p < 0.05) of any of elements analyzed except Mn, in NH4OAc/water extraction procedure and Fe and Al by water extraction procedure. Contamination of Al, B, Cd, Cr, Cu, Fe, Mn, Mo, Ni, Pb, Si, and Zn in oyster tissue ranged from 399 to 1460, 231 to 254, <1.5 to 2.9, <1.5 to 8.0, 67 to 121, 232 to 1357, 17 to 54, <0.5 to 0.64, <1.5 to 2.5, <1.5 to 4.0, 241 to 381, and 978 to 2428 μg/g dry weight (dw), respectively. Greatly elevated concentrations of elements such as P, Ca, Mg, K, and S were noticed in oyster tissue. The concentration range of Hg and As in sediment was 1.2–1.9 and 11–55 μg/g dw, respectively. The concentration range of Hg and As in oyster tissue was 130–908 and 200–912 ng/g dw, respectively. With the exception of As and Hg, other elements are several orders of magnitude greater in oyster tissue. There is no significant (p < 0.05) contamination variation in target analyses between the nine selected sites. Concentrations of heavy metals in sediment and oyster were either comparable or lower than those of other countries. Greater biota-sediment accumulation factor was noticed for P and Zn. Concentrations of Hg and P in oyster tissue were higher than the threshold limit for human consumption. Overall, the baseline data can be used for regular ecological monitoring, considering the domestic and industrial growth around this important marsh/estuarine ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trace elements and heavy metals are natural constituents of the earth and are present in varying concentrations in all ecosystems (Kannan et al. 1993, 1995, 1998; Sakai et al. 2000; Ichihashi et al. 2001; Ikemoto et al. 2004; Karadede et al. 2004; Storelli et al. 2005; Amaraneni 2006; Alquezar et al. 2006; Sankar et al. 2006). However, anthropogenic activity has altered elements geochemical cycles, thus generating further cause for environmental concern. Trace elements that exist naturally at background levels in the environment include chromium (Cr), cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), vanadium (Va), strontium (Sr), and zinc (Zn), which are essential elements in living organisms. However, some trace elements or heavy metals such as mercury (Hg), cadmium (Cd), arsenic (As), chromium (Cr), thallium (Ti), and lead (Pb) are not required for metabolic activity and are toxic. The term heavy metal refers to any metallic chemical element that has a relatively high density and is toxic or poisonous at low concentrations. Therefore, heavy metals have the potential to accumulate in the soils, seawater, freshwater, and sediments (Moss and Constanza 1998; Mathew et al. 2003; Chindah et al. 2004; Kongchum et al. 2006), and tend to bioaccumulate and biomagnify in living organisms. The main anthropogenic sources of heavy metals include industries, mining activities, foundries and smelters, and diffuse sources such as piping, constituents of products, combustion byproducts, traffic, municipal solid waste, urban run-off, domestic waste, and sewage disposal.
Sediments play a key role in the geochemical and biological processes of an estuarine ecosystem. In particular, sediments act as sinks for toxic metals that enter the estuary. In doing so, they regulate the concentration of these minerals and compounds in the water column (De Groot et al. 1976). Sediment also plays a very important role in the physicochemical and ecological dynamics of trace metals in aquatic ecosystems. The physicochemical nature of sediment bound trace metals is important in the bioaccumulation of aquatic organisms. Heavy metals have multitude of toxic effects, such as carcinogenic effects, acute syndrome, neurological dysfunction, that ultimately cause diseases in the brain, kidney, skin cancer, and such like places.
Aquatic animals accumulate large quantities of xenobiotics, and the accumulation depends upon the intake and the elimination from the body (Karadede et al. 2004). Among different aquatic organisms, clams, oyster, and mussels accumulate large quantities of heavy metals. Oysters are often used as indicators of marsh and estuary health. Because oysters are suspension feeders that filter water, they can retain small particles within their body (Day et al. 1989). Particularly, American oyster, Crassostrea virginica, filters large volumes of seawater and concentrated metals and other pollutants. Therefore, they are ideal pollution indicators and are frequently used in environmental assessment and monitoring (Haye et al. 2006). The ability of the American oyster to accumulate high concentrations of metals in its soft tissues is well documented (Machado and Zalmon 2005; Apeti et al. 2005a, 2005b; Elston et al. 2005).
Savannah River estuary receives domestic wastewater and other contaminants from several industries and mosquito control operations along its upstream waters. Considering the significant quantities of wastes, both of industrial and domestic origin, being released into the Savannah River estuary every year, it is of particular interest to evaluate the presence of pollutants mainly from anthropogenic source. Considering those facts, in this study we monitored trace elements and heavy metals including highly toxic As, Cd, Hg, Ni, and Pb in archived sediments and American oyster (Crassostrea virginica) tissue collected in nine selected locations along the Savannah River marsh/estuarine ecosystem. Here harvesting oysters was found to be significant; also, several industrial activities at these locations in which they influence toxic metal accumulation by oysters ultimately end up as human consumption. Another significant objective of this study was to also identify efficient extraction procedures for sediment samples, using three newly developed methods, and to find out robust methods for future analysis.
Materials and Methods
Study Design
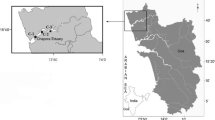
The purpose of this research was to acquire baseline chemical data necessary to provide an evaluation of the ecological health of the salt marsh estuarine ecosystem. Specifically, this study was designed to evaluate the levels of variety of chemical pollutants such as trace elements and heavy metals, in marsh/estuarine sediment and oyster tissue. For this particular study, nine sampling sites (Fig. 1 and Table 1) were chosen within or along Cockspur Island and McQueen’s Island and within the Fort Pulaski National Monument salt marsh ecosystem. These sites are frequently used by people for intense oyster harvesting for consumption purposes. Three of these sites were within Oyster Creek (Sites 1, 2, 3), two sites were along Bull River (Sites 4, 9), and one site was at the mouth of the Savannah River (Site 5) at an inlet opening to the Atlantic Ocean. The remaining three sites were near the northeastern entrance of Lazaretto Creek (site 6), whereas the other was along the north shore of Cockspur Island in the Savannah River (site 7), and the last site was at the western entrance of Lazaretto Creek on Tybee Island (Site 8). All of these selected sites were based on the undisclosed industries, factories, domestic influence, and also were nonpolluted in these areas.
