Abstract
The dynamic nature of the annual volume of water discharged down the Carson River over a 10-year period, which included a century flood and drought, was examined in order to gain a better understanding of mercury movement, biological availability, and exposure to waterbirds nesting at Lahontan Reservoir. Total annual water discharge directly influenced total mercury (THg) in unfiltered water above the reservoir and downstream of a mining area, whereas methyl mercury (MeHg) at the same site was negatively related to annual discharge. Annual water storage at Lahontan Reservoir in the spring and early summer, as expected, was directly related to annual Carson River discharge. In contrast to the findings from above the reservoir, annual MeHg concentrations in water sampled below the reservoir were positively correlated with the total discharge and the amount of water stored in the reservoir on 1 July; that is, the reservoir is an important location for mercury methylation, which agrees with earlier findings. However, unfiltered water MeHg concentrations were about 10-fold higher above than below the reservoir, which indicated that much MeHg that entered as well as that produced in the reservoir settled out in the reservoir. Avian exposure to mercury at Lahontan Reservoir was evaluated in both eggs and blood of young snowy egrets (Egretta thula) and black-crowned night-herons (Nycticorax nycticorax). Annual MeHg concentrations in unfiltered water below the reservoir, during the time period (Julian Days 90–190) when birds were present, correlated significantly with mercury concentrations in night-heron blood (r 2 = 0.461, p = 0.027), snowy egret blood (r 2 = 0.474, p = 0.024), and night-heron eggs (r 2 = 0.447, p = 0.029), but not snowy egret eggs. A possible reason for lack of an MeHg water correlation with snowy egret eggs is discussed and relates to potential exposure differences associated with the food habits of both species. THg concentrations in water collected below the reservoir were not related to egg or blood mercury concentrations for either species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
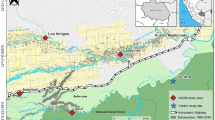
Anthropogenic mercury (Hg) has contaminated the Lower Carson River System (LCRS) in western Nevada from the Comstock mining area above Dayton to the river’s terminus in a network of wetlands about 120–128 km downstream (Fig. 1; Hoffman and Thomas 2000; Van Denburgh 1973). During the Comstock Lode era of 1859–1890, an estimated 6.8 × 166 kg of liquid mercury (Hg°) were released in mill tailings from gold and silver mining into the Carson River and its tributary canyons (Smith 1943). Thus, for more than 140 years, Hg-laden tailings have been washed into the floodplains of the LCRS, resulting in some of the highest Hg concentrations ever reported in a natural system (Wayne et al. 1996).
In 1915 the extent and degree of Hg deposition in the floodplains of the LCRS was modified with the construction of Lahontan Reservoir. The resultant serpentine-shaped 27-km-long irrigation reservoir became a sink for most of the sediment-bound Hg that washed downstream. For example, during a major winter flood in 1996–1997, about 90% of the 4.9 × 1011 kg of sediment and about 80% of the 4.5 × 103 kg of Hg that washed into the reservoir were retained (Hoffman and Taylor 1998). The sink effect of Lahontan Reservoir spared the agricultural and wetlands downstream from much additional Hg loading over time, but rendered the reservoir and piscivorus gamefish excessively contaminated. In 1990, the US Environmental Protection Agency (EPA) placed much of the Carson River Basin from Dayton through Lahontan Reservoir on the EPA’s National Priorities List (i.e., Superfund) for research and cleanup.
The initial phase of this study was a 2-year evaluation of the effects of Hg on fish-eating birds nesting in the LCRS at Lahontan Reservoir and Carson Lake and at reference areas in northeastern Nevada (Henny et al. 2002). In the first year (1997), reproduction of snowy egrets (Egretta thula) and black-crowned night-herons (Nycticorax nycticorax) was studied via the “sample egg” technique (Blus 1984) (i.e., evaluate reproductive success of each clutch in relationship to the total Hg [THg] concentrations in the sample egg collected from that clutch). Both species laid eggs with comparatively low THg concentrations (Henny et al. 2002). Most eggs had THg concentrations below 0.80 μg/g wet weight (ww), the putative threshold concentration at which reproductive problems might be expected (Heinz 1979; Newton and Haas 1988).
Snowy egret and night-heron nestlings were collected at about 2 weeks of age in 1997 for residue and physiological analyses, with studies in 1998 expanded to include young just prior to flight and adults. The main findings in 1997–1998 with respect to the birds collected were detection of putative cellular toxicity (i.e., histopathologic and physiologic) in the nervous, immune, hepatic, and renal systems of young night-herons and snowy egrets at about 2–6 weeks of age (Henny et al. 2000, 2002). In contrast, adults with much higher concentrations of THg in the blood, brain, liver, and kidneys did not develop injury to the extent noted in the young. This difference is probably due to maturation of key physiologic systems and the ability of adults to demethylate the organic Hg and sequester resultant inorganic mercury (IoHg).
Clearly, the 1997–1998 studies indicated potential Hg toxicity to young piscivorus birds of the LCRS, although no overt clinical signs were noted in either nestlings or older young moving among the branches prior to dispersal from the natal area. To further investigate Hg toxicity to young snowy egrets and night-herons, a limited version of the 1997–1998 study was continued in 1999 and 2000. Emphasis was placed on Hg residues in eggs and blood of young, reproductive success, and observations of young for evidence of overt toxicity. These latter studies were focused on Gull Island in Lahontan Reservoir, the primary sink for Hg from the Comstock mining area (Fig. 1). Considering 4 years (1997–2000) of observing young piscivorus birds at Lahontan Reservoir through the time of dispersal without noting classic evidence of Hg toxicity, it appeared that the substantial Hg exposure encountered while at the colony did not result in obvious behavioral problems despite cellular lesions in critical physiologic systems (Henny et al. 2002).
Several critical questions were raised: (1) How long would young continue to forage in the Hg-contaminated LCRS? (2) What additional effect would continued postdispersal Hg exposure (∼50 days) have on cellular lesions in the nervous, immune, and detoxicating systems? (3) Would putative immune deficiencies and neurological impairment of young affect survivability during the postdispersal period from the colony? To address these questions, which are the subjects of individual reports in this series, research was continued through 2006 for a total of 10 reproductive cycles. The plan was to continue the multispecies studies of reproduction and toxicity per 1997–1998 (Henny et al. 2002) but also expand the study via radiotelemetry to document movements and survival of young snowy egrets after their dispersal from the natal colonies at Lahontan Reservoir through fall departure from the LCRS. Although night-herons were continuously studied, the emphasis was on the snowy egret for telemetry studies because of its near total diet of aquatic invertebrates and fish and the species’ propensity to probe sediments for invertebrates.
This article evaluates the influence of annual water conditions on Hg exposure to snowy egrets and night-herons in the very dynamic Great Basin, specifically at Lahontan Reservoir in the LCRS from 1997 through 2006. Factors that might have influenced the nesting waterbird populations included: (1) annual changes in water discharge on the Carson River (Fig. 2) and resultant monthly water storage levels at Lahontan Reservoir (Fig. 3) (i.e., drought effect) and (2) annual changes in THg and methyl mercury (MeHg) concentrations in water and the resultant annual exposure to birds as measured in eggs and blood of young (i.e., Hg effect). The study began in the spring following a “Century Flood” on the Carson River during the winter of 1996–1997 and then followed the dynamic nature of the LCRS through drought, but then a return to years with more water. When drought prevailed over northern Nevada during several middle years of the study, it severely reduced wetlands distribution and quality in the LCRS. Because demethylation and sequestration processes appeared to reduce the amount of MeHg redistributed by adult birds to their eggs (Henny et al. 2002), we also compared whole-blood concentrations of young with egg concentrations to evaluate which sampling procedure better reflected annual water concentrations and Hg exposure to birds.
This article also provides a framework for subsequent reports in this series including (1) drought and Hg effects on snowy egret and night-heron reproduction, (2) Hg effects on the health (toxicology and pathology) of young snowy egrets and night-herons, (3) dispersal patterns of young snowy egrets from their natal area and length of time Hg exposure continues, and (4) survival of young to the time of their first migration. In all cases, findings from the LCRS are compared to findings from a reference area in eastern Nevada.
Methods and Techniques
Study Sites
Sampling, per 1997–1998 (Henny et al. 2002) was restricted to the Gull Island and Evans Island nesting colonies in the northeastern portion of Lahontan Reservoir, western Churchill County, NV, and to the nesting colony at our reference site about 16 km east of Elko, NV on the Humboldt River in central Elko County, NV.