Sample Collection
At each site, water, sediment, and American oyster (C. virginica) were collected during a 5–day period in November 2000 and 2001. For water, approximately 2 liters of water was collected from 1 m below the surface. In the field, the following water quality parameters were measured: Secchi disk depth, temperature, salinity, total dissolved solids, conductivity, dissolved oxygen, and dissolved oxygen percent saturation (Table 2). Water samples were transported back to the laboratory for additional analysis, which included pH, turbidity, settleable solids, nitrate (alkaline KMnO4 method), and phosphate (stannous chloride method) (Table 2). In the case of the sediments, at each sampling site, five subsamples of sediment from depths of 1–5 m (Table 3) were collected using a bottom-grab sampler (clamshell type). Clean stainless steel scoops were used to remove the top 0–5 cm of sediment from the grab sample. Sediment from each subsample was immediately placed in acetone-washed I-Chem bottles (Ben Meadows Co., WI, USA), sealed, labeled, and transported to the laboratory with ice and placed in a deep freezer until chemical analysis. Sediment moisture content (sediment dry method) and pH (sediment and water with 1:1 suspension) were analyzed in the laboratory (Table 3) at a later period. The American oysters were collected by hand or rake at the same sites, from the mid and lower intertidal zone. Oysters were immediately opened, and edible tissue was directly transferred into acetone-washed I-Chem bottles, sealed, labeled, and transported to the laboratory with ice, and placed in a deep-freezer until chemical analysis. Edible tissue of 25–36 oysters were collected at each site to form a single composite oyster sample from each site.
Chemical and Instrumental Analysis
The literature suggests that there are several methods used for soil and biological tissue extraction. However, for sediments there is no specific extraction method is recommended. Therefore, we tried to compare three extraction methods to compare their extractability of elements. Collected sediment samples for elemental analysis were air dried in a greenhouse. The moisture content (%) was measured. The pH was determined using Fisher Accumet (Thermo Fisher Scientific Co.) model-15 pH meter (McLean 1982), and then the sediments were subjected to extractable elements analysis by three extraction procedures, ammonium acetate (NH4OAc) extraction, Mehlich-3 (M-3) extraction, and water extraction procedures, in order to understand the robust method for future sediment analysis.
Ammonium Acetate Extraction
Five grams of air-dried sediment sample were placed in a 50-mL screw-capped polypropylene centrifuge tube, and 40 mL of 1 M NH4OAc was added and shaken for 30 min (Thomas 1982). The samples were centrifuged and the supernatant was filtered through Whatman 42 (LabX Whatman Plc., Canada) filter paper. The clear supernatant was saved and analyzed for various metals using the Perkin Elmer RL 3300 (Perkin Elmer Inc., USA) Inductively Coupled Plasma-Optical Emission Spectrophotometer (ICP-OES).
Mehlich-3 (M-3) Extraction
Two and a half grams of air-dried sediment sample was placed in a 50-mL screw-capped polypropylene centrifuge tube, and 25 mL of Mehlich-3 extractant (Mehlich 1984) was added and shaken for 5 min. The samples were centrifuged and the supernatant was filtered through Whatman 42 filter paper. The clear supernatant was saved and analyzed for various metals using Perkin Elmer RL 3300 (ICP-OES).
Water Extraction
Two and a half grams of air-dried sediment sample was placed in a 50-mL screw-capped polypropylene centrifuge tube, and 25 mL of deionized water was added and shaken for 30 min. The samples were centrifuged and the supernatant was filtered through Whatman 42 filter paper. The clear supernatant was saved and analyzed for various metals using Perkin Elmer RL 3300 (ICP-OES). With all three extraction procedures, only the total mercury (Hg) and total arsenic (As) from sediment samples were analyzed by Instrumental Neutron Activation Analysis.
Chemical and Instrumental Analysis of Oyster
Prior to analysis, the oysters’ edible tissue samples moisture content was determined and then freeze-dried. Lipid contents were analyzed by gravimetric method during organohalogen compound analysis (Sajwan et al. 2007; Senthil Kumar et al. 2007). Heavy metals (except Hg and As) were analyzed by digesting the homogenized samples in a mixture of nitric, perchloric, and sulfuric acids (Honda et al. 1982). One subset of oyster tissue composite was prepared for analysis of arsenic and mercury by a hot acid digestion method (Akagi and Nishimura 1991). All elements were analyzed using ICP-OES with the instrumental programming being the same as described in Ichihashi et al. (2001).
Quality Assurance and Quality Control
Chemical analysis was conducted in a clean environment (in the laboratory with acid-washed glasswares and pure chemicals/solvents that were purchased from Sigma-Aldrich Co). For each batch of 5 sediments, 1 blank sample was analyzed (total 9 blanks). Similarly, 2 blanks were run for 9 oyster tissue homogenates. None of the blank samples (n = 11) contained detectable limits of target analytes. All 45 sediment samples were also analyzed in the Nuclear Reactor Facility (NRF) at University of Florida, for intercalibration. The data evolved from our laboratory and NRF found the same standard deviations within the range of 5–9%. Similarly, oyster tissue was also analyzed in the Georgia Soil, Plant and Water Laboratory (GSPWL). The data evolved from our laboratory, and GSPWL found the same standard deviations within the range of 3–8%. The concentrations for all the elements and metals were expressed as μg/g dry weight (dw), unless specified otherwise. Statistical analysis was performed using SAS 9.1.3 Software Edition Rev.22 version (SAS Software Inc., Luxemburg, Belgium).
Results and Discussion
Water Quality
Water quality parameters (Table 2) were normal and typical of Georgia salt marsh ecosystems during autumn. At this location near the Savannah River inlet, and Atlantic Ocean, salinity would be expected to be relatively high (mid to high 20s). Water clarity as indicated by Secchi disk depth was typical of Georgia estuarine river and marsh creeks. Dissolved oxygen levels were high, which is typical in autumn and winter conditions in these habitats. Nutrients, particularly nitrate, were slightly elevated. However, during a previous study (Richardson, unpublished data), slightly elevated nitrate levels were noticed in water samples from Savannah River estuary, in comparison to samples from Wilmington River/Wassaw Sound estuary further south. Elevated nitrates, nitrites, and phosphates were also found in water from wetland in India (Amaraneni 2006). Overall, water quality parameters measured during this study, and taken during the limited time span of sampling, were typical and normal and met the criteria set down in the directives in all cases, which is true for Irish waters (Smyth et al. 1995). Continued water quality monitoring with more frequent sampling should be conducted in order to reveal any temporal trends in quality. More or less similar water qualities have been noticed in aquatic ecosystems in India (Jayaprakash et al. 2005; Amaraneni 2006; Kuppusamy and Giridhar 2006), and typical estuarine water qualities from Australia (Alquezar et al. 2006).
Sediment and Oyster
Mean depth of sediment collection varied from 1.4 to 3.8 (average of 5 subsamplings) at the 9 sites (Table 3). Average sediment moisture content was 47–73% (average of 5 subsamplings), whereas pH was between 5.0 and 8.1. The lowest pH (3.5) was noticed in site 6, whereas the highest (8.3) was noticed at site 4. Most sediment samples had pH values of 7–8. Site 6 was different from the other sites in having relatively low pH values in 4 of 5 sediment subsamples collected at the site. Differences in sediment pH may influence bioavailability of metals; therefore, sites and estuaries were selected with similar physicochemical parameters to minimize variability (Peakall and Burger 2003). Sediment pH ranges of 7.1–9.3 were also noticed from urban wetlands in Coimbatore, India (Mathew et al. 2003). Most of the sediment samples were classified as pelite (silt–clay) (<0.063 mm in diameter of grains), sand (0.063–2 mm), and gravel (>2 mm). Fat percent in oyster tissue was between 1.2 and 1.5, whereas site 6 oyster showed a very low fat percentage of 0.005. Sites 7–9 fat contents were not determined. Therefore, the fat weight basis of organic compounds should be ignored for further discussions. Moisture content in oyster tissue was between 20 and 21% (Table 3).
Trace Elements in Sediment
Trace elements such as calcium (Ca) were high followed by magnesium (Mg) and phosphorus (P) (Table 4). The potassium (K) and sulfur (S) were not analyzed in sediment. Extraction by M-3 method yielded greater Ca (948–31,200 μg/g dw) followed by NH4OAc (250–4000 μg/g dw) extraction and water extraction (199–2810 μg/g dw) procedures (Fig. 2). Similarly, Mg was in the range of 381–3430, 226–2752, and 165–2640 μg/g dw using M-3, NH4Oac, and water extraction methods, respectively (Table 4). Percentage contributions of elements were higher in the M-3 extraction procedure (Fig. 2). Phosphorus was very low with 0.11–147 μg/g dw using all 3 extraction procedures. However, p was significantly (p > 0.01) higher in sites 4 and 6 by the M-3 method. Phosphorus in the sediments from a marine environment (Cochin, India) showed more elevated levels than those in this study (Ashraf et al. 2006). Considering the robust results of elements by M-3 extraction methods in the present study, we consider only these data for further interpretations unless otherwise specified. Overall, concentrations of elements were slightly higher in sites 1–5. However, this is not statistically significant (p > 0.05).
Among other elements, boron (B), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), and zinc (Zn) are of particular interest. Basically, in sediments, B and Mo were not analyzed, Cu and Ni were below the detection limit through any of the three extraction procedures, whereas Fe (91–1200), Mn (5.2–140), and Zn (1.1–15) were detected greatly by M-3 extraction, followed by the water extraction procedure [Fe (0.15–313), Mn (0.03–103), and Zn (0.02–11)], and NH4OAc Fe [(0.02–0.54), Mn (0.91–118) and Zn (0.07–4.1)] (Table 4 and Fig. 2) procedure on μg/g dw. Significantly (p = 0.001) greater concentrations of Fe, Mn, and Zn were observed in site 6 when compared to other sites, particularly by water extraction procedure. Similarly, Mn was significantly higher (p = 0.003) in site 6 by NH4OAc extraction procedure.
Concentrations of Zn (data of M-3) observed in sediments in this study were several orders of magnitude less than industrial regions of New Zealand; India; Brazil (Deely et al. 1992; Baptista et al. 2000; Crapez et al. 2003; Mathew et al. 2003; Amaraneni 2006); Apalachicola Bay, Florida; Chesapeake Bay; and Mississippi Bay (Frazier 1975; Rodrigo 1989; Apeti et al. 2005b; Elston et al. 2005), but similar to sediments from Nigeria (Chindah et al. 2004). Similarly, Mn was several orders of magnitude lower than soil from urban wetland in India (Mathew et al. 2003), but similar to or, slightly higher than, that of those from Chesapeake Bay (Frazier 1975), whereas Fe was very low in India and at Chesapeake Bay (Frazier 1975; Mathew et al. 2003).