Lahontan Reservoir
Snowy egrets and night-herons nested primarily in the main stand of saltcedar (tamarisk; Tamarix sp.) on the eastern perimeter of Gull Island just above the high-water mark when the reservoir is at capacity. During good water years, the saltcedar was nearly impenetrable and provided protection of eggs and nestlings from avian predators such as ring-billed and California gulls (Larus delawarensis and L. californicus) that nest in large, dense colonies on the open ground. However, during drought years, the foliage in the main saltcedar stand was conspicuously less dense. The sparse foliage left many nests exposed to midday sun, high winds, and possible avian predation. Small younger clusters of more heavily foliated saltcedar flourished along the southern and southwestern perimeter (lower elevation) of Gull Island and were favored for nesting by night-herons during the drought. Snowy egrets continued to nest in the main saltcedar stand in all years.
Evans Island, located about 0.4 km to the northeast is similar to Gull Island, but is much closer to the mainland (∼0.26 vs. 1.10 km) off Horseman’s Point. Per Gull Island, much of the open ground on Evans Island was regularly utilized by nesting gulls throughout the study. Night-herons nested in the perimeter saltcedar strands on Evans Island during 2001–2003 and in 2004 and 2006, but not in 2005. Snowy egrets did not nest on Evans Island.
Neither Gull Island nor Evans Island is well vegetated around their perimeters due to extreme annual and temporal fluctuations in the water level of this irrigation reservoir (Fig. 3). Vegetated shallows around the islands that would normally harbor prey for wading birds are generally absent by the time the young snowy egrets and night-herons can leave their nests in search of food. Therefore, both species, but especially the later hatched snowy egrets, are entirely dependent on their parents for food while on the island. Double-crested cormorants, great blue herons (Ardea herodias), and great egrets (Ardea alba) nest in the upper branches of partially dead cottonwood (Populus fremontii) trees on the eastern perimeter of the island.
Humboldt River-Ryndon
The nesting colony on the Humboldt River is located in a dense stand of willows (Salix sp.) within the floodplain and is separated from the river by a densely vegetated sandbar. The colony (composed of great, snowy, and cattle egrets [Bubulcus ibis] and night-herons) was mostly located over water in years of above normal precipitation. As the river flow decreased at about the time of dispersal for most of the birds, only about one-third of the nests remained over water. In 2003–2004, precipitation was less than normal and nearly all nests were over dry ground at heights of 2–3 m.
Water Conditions and Sampling
Water conditions were evaluated for the annual flow of the Carson River into Lahontan Reservoir and the reservoir’s storage on 1 April, 1 May, 1 June, and 1 July. These dates coincide with egg and bird sampling and precede widely variable drawdown for irrigation. River flow was measured at the Fort Churchill gaging station (USGS Gaging Station #10312020) ∼13 km upstream of Lahontan Reservoir. Storage was based on the reservoir’s surface elevation (USGS Water Resources Discipline, Carson City, NV).
Water was sampled for unfiltered THg and MeHg concentration at Weeks Bridge just downstream of the Fort Churchill gaging station. The general scheme was to collect two water samples prior to Julian Day 90 (early), three to eight samples between Julian Days 90 and 190 (middle), and two to three samples after Julian Day 190 (late). Each sample was a composite taken from several verticals and thoroughly mixed prior to subsampling and transport at 4°C within 24 h for chemical analysis. Details of sampling procedures are described by Hoffman and Thomas (2000). Another water sampling station was 1.76 km below Lahontan Dam (USGS Gaging Station #10312150).
Egg Sampling
Lahontan Reservoir (Gull Island and Evans Island) was surveyed for nests of night-herons and snowy egrets from the beginning of nesting through the time the young began to leave their nest at about 2–3 weeks of age. Night-heron and snowy egret nests were identified, evidence of incubation and clutch size was recorded, and a randomly selected egg was removed from each of the first 10 (2002–2006) or 15 (1999–2001) nests encountered with a minimum three-egg clutch. The sample size was reduced after 2001 when 10 eggs were determined adequate and representative of the colony. When an egg was collected, it was inscribed with a No. 2 pencil for identification and placed in a protective egg carton for transport at ambient temperature to the laboratory. Eggs were refrigerated pending further processing for THg analysis. Using the same techniques, eggs of each species were sampled for THg analysis at the reference site on the Humboldt River.
Eggs were weighed and volume determined (by water displacement). Each egg was opened at the equator, and the contents transferred to a chemically clean jar and weighed. Samples were then placed in a standard freezer until they were analyzed.