Trace Elements in Oyster
Elements in oyster tissue were in the following order: Ca ≥ S > K > P > Mg (Table 5). It is worth indicating that S, K were analyzed only from sites 7, 8, and 9. Overall, Ca was predominant (19, 920–986, 970), followed by a decreasing order of Na (32, 210–244, 260) > S (15, 500–517, 450) > K (9951–9912, 060) > P (2836–7868) ≥ Mg (2752–6440) on μg/g dw. Concentrations of the selected elements in oyster tissues were 3 to 4 orders of magnitude higher than the concentrations in the sediment, suggesting efficient tissue bioaccumulation. Correlations between oyster and sediment, though positive, were significantly low Ca (r2 = 0.13) and Mg (r2 = 0.11) (Fig. 3), and these results suggest that oyster may accumulate elements not only from sediment, but also from the water column. Earlier studies demonstrated that heavy metal bioaccumulation in filter feeders such as American oyster is the net result of uptake, and elimination of the metals in ambient water (Apeti et al. 2005a, 2005b).
Elements such as Cu, Fe, Mn, and Zn in oyster tissue were analyzed in all 9 sites, whereas B and Mo were analyzed only from sites 7, 8, and 9. Concentrations of essential elements in oyster tissue were in increasing order from Mo (<0.5–0.64), Mn (17–52), Cu (67–121), B (231–254), Fe (232–1357), and Zn (978–2428), on μg/g dw (Table 5). There was no apparent site-specific bioaccumulation of elements noticed in oysters. Zn, Mn, and Cu were greater in site 4, whereas Fe was slightly high in site 8. Overall, concentrations of trace elements were several folds greater in oyster when compared to sediment. Particularly, Zn was highly bioaccumulated in oyster when compared to sediment. The well-known element Zn, which easily accumulates in oysters, by far surpasses the maximum limit of tolerance (MLT). The results confirm this trend showing the capacity of oysters to metabolically control high Zn concentrations (Frias-Espericueta et al. 1999). As mentioned earlier, living organisms require trace amounts of some elements, including Co, Cu, Fe, Mn, Mo, Va, Sr, and Zn. Many of these heavy metals exist naturally, at background levels in the environment. Zn, Fe, Cu, and Mn are biologically essential and play an important role as cofactors in enzymatic processes (Singh and Steinnes 1994).
Zn is well documented as an essential trace metal in the physiology of bivalves. This suggests that physiologically, Zn should have higher assimilation efficiency as compared to that of other elements. Differences in efflux rate could also be the cause of the difference between levels of the concentrations of other elements and Zn in oysters. Zn efflux rate being slightly smaller than that of other elements, the bioaccumulation rate of Zn is consequently greater. The second argument that explains the difference is based on the presence in bivalve tissues of low molecule sulphur-rich metal binding proteins such as metallothionein (MT). MT is known to selectively complex certain heavy metals such as Cu and Zn, making these elements the most abundant in oyster tissue. Finally, the selective retention of Zn to a different degree is caused by the chemistry of the characteristics of a metal, and is the octanol/water partition coefficient Kow. The partition coefficient is an important parameter that determines the bioavailability of metal in ingested food and water.
Concentrations of Zn and Cu observed in oyster tissue in this study were less than those in Chesapeake Bay (Frazier 1975), but similar to or greater than those in Ireland, Mexico, Brazil, and other U.S. estuaries (Goldberg et al. 1983; Phelps and Mihursky 1986; Smyth et al. 1995; Bloxham et al. 1998; Frias-Espericueta et al. 1999; Machado and Zalmon 2005; Apeti et al. 2005a, 2005b; Elston et al. 2005). Likewise, Mn and Fe concentrations were greater in the present study when compared to those in Chesapeake Bay, Gulf Coast, Irish Coast, and Brazil Coast (Frazier 1975; Frias-Espericueta et al. 1999; Machado and Zalmon 2005). It has been well documented that the American oyster is capable of bioaccumulating heavy metals in its soft tissue, to levels that are greater than in its corresponding environment.
Heavy Metals in Sediment
Among nonessential trace elements or heavy metals, aluminum (Al), arsenic (As), cadmium (Cd), cobalt (Co), chromium (Cr), lead (Pb), mercury (Hg), nickel (Ni), and silicon (Si) are of major concern. In sediment, Co and Si were not analyzed; Ni was not detected in all 3 extraction procedures. As and Hg were exempted from NH4OAC and water extraction procedures because they were only extracted by the M-3 procedure. Excluding As and Hg, analysis of sediment by the NH4OAC extraction procedure yielded below the detection limit of Cr, whereas contamination status of other elements was in the decreasing order Cd (ND-2.1), Al (ND−1.8), Pb (ND−0.53) on μg/g dw (Table 4). There is no significant (p < 0.04) difference of contamination of any of these elements at the 9 studied sites. The M-3 extraction procedure in general showed the following decreasing order: Al (9.6–1310) >> As (ND−98) > Hg (0.10–3.6) ≥ Cd (ND−3.5) > Pb (0.24–2.6) ≥ Cr (ND−2.8) on μg/g dw (Tables 4 and 6). Water extraction procedure yielded the following decreasing order Al (0.03–177), Cd (ND−0.43), Pb (ND−0.18), and Cr (ND−0.02) on μg/g dw (Table 4). Mean Al in site 6 showed significantly higher (p < 0.010) concentration than other sites. Because the M-3 extraction method yielded higher element concentrations than NH4OAC and water extraction methods, the data from M-3 was considered for further discussions. When considering M-3 data, in general, there is no significant difference in concentrations between sites, and therefore mean concentrations (average of 45 sediment samples) of Al, Cd, and Pb by different extraction method is illustrated by Fig. 4, because As and Hg were analyzed only by the M-3 method, and Ni was below the detection limit. It is apparent that the M-3 extraction procedure is considered ideal for sediment analysis. The mercury concentration in sediments is a good indication of contamination of an ecosystem, because sediments often behave as a sink of this heavy metal. Although there is no legislation concerning mercury pollution in sediments, a site can be considered contaminated when the mercury content exceeds its natural level (20–100 ng/g). High concentration of Hg (1220–1860 ng/g dw) in sediment is of major concern.
Metals in contaminated sediments may persist and impact upon estuaries for decades. Sediments are important substrates for heavy metal attachment in any aquatic environment (Ackermann et al. 1983; Horowitz 1985; Forstner 1989). Sediments are composed of oxides of Fe and Mn, minerals, terrigenic material, and organic matter. Heavy metals ions accumulate in estuarine sediments as a result of either the deposition of metal-enriched allochthonous particles, or the adsorption of dissolved heavy-metal ions from the water column (Rodrigo 1989). Adsorption of heavy-metal ions on sediment particles is, in general, a function of 2 factors: total surface area per unit weight of sediment, and the adsorption potential of sediment materials. In addition, the degree to which water systems withstand heavy-metal pollution is frequently dependent on the concentration of suspended sediment in the water column. Suspended sediment, particularly clays (<4 μm), act as sponges adsorbing metals directly from the dissolved phase (Deely et al. 1992). Sediment is composed of a combination of lithogenic, authigenic, and biogenic components such as mineral grains, organic matter, Fe and Mn oxides, sulphides, and carbonates. Heavy metals may be attached to any of these phases in proportions that depend on the physicochemical conditions prevailing in the sediment and associated water (Deely et al. 1992). Generally, as grain size decreases, the concentration of metals adsorbed onto sediment component increases, particularly across the transition zone from silt (4–63 μm) to clay (<4 μm). The flat platy structures of clay minerals have high surface areas, surface charges, and cation exchange capacities that readily attract metals and metal-carrying substances (Forstner and Wittmann 1981; Horowitz 1985; Cauwet 1987).
Chromium and Pb analyzed in sediment from the present study were slightly higher than Nigerian sediment (Chindah et al. 2004). On the other hand, several other estuaries in the United States, Brazil, New Zealand, and India had elevated levels (Frazier 1975; Rodrigo 1989; Deely et al. 1992; Baptista et al. 2000; Crapez et al. 2003; Mathew et al. 2003; Elston et al. 2005; Apeti et al. 2005b; Amaraneni 2006). Data on Al in sediment from other areas is not available, and therefore comparisons cannot be made for Al. Nickel in this study was not detected, whereas other studies showed concentrations from 1 to 168 parts per million (Frazier 1975; Rodrigo 1989; Crapez et al. 2003; Mathew et al. 2003; Elston et al. 2005; Amaraneni 2006). Cadmium analyzed in this study was greater than concentrations in Chesapeake Bay, India, Nigeria, Mississippi Bay, and Apalachicola Bay in Florida (Frazier 1975; Mathew et al. 2003; Chindah et al. 2004; Elston et al. 2005; Apeti et al. 2005b; Amaraneni 2006). Mercury and As concentrations in this study were greater than studies from New Zealand, Thousand Islands Florida and Mississippi (Magalhaes et al. 1997; Baptista et al. 2000; Burton 2002; Elston et al. 2005; Kongchum et al. 2006).
Adriano (2001) indicated a normal soil concentration of mercury ranging from 20 to 250 ng/g. Kannan et al. (1998) reported total mercury concentrations in sediments from south Florida estuaries ranging from 1 to 219 ng/g dw. These comparisons show that mercury concentration in Savannah estuary is much higher than Florida coast. Mean arsenic concentrations in sediment (sites 1–6) ranged from 11 to 55 μg/g dw (Table 6). By comparison, Adriano (2001) reported noncontaminated U.S. soils having an average arsenic concentration of 7.4 μg/g, and ocean sediments as having average arsenic concentration of 33.7 μg/g (with a range from <0.40 to 455 μg/g). Arsenic is often a component of pesticides, and the highest sediment concentration found during this study was from site 6, which is the site closest to residential development areas. The higher sediment arsenic concentrations at this site might be related to non-point-source pollution origins of arsenic into Savannah River and its tributaries. However, geographical comparison for Hg and As is not withstanding due to no data for sites 7, 8, and 9.
Heavy Metals in Oyster
Concentrations of heavy metals in oyster were in the following order: Si (241–381) > B (231–254) >> Pb (<1.5–4.0) > As (0.20–3.0) > Cd (<1.5–2.9) > Ni (<1.5–2.5) > Mo (<0.5–0.64) > Hg (0.09–0.91) (Table 5 and 6) μg/g dry wt basis. Except for As and Hg, nonessential elements in oyster tissue were greater than sediments. The results indicate that bioaccumulation of heavy metals in oyster varies depending upon their chemical properties and logK ow efficiency.
International comparison of heavy metals in oyster tissue comprehended that Savannah coastal waters had higher Pb, Al, Cd, Cr, Ni, Hg, and As when compared to Irish Coast UK, Mexico, Brazil, and some U.S. estuaries (Smyth et al. 1995; Bloxham et al. 1998; Frias-Espericueta et al. 1999; Machado and Zalmon 2005; Elston et al. 2005).
Overall, no statistically significant (p < 0.05) results were obtained when comparing trace elements and heavy metals between sites. Only sites 1–5 contained slightly higher trace elements than sites 6–9. Comparison of trace elements between sites should be ignored. Nevertheless, statistically significant (p = 0.01 and p = 0.05) results were obtained when comparing the M-3 extraction to water extraction and M-3 extraction to NH4OAC method. Consequently, the M-3 extraction method should be adopted for sediment analysis.