Bird Sampling
Sampling per 1998 was to evaluate Hg residues in blood of night-herons and snowy egrets at about the age they began to wander from the nest (2–3 weeks). The goal was to sample a minimum of 10 birds of each species per year at Gull Island and the reference area. The general criterion for specimen selection for blood samples was that either bill length or body mass would exceed 5.5 cm or 600 g for night-herons and 4.0 cm or 250 g for egrets. The basic procedure was to capture the young on the nest or nearby, measure its bill length to estimate its age in days (Custer and Peterson 1991), check its weight, compare results to size criterion, and either return the bird immediately to its nest if it did not meet criteria or draw a blood sample and then return it to its nest. Rarely, slightly smaller birds were bled to assure adequate sample size; blood THg reflects contemporary dietary exposure. Blood samples (∼2 mL) were drawn by jugular venipuncture (23-G needle) into a 4.5-mL lithium-heparinized bead monovette syringe. The sample was capped, gently inverted several times for mixing with heparinized beads, and immediately stored on wet ice. Chilled blood samples were thoroughly mixed on an electric rocker for 5 min and then pipetted into a 2.5-mL cryotube (∼0.5 mL) for THg residue analysis. Whole blood was preserved as soon as possible in a standard freezer until analyzed.
Analytical Chemistry
All samples were analyzed by laboratories under contract to the EPA. Hg tissue analyses for years 1997 and 1998 were reported in Henny et al. (2002). Because eggs and blood contain essentially 100% MeHg (Henny et al. 2002), they were only analyzed for THg, but values actually represent MeHg. Blood and egg samples were analyzed for THg at ToxScan Inc. (Watsonville, CA) using EPA methods 245.7 (1999, 2001, 2004, 2005) and 7471 (2000, 2002, 2003) of the EPA 6000/7000 series methods, with solids determination done using EPA method 160.3. Blood and eggs in 2006 were analyzed for THg at EPA Region 9 Laboratory (Richmond, CA) using EPA methods 245.1 and 7473/SOP535. All Hg concentrations are reported in micrograms per gram (ww) except as noted. Egg residue concentrations were adjusted to fresh wet weight based on egg volume (Stickel et al. 1973). Quality assurance and quality control were acceptable at each of the analytical laboratories according to EPA guidelines. Samples of double-distilled deionized water used for the final rinse of surgical instruments and to check syringes and needles were analyzed each year for Hg contamination. The detection limits for THg in blood and eggs ranged from 0.02 to 0.10 μg/g ww, and samples were seldom encountered below the detection limit. One-half the detection limit was used for calculation purposes when nondetections were encountered.
Unfiltered water samples were chemically analyzed for THg and MeHg by Frontier Geosciences (Seattle, WA) according to methods described by Bloom (1989) and Gill and Bruland (1990). Minimum analytical reporting limits for THg and MeHg were 0.04 and 0.02 ng/L. Details of laboratory analyses are reported by Hoffman and Thomas (2000).
Statistical Procedures
Mercury residue concentrations were log-transformed for summarization as geometric means and for statistical analyses. Due to unequal sample sizes, the General Linear Models Procedure (SAS Institute 1999) was used for analysis of variance. Tukey’s Studentized range test (α = 0.05) was used to separate means. Regression analysis was used to define Hg relationships associated with egg and blood concentrations and water concentrations during the multiyear study. An adjusted r 2 was used (but shown as r 2 in the text), which is the squared correlation coefficient corrected for the number of independent variables in the equation.
Results
Annual Water Discharge and Mercury Concentrations in the Carson River, 1997–2006
Increased flow on the Carson River, especially in high-discharge years, increased the concentration of Hg (mostly particle-adsorbed IoHg) in the water column because of bank erosion, which included historic tailings from milling operations at many locations along the river (Hoffman and Taylor 1998; Hoffman and Thomas 2000). We therefore theorized that the annual changes in flow of this dynamic riverine system might provide a practical indication of annual Hg exposure in waterbirds of the LCRS and perhaps serve as an indicator of potential hazard to such species (as previously shown during high-flow years of 1997–1998; Henny et al. 2002).