Tolerable Intake of Trace Elements and Heavy Metals
The World Health Organization (WHO) has established a provisional tolerable weekly intake (PTWI) for Cd at 7 μg/kg of body weight. This PTWI weekly value corresponds to a daily tolerable intake level of 70 μg of Cd per day for the average 70-kg man and 60 μg of Cd per day for the average 60-kg woman. Clearly, the daily Cd intake for the general population from oyster, which is by far the dominant source of Cd, is well below the guidelines established by WHO. The United States Food and Drug Administration (USFDA 1993) set an estimated safe and adequate daily dietary intake for Cr 200 μg/person/day as an allowed consumption level, and for Ni 1200 μg/person/day as an allowed consumption level. Concentrations of Cr and Ni in oyster tissues were <1.5–8.0 μg/g and <1.5–2.5 μg/g on a dw basis, respectively. The observed Cr and Ni values do not have any significant effect on humans. The tolerable daily intake of Pb was 25 μg/kg body weight, which is well below the concentration observed in this study. United States Environmental Protection Agency (USEPA) has established an interim reference dose for methylmercury (MeHg) of 1 × 10−4 μg per kg body weight/day. Although the EPA recommends that the conservative assumption be made that all mercury is present as MeHg, in order to be most protective of human health, the EPA indicates that the typical U.S. consumer, eating less than 10 g of fish and shellfish per day, having average mercury concentrations between 100 and 150 ng/g, may have an adverse impact. Concentrations of Hg in 1 g oyster were between 93 and 908 ng/g, and therefore oyster collected from sites 1, 3, 4, 6, and 7 were above the 150 ng/g limit, and were considered to pose adverse effects. However, with limited samples we cannot derive any final conclusion. Arsenic was detected in all oyster tissue samples to a greater degree than Hg, with a range from 200 to 3042 ng/g dw (Table 6). Based on WHO data, the lethal dose of arsenic trioxide is 10–180 μg, and for arsenide is 70–210 μg, which is much higher than the observed As concentration in oyster tissue analyzed in this study. Further studies are needed to delineate the degree of mercury problems in Savannah coastal waters.
Biota-Sediment Accumulation Factors
Biota-sediment accumulation factors (BSAF) have been proposed as a simple model for predicting the bioaccumulation of sediment-associated contaminants by infaunal invertebrates (Kannan 1999). BSAF can be estimated based on the dry weight normalized concentrations of contaminants in oyster divided by dry weight concentrations of contaminants in sediment, basically using BSAF = Oyster dw/Sediment dw formula. This simple construct of contaminant partitioning in infaunal organisms–sediment systems was based on the assumption that no kinetic or structural barriers to the establishment of equilibrium are present. BSAFs varied depending on the species (both chemical and biological), sediment organic carbon, and contaminant concentrations, which suggested the need for site-specific evaluation of BSAFs.
Based on the sediment data from the M−3 extraction procedure, BASF calculation can be made for elements such as P, Ca, Mg, Zn, Mn, Fe, and Al (for 9 sampling locations) and for As and Hg (for 6 sampling locations). The BSAF were in the range of 89–1891 (P), 1.5–37 (Ca), 1.1–8.8 (Mg), 185–639 (Zn), 0.3–1.9 (Mn), 0.4–2.4 (Fe), 0.7–86 (Al), 0.004–0.02 (As), and 0.007–0.07 (Hg), respectively. Greater bioaccumulation potential of P and Zn by oyster deserves a specific interpretation. These results imply specific accumulation of zinc in oyster in the Savannah estuarine ecosystem. On the other hand, lower BSAF of toxic elements such as Al, As, and Hg were noticed. Recommended Dietary Allowance (RDA) for Zn is 15 mg a day for men (15 mg/day); 12 mg/day for women; 10 mg/day for children; and 5 mg/day for infants. Based on this information, consumption of oyster from Savannah coastal waters may present less than RDA levels. MLT for P from oyster is 4000 μg/day (Dietary Reference Intake “DRI” 1997). Concentrations of P in oysters in sites 2, 4, 5, 7, 8, and 9 were >4000 mg/g, and thus consumption of even 1 g of oyster may lead to high accumulation of P in humans.
Although BSAF was found to be >1 for all elements, correlation of sediment to oyster showed nonsignificant correlation (Fig. 5) for 3 essential and 3 nonessential elements. In particular, Mn, Fe, and Al showed negative correlation with r2 values of 0.0716, 0.0819, and 0.2179, respectively, while Hg, Zn, and As showed positive but very weak correlation of 0.3061, 0.0572, and 0.0003, respectively. Because it was already discussed that oysters may intake elements and heavy metals mainly by water column rather than sediment. Consequently, studies should be conducted with waters collected from several areas of Savannah coast along with sediment.
Conclusions
Water quality parameters from all sampling locations were according to proposed directives for estuarine water. Sediment pH in sediments was similar to that of several estuarine ecosystems. Among the three extraction procedures, the Mehlich−3 (Mehlich 1984) extraction procedure should be adopted for future sediment extraction. Concentrations of trace elements and heavy metals (except Hg and As) were lower in sediment. Higher concentrations of Al, As, Cd, Cr, Pb, and Zn in oyster tissue than sediment suggests their efficient bioaccumulation characteristics. Greater bioaccumulation of Zn and P in oyster especially has been noticed. The bioaccumulation of elements and metals comes mainly from water rather than the sediments, which we found weakly correlated. International and geographical comparison of concentrations was either comparable, lower, or higher for sediments and oyster. Intake of oyster was found to be unsafe as far as mercury and phosphorus concentrations and guidelines set by WHO and DRI, respectively. Continued monitoring is needed at the Savannah River because of rapid development of industries in this river basin and dumping of all domestic sewage and industrial effluents.