To test this concept, the annual water discharge for the Carson River at Fort Churchill was first compared to mean unfiltered THg and MeHg concentrations immediately below Fort Churchill at Weeks Bridge, downstream of the mining area but above Lahontan Reservoir (Table 1). Water samples were collected annually prior to Julian Day 90 (< 1 April, early) between Julian Day 90 and 190 (1 April–9 July, middle), and after Julian day 190 (> 9 July, late). These early, middle, and late water THg and MeHg concentrations were regressed against total annual water discharge. THg water concentrations at Weeks Bridge for the middle water sampling period, which represents the time period with the largest annual water sample dataset, were positively related to total discharge (Fig. 4A), whereas the MeHg water concentrations were negatively related to total discharge during the same sampling period (Fig. 4B) and the late sampling period (Y = 393.31 − 109.55X, r 2 = 0.463, p = 0.038). It is of interest that the relationship between THg in water during the middle sampling period at Weeks Bridge was improved when the annual combined monthly water discharge for April–July was used instead of the total annual water discharge (Y = −137.8 + 0.281X, r 2 = 0.918, p = 0.0001). No similar improvement was noted for MeHg in water when April–July water discharge was used.
The total discharge from the Carson River obviously relates to the amount of water stored in Lahontan Reservoir. Water stored in Lahontan Reservoir on 1 July follows a strong exponential function with a rise to its maximum storage capacity in relation to water discharged down the Carson River (Fig. 5). Thus, we also compared THg and MeHg water concentrations (early, middle, and late) at Weeks Bridge (above reservoir) with acre feet of water stored at Lahontan Reservoir on 1 July. The acre feet (at Lahontan Reservoir) relationships with THg and MeHg were similar to total discharge findings: THg (early), not significant; THg (middle), Y = 147,426 + 30.45X, r 2 = 0.819, p = 0.0012; THg (late), not significant; and, similarly, MeHg (early) not significant; MeHg (middle) Y = 362,345 – 38,730X, r 2 = 0.574, p = 0.018; MeHg (late) Y = 428,804 − 77,596X, r 2 = 0.028.
In summary, the higher the annual discharge or the more water at Lahontan Reservoir on 1 July, the higher the THg concentrations (a positive relationship) in the water at Weeks Bridge. In contrast, in years with higher discharges or more water in Lahontan Reservoir on 1 July, the MeHg concentration in the water at Weeks Bridge was lower (a negative relationship) based on both the middle sampling period (i.e., time when most water samples collected) and the late sampling period. Thus, higher water discharge does not have an immediate positive effect on MeHg availability in upstream waters like at Weeks Bridge.
Of special interest, because MeHg readily moves through the food chain to waterbirds, MeHg in unfiltered water immediately downstream of Lahontan Reservoir and the nesting colony on Gull Island (Table 2) was evaluated in relation to annual water discharge of the Carson River and acre feet of water stored in the reservoir on 1 July. Both total water discharge and acre feet of water in the reservoir on 1 July were compared to MeHg in water below the reservoir (Fig. 6). In contrast to the negative relationships between MeHg concentrations in water at Weeks Bridge above the reservoir and water discharge or acre feet of water in the reservoir, MeHg in water sampled below the reservoir was positively correlated with both total discharge and acre feet of water in the reservoir (Fig. 6); however, the MeHg concentrations were reduced about 10-fold in water leaving the reservoir compared to water entering the reservoir (Table 1 and 2).
Mercury in Water and Mercury in Bird Eggs and Blood, 1997–2006
With MeHg in unfiltered water collected below Lahontan Reservoir strongly associated with annual discharge of the Carson River and acre feet of water in Lahontan Reservoir on 1 July each year (Fig. 6), it became important to determine if Hg concentrations in water were related to the annual Hg exposure of snowy egrets and night-herons. We measured exposure by analyzing eggs laid at the reservoir and blood from young birds produced there. Mean annual concentrations of MeHg in eggs (measured as THg) collected at Lahontan Reservoir for both snowy egrets and night-herons (Table 3) showed generally similar year-to-year variability (coefficient of variation [CV] = 77.4% and 55.2%) when compared to MeHg in blood samples (measured as THg) from the same species (CV = 60.2% and 79.8%) at the same location (Table 4). Only a few years for both species showed egg concentrations with significant differences (Table 3), whereas blood samples, especially for snowy egrets, showed more years significantly different, especially the lower concentrations in 2000–2002 (Table 4).
The percentage of the eggs sampled with THg ≥0.80 μg/g (the putative threshold of reproductive toxicity) is also presented. During the years 1997–2006, 27 of 113 snowy egret eggs (23.9%) from Lahontan Reservoir contained THg concentrations ≥0.80 μg/g, whereas at the reference area, only 1 of 58 (1.7%) snowy egret eggs contained ≥0.80 μg/g (Table 3). Night-heron eggs from the Lahontan Reservoir and the reference area showed a similar pattern with 20 of 122 (16.4%) and 0 of 28 (0%) containing THg ≥0.80 μg/g.
In addition to reviewing annual geometric mean concentrations of Hg in blood from these young birds, we compared the percentage of samples equal to or above a given concentration. We chose to use 2.00 μg/g (an arbitrary value one-half the 4.00 μg/g classified as extrahigh-risk for adult loons [Evers et al. 2004]); although it should not be construed as an effect level, it is mainly used to illustrate distributions (Table 4). Overall, 88 of 142 (62.0%) snowy egret blood samples collected between 1997 and 2006 at Lahontan Reservoir contained ≥2.00 μg/g THg. The percentage of samples with ≥2.00 μg/g THg varied annually from 100% in 1997, 1998, 2003, and 2005 to lows of 13% in 2000, 0% in 2001, and 27% in 2002. None of the 67 snowy egret blood samples from the reference site on the Humboldt River contained THg concentrations ≥2.00 μg/g.
Young night-herons were also bled at Lahontan Reservoir between 1997 and 2006 (Table 4). The pattern of annual changes in whole-blood THg concentrations at Lahontan Reservoir for night-herons was similar to the snowy egrets, although fewer years were statistically separable for night-herons compared to egrets. Between 1997 and 2006, 38 of 74 (51.4%) night-heron blood samples collected at Lahontan Reservoir contained ≥2.00 μg/g THg (Table 4). Of 37 blood samples from night-herons collected at the Humboldt River reference area, none had concentrations ≥2.00 μg/g THg. In 1997, an additional four young night-herons bled south of the Humboldt River at Ruby Lake NWR contained THg concentrations (0.72 μg/g), similar to the Humboldt River site in 1997 (0.56 μg/g) (p = 0.45) with none ≥2.00 μg/g.
Annual water samples from Lahontan Reservoir were not collected. Given the above information on THg (actually MeHg) concentrations in eggs and blood samples of birds at Lahontan Reservoir, were these annual concentrations from snowy egrets and night-herons related to MeHg concentrations in unfiltered water collected below the dam when birds were present? Annual MeHg concentrations in unfiltered water from below the reservoir (although lower than expected in the reservoir, see above) during the “early” water sampling period (when birds were present) was significantly and positively related to THg in blood of young night-herons (p = 0.0265) and blood of young snowy egrets (p = 0.0241). It was also related to THg in eggs of night-herons (p = 0.0293) but not eggs of snowy egrets (p = 0.2698) (Table 5, Fig. 7).
Significant relationship between THg in black-crowned night-heron eggs (A) and blood (B) and snowy egret blood (C) collected at Lahontan Reservoir and MeHg concentrations in water below the reservoir, 1997–2006. Note: Blood and egg concentrations analyzed as THg but represent MeHg (see Methods and Techniques section)
Total Hg in unfiltered water below the reservoir during the “early” sampling period was not related to THg in blood or eggs of either species (Table 5). A significant or nearly significant relationship between acre feet of water in Lahontan Reservoir on 1 April (p = 0.0053), 1 May (p = 0.0788), 1 June (p = 0.0056), and 1 July (p = 0.0563) and THg in night-heron eggs (Fig. 8) was of interest and might help explain observed THg concentrations in eggs of night-herons that begin nesting several weeks earlier than snowy egrets. Acre feet of water in the reservoir on 1 April, 1 May, and 1 June was not related to snowy egret blood or egg THg concentrations or to night-heron THg concentrations in blood; however, THg in snowy egret blood was related to acre feet in the reservoir on 1 July (Table 5).
Discussion and Conclusions
The dynamic nature of the LCRS was initially thought to complicate an understanding of Hg exposure to waterbirds and its possible effects. However, the flood-to-drought cycle during this 10-year study provided an opportunity to understand (1) the annual magnitude of Hg movement through the system, (2) how Hg movement related to Carson River discharge, (3) the relationship between water discharge from the Carson River and amount of water stored at Lahontan Reservoir, and (4) annual Hg exposure to waterbirds in relation to Hg in water and amount of water stored at Lahontan Reservoir.