References
Ackermann F, Bergmann H, Schliechert U (1983) Monitoring of heavy metals in coastal and estuarine sediments-a question of grain size: <20 μm versus <60 μm. Environ Sci Technol Lett 4:317–328
Adriano DC (2001) Trace elements in terrestrial environments: Biogeochemistry, bioavailability, and risk of metals, second edition. Springer-Verlag, New York
Akagi H, Nishimura H (1991) Speciation of mercury in the environment. In: Suzuki T, Imura N, Clarkson TW (eds) Speciation of mercury in the environment. Plenum Press, New York, pp 53–76
Alquezar R, Markich SJ, Booth DJ (2006) Metal accumulation in the smooth toadfish, Tetractenos glaber, in estuaries around Sydney, Australia. Environ Pollut 142:123–131
Amaraneni SR (2006) Distribution of pesticides, PAHs and heavy metals in prawn ponds near Kolleru lake wetland, India. Env Int 32:294–302
Apeti DA, Johnson E, Robinson L (2005a) A model for bioaccumulation of metals in Crassostrea virginica from Apalachicola Bay, Florida. Am J Environ Sci 1:239–248
Apeti DA, Robinson L, Johnson E (2005b) Relationship between heavy metal concentrations in the American oyster (Crassostrea virginica) and metal levels in the water column and sediment in Apalachicola Bay, Florida. Am J Environ Sci 1:179–186
Ashraf MP, Edwin L, Meenakumari B (2006) Studies on the seasonal changes of phosphorus in the marine environment off Cochin. Env Int 32:159–164
Baptista NJA, Smith BJ, McAlister JJ (2000) Heavy metal concentrations in surface sediments in a nearshore environment, Jurujuba Sound, SE Brazil. Environ Pollut 109:1–9
Bloxham M, Rowe A, McGovern E, Smyth M, Nixon E (1998) Trace metal and chlorinated hydrocarbon concentrations in shellfish and finfish from Irish waters—1996. Marine environmental series 2/98, fishery leaflet 179, Marine Institute: 1–18
Burton M (2002) Copper and mercury in an oyster reef system, Ten Thousand Islands, Southwest Florida. http://www.216.109.125.130/search/cache?ei=UTF-8&fr=slv8-yie7&p=BURTON+MARGO++Geology+Dept&u=keck.wooster.edu/archives/symposium/02/Florida01_pdfs/burtonabs.pdf&w=burton+margo+geology+dept&d=fRUmFBIeOSGi&icp=1&.intl=us
Cauwet G (1987) Influence of sedimentological features on the distribution of trace metals in marine sediments. Marine Chemistry 22:221–234
Chindah AC, Braide AS, Sibeudu OC (2004) Distribution of hydrocarbons and heavy metals in sediment and crustacean (Shrimps-Penaeus notialis) from the Bonny/New Calabar River Estuary, Niger Delta. AJEAM-RAGEE 9:1–17
Crapez M, Neto JAB, Bispo MGS (2003) Bacterial enzymatic activity and bioavailability of heavy metals in sediments from Boa Viagem Beach (Guanabara Bay). Anuario Inst Geociencias UFRJ 26:60–68
Day JW, Hall CAS, Kemp WM, Alejandro YA (1989) Estuarine ecology. John Wiley & Sons, New York, 558 p
Deely JM, Tunnicliff JC, Orange CJ, Edgerley WHL (1992) Heavy metals in surface sediments of Waiwhetu Stream, Lower Hutt, New Zealand. N Z J Marine Freshwater Res 26:417–427
De Groot AJ, Salmons W, Allersma E (1976) Processes affecting heavy metals in estuarine sediments. In: Burton JD, Liss PS (eds). Estuarine chemistry. Academic Press, London, pp 131–157
DRI (1997) http://www.en.wikipedia.org/wiki/Dietary_Reference_Intake
Elston R, Cake EW Jr, Humphrey K, Isphording WC, Rensel JE (2005) Dioxin and heavy-metal contamination of shellfish and sediments in St. Louis Bay, Mississippi and adjacent marine waters. J Shellfish Res 24:227–241
Forstner U (1989) Contaminated sediments: Lectures on environmental aspects of particle-associated chemicals in aquatic systems. Springer-Verlag, Berlin, Heidelberg, New York, 157 p
Forstner U, Wittmann GTW (1981) Metal pollution in the aquatic environment. 2nd edition. Springer-Verlag, Berlin, Heidelberg, New York, p 486
Frazier JM (1975) The dynamics of metals in the American oyster, Crassostrea virginica. I. Seasonal effects. Chesapeake Sci 16:162–171
Frias-Espericueta MG, Ortiz-Arellano MA, Osuna-Lopez JI, Ronson-Paulin YJA (1999) Heavy metals in the rock oyster Crassostrea iridescens (Filibranchia: Ostreidae) from Mazatlan, Sinaloa, Mexico. Rev Biol Trop 47:843–849
Goldberg ED, Koide M, Holdge V, Flegal AR, Martin J (1983) U.S. mussel watch: 1977–1978 results on trace metals and radionuclides. Estuarine Coastal Shellfish Sci 16:69–93
Haye JM, Santschi PH, Roberts KA, Ray S (2006) Protective role of alginic acid against metal uptake by American oyster (Crassostrea virginica). Environ Chem 3:172–183
Honda K, Tatsukawa R, Fujiyama T (1982) Distribution characteristics of heavy metals in the organs and tissues of striped dolphin, Stenella coeruleoalba. Agric Biol Chem 46:3011–3021
Horowitz AJ (1985) A primer on trace element-sediment chemistry. US Geological Survey Water Supply Paper 2277, 67 p
Ichihashi H, Nakamura Y, Kannan K, Tsumura A, Yamasaki S (2001) Multi-elemental concentrations in tissues of Japanese common squid (Todarodes pacificus). Arch Environ Contam Toxicol 41:483–490
Ikemoto T, Kunito T, Watanbe I, Yasunaga G, Baba N, Miyazaki N, Petrov EA, Tanabe S (2004) Comparison of trace element accumulation in Baikal seals (Pusa sibirica), Caspian seals (Pusa caspica) and northern fur seals (Callorhinus ursinus). Environ Pollut 127:83–97
Jayaprakash M, Srinivasalu S, Jonathan MP, Ram Mohan V (2005) A baseline study of physico-chemical parameters and trace metals in waters of Ennore Creek, Chennai, India. Mar Pollut Bull 50:583–608
Kannan K (1999) Clam-sediment accumulation factors for polychlorinated biphenyl congeners at a contaminated estuarine marsh. Toxicol Environ Chem 68:159–167