Total Hg in unfiltered water on the Carson River at Weeks Bridge upstream of Lahontan Reservoir, but downstream of the mining and milling areas, clearly showed that in years with higher discharge, more THg was present in the water (Fig. 4A). The annual discharge accounted for 83.1% of the yearly THg variability in water at Weeks Bridge, or 91.8% of the yearly variability if April–July discharge was used instead of total annual discharge. Most of the THg was reported in an IoHg form, which is not readily absorbed by vertebrates. MeHg in the water at Weeks Bridge was negatively related to annual discharge (Fig. 4B). In high-discharge years, the additional water seemed to dilute the limited amount of MeHg in the system at Weeks Bridge above Lahontan Reservoir.
The annual quantity of water stored in Lahontan Reservoir on 1 July followed an exponential rise to storage capacity in relation to water discharged down the Carson River, which accounted for 91.4% of the annual variability (Fig. 5). With the strong correlation between annual water discharge of the Carson River and acre feet of water stored at Lahontan Reservoir on 1 July, both were compared to MeHg concentrations in unfiltered water collected below the dam. In contrast to the water findings upstream at Weeks Station (negative relationship to water discharge), MeHg in water below the reservoir was positively correlated with both total annual discharge and acre feet of water stored in the reservoir on 1 July, accounting for 79.0% and 78.4% of the annual MeHg variability (Fig. 6). Clearly, the reservoir is an important location for Hg methylation, with higher MeHg concentrations in unfiltered water in years when more THg enters the reservoir from upstream locations. However, the MeHg concentrations in unfiltered water below the reservoir are about 10-fold lower that at Weeks Bridge above the reservoir. Thus, in all years, much of the MeHg (and also THg) in water does not pass the dam (see Tables 1 and 2). More specifically, an estimated 3.6 × 103 kg of THg washed downstream during the flood of 1996–1997 and settled out of the water column into Lahontan Reservoir (Hoffman and Taylor 1998). Due to the configuration of the Lahontan Reservoir and sharp changes in the direction of the river flow, a large portion of the contaminated sediments (mostly old mine tailings) were deposited near the head of the reservoir (Marvin-DiPasquale et al. 2000) and most likely throughout the floodplain from the Carson Delta to Silver Springs Bay. This area, and especially the 3–5-km zone from Fisherman’s Point to Silver Springs Bay, represents the main feeding grounds for wading birds at Lahontan Reservoir. The degree of contamination in this part of the reservoir was indicated through residual Hg analysis of unfiltered water samples from the Carson Delta in September 1998 (20 months after the 1996–1997 flood; Hoffman and Thomas 2000). Both THg (9000 ng/L) and MeHg (7.8 ng/L) concentrations were among the highest of all sampling stations along the Carson River between Dayton (the diffuse source of the mine-Hg contamination) and Stillwater (the northeastern terminus of the river). In another 1998 study, the southern delta (Carson Delta) was shown to be an important zone of MeHg production for the Lahontan Reservoir as a whole; both THg and MeHg were more than 20 times higher in the delta at the head of the reservoir than at the dam (Marvin-Di Pasquale et al. 2000). MeHg is of primary concern because it moves through the food chain and is readily accumulated by birds and other vertebrates.
From a biological perspective, it was important to understand whether the MeHg in unfiltered water collected below the reservoir (no annual water data collected in reservoir) was related to night-heron and snowy egret exposure to Hg. Both blood samples from young birds and eggs were collected annually to measure exposure. Demethylation and sequestration processes appeared to reduce the amount of MeHg redistributed by adult birds to their eggs based on the first 2 years of this study (Henny et al. 2002); therefore, we were especially interested to know whether blood or eggs provided the best correlation with MeHg in water. Annual MeHg concentrations in water below the dam (during the time that birds were in the area) were significantly correlated to MeHg (again measured as THg) in eggs and blood of young night-herons and blood of young snowy egrets (Fig. 7), but not eggs of snowy egrets. The water findings also provide insight into why blood samples collected in drought years, especially snowy egrets, contained some of the lowest Hg concentrations. In contrast to this study of one dynamic system over time, Brumbaugh et al. (2001) studied many basins in the United States and reported Hg bioaccumulation (mostly MeHg in fish fillets) strongly and positively correlated with MeHg in unfiltered water, but only moderately with MeHg in sediment or THg in water.