Kannan K, Sinha RK, Tanabe S, Ichihashi H, Tatsukawa R (1993) Heavy metals and organochlorine residues in Ganges River dolphins from India. Mar Pollut Bull 26:159–162
Kannan K, Smith RG Jr, Lee RF, Windom HL, Heitmuller PT, Macauley JM, Summers JK (1998) Distribution of total mercury and methyl mercury in water, sediment, and fish from south Florida estuaries. Arch Environ Contam Toxicol 34:109–118
Kannan K, Yasunaga Y, Iwata H, Ichihashi H, Tanabe S, Tatsukawa R (1995) Concentrations of heavy metals, organochlorines, and organotins in horseshoe crab, Tachypleus tridentatus, from Japanese coastal waters. Arch Environ Contam Toxicol 28:40–47
Karadede H, Oymak SA, Unlu E (2004) Heavy metals in mullet, Liza abu, and catfish, Silurus triostegus, from the Ataturk Dam Lake (Euphrates), Turkey. Env Int 30:183–188
Kongchum M, Devai I, DeLaune RD, Jugsujinda A (2006) Total mercury and methylmercury in freshwater and salt marsh soils of the Mississippi river deltaic plain. Chemosphere 63:1300–1303
Kuppusamy MR, Giridhar VV (2006) Factor analysis of water quality characteristics including trace metal speciation in the coastal environmental system of Chennai Ennore. Env Int 32:174–179
Machado FAG, Zalmon IR (2005) Temporal and spatial variation on heavy metal concentrations in the oyster Ostrea equestris on the Northern Coast of Rio De Janeiro State, Brazil. Brazil J Biol 65:67–76
Magalhaes CC, Krug FJ, Fostier AH, Berndt H (1997) Dietary determination of mercury in sediments by atomic adsorption spectrometry. J Anal Atomic Spectrometry 12:1231–1234
Mathew M, Mohanraj R, Azeez PA, Pattabhi S (2003) Speciation of heavy metals in bed sediments of wetlands in Urban Coimbatore, India. Bull Environ Contam Toxicol 70:800–808
McLean GW (1982) Hydrogen-ion activity. In: Page AL et al. (ed.) Methods of soil analysis. Part II, 2nd ed. Agronomy 9:159–164
Mehlich A (1984) Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Comm Soil Sci Plant Ana 15:1409–1416
Moss A, Constanza S (1998) Levels of heavy metals in the sediments of Queensland rivers, estuaries and coastal waters. Environmental technical report no. 20- ISSN 1037-4671, Queensland Government, Australia
Peakall D, Burger J (2003) Methodologies for assessing exposure to metals: speciation, bioavailability of metals and ecological host factors. Ecotoxicol Environ Safety 56:110–121
Phelps HL, Mihursky JA (1986) Oyster (Crassostrea virginica, Gmelin) spat settlement and copper in aufwuchs. Estuaries 9:127–132
Rodrigo AG (1989) Surfacial sediment-heavy metal associations in the Avon-Heathcote Estuary, New Zealand. N Z J Marine Freshwater Res 23:255–262
Sajwan KS, Senthil Kumar K, Suresh N, Fowler A, Richardson JP, Loganathan BG (2007) Persistent -organochlorine pesticides, -polychlorinated biphenyls and -polybrominated diphenyl ethers in fish from coastal waters off Savannah, Georgia, USA. Toxicol Env Chem (DOI: 10.1080/02772240701270047)
Sakai H, Saeki K, Ichihashi H, Kamezaki N, Tanabe S, Tatsukawa R (2000) Growth-related changes in heavy metal accumulation in green turtle (Chelonia mydas) from Yaeyama Islands, Okinawa, Japan. Arch Environ Contam Toxicol 39:378–385
Sankar TV, Zynudheen AA, Anandan R, Viswanathan Nair PG (2006) Distribution of organochlorine pesticides and heavy metal residues in fish and shellfish from Calicut region, Kerela, India. Chemosphere 65:583–590
Senthil Kumar K, Sajwan KS, Richardson J (2007) A baseline monitoring of heavy metals, organochlorine pesticides, polycyclic aromatic hydrocarbons and alkylphenols in sediment and oyster collected from marsh/estuarine Savannah, GA. Mar Pollut Bull (in press)
Singh BR, Steinnes E (1994) Soil and water contamination by heavy metals. In: Lal R, Stewart BA (eds) Soil processes and water quality. Lewis, Boca Raton, Florida, pp 233–272
Smyth M, Rowe A, McGovern E, Nixon E (1995) Monitoring of shellfish growing areas—1995. Marine environmental series 1/97, fishery leaflet 174, Marine Institute, pp 1–11
Storelli MM, Storelli A, D’Addabbo R, Marano C, Bruno R, Marcotrigiano GO (2005) Trace elements in loggerhead turtles (Caretta caretta) from the eastern Mediterranean Sea. Overview and evaluation. Environ Pollut 135:163–170
Thomas GW (1982) Exchangeable cations: In: Page AL (ed) Methods of soil analysis, part 2, 2nd ed. Agronomy 9:159–164
USFDA (1993) Guidance document for arsenic, chromium, nickel in shellfish. Center for Food Safety and Applied Nutrition. United States Food and Drug Administration, Washington, DC
Acknowledgments
Part of this study was financially supported by the U.S. National Park Service, Department of Interior (grant/order number P5420050005). Part of this research was performed under the auspices of contract number DE-FG09-96SR18558, United States Department of Energy and Environmental Protection Agency. We wish to thank the management and staff of Bull River Marina for allowing us to use space at their marina for overnight boat docking and for allowing us to load and unload equipment and personnel during the field sampling period. We thank Captain Jay Rosenzweig, Savannah State University, for his cooperation and skill during boat sampling trips.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sajwan, K.S., Kumar, K.S., Paramasivam, S. et al. Elemental Status in Sediment and American Oyster Collected from Savannah Marsh/Estuarine Ecosystem: A Preliminary Assessment. Arch Environ Contam Toxicol 54, 245–258 (2008). https://doi.org/10.1007/s00244-007-9033-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-007-9033-1