The lack of an apparent relationship between MeHg in water and snowy egret eggs (which generally contained higher concentrations) tends to support the concept that demethylation and sequestration of MeHg in the liver and kidney by adult birds limits the amount of MeHg deposited in their eggs (Henny et al. 2002), especially when liver concentrations become extremely high. Much more demethylation occurs after THg concentrations in the liver reach a threshold of about 8 μg/g ww and IoHg is sequestered with selenium and is not readily distributed throughout the bird, including its eggs (Henny et al. 2002). Thus, the egg has limitations for monitoring purposes. Hughes et al. (1997) found that feather samples from nestling ospreys (Pandion haliaetus) (∼28–35 days old) appear to be better indicators of local contaminant conditions, because spatial patterns of THg in known prey collected in the same areas resembled those in nestling feathers rather than those in eggs. This finding is important because THg concentrations in feathers of young are strongly related to concentrations in blood of young (DesGranges et al. 1998; this study; unpublished data). However, another concern regarding MeHg is evaluating its effect on productivity, and the collection of sample eggs (although concentrations might be impeded), remains effective for that purpose, as later reported in this series of articles.
Another argument for lower than expected Hg concentrations in eggs is that birds arrive only a short time before egg laying and, thus, do not have adequate time to accumulate Hg. However, Heinz and Hoffman (2004) showed that once a bird began ingesting methyl mercury chloride in its diet, it took only a few days to deposit elevated concentrations of Hg in its eggs. The laboratory diet (5, 10, and 20 μg/g, but only ∼10% moisture) was high compared to actual MeHg intake during this study based on stomach contents (0.48 and 0.88 μg/g ww, for night-herons and snowy egrets at Lahontan Reservoir [Henny et al. 2002]). Although the critical time period from exposure to peak egg concentrations was not specifically determined in the laboratory study, just a few days difference during this short pre-egg-laying period might influence the amount of Hg deposited in eggs. To address this question during our study, a comparison was made of Hg in eggs from early- and late-nesting night-herons and snowy egrets in 1999 at Lahontan Reservoir. Regular collections (10 eggs) were completed on 18 May for night-herons and 2 June for snowy egrets, and an additional 5 eggs per species were collected on 30 June. Results from night-herons for early and late collections were similar (p = 0.27), with geometric means and extreme concentrations of 0.53 μg THg/g (0.39–0.81, n = 10) for early and 0.43 μg THg/g (0.19–0.60, n = 5) for the later collection. Likewise, snowy egret eggs were similar (p = 0.16), with geometric means and extreme concentrations during the early and late collections of 0.36 μg THg/g (0.17–1.43, n = 10) and 0.61 μg THg/g (0.24–1.63, n = 5). No significant differences were found for either species. The late clutches might have included some renesting attempts. Even if some renesting birds were included in the late samples, a rapid accumulation would occur during the 2-week recycling period required between clutches for most bird species. The evidence presented above suggests that eggs (when exposed to Hg in the diet as in this study and not as high as in the laboratory study of Heinz and Hoffman [2004]) are less useful than blood of young to study annual Hg exposure to birds at a given location.
Why the Hg relationship between night-heron eggs and MeHg in water below the reservoir along with acre feet of water in the reservoir (Table 5), but no relationship with snowy egret eggs? We believe the difference relates to diet because the two species are nesting side by side. Night-herons are generally characterized as feeding on a much broader variety of foods and most authors consider them very opportunistic (Davis 1993; Hancock and Kushlan 1984). Wolford and Boag (1971) noted that night-herons fed primarily on fish when available but were quick to take advantage of other potential prey, especially small mammals, young birds, and amphibians. In contrast, the snowy egret feeds in open aquatic habitats on fish and invertebrates but does not feed on birds or small mammals (Hancock and Kushlan 1984). During 1997 and 1998 (wet years), based on 16 night-heron stomachs, the average diet included 34% fish, 34% insects, 20% mammals, 6% crayfish, 6% birds, and <2% vegetation and sediment, whereas the snowy egret diet was 49% fish, 45% insects, and 6% vegetation and sediment (Henny et al. 2002). Furthermore, night-heron stomachs with mammals contained much lower Hg concentrations. We strongly suspect that as the water supply became more limited during drought years, the night-herons foraged more frequently outside the riverine system to find adequate food; thus, a food shift occurred as acre feet of water in Lahontan Reservoir and vicinity decreased, with a progressively higher percentage of nonaquatic mammals and birds (with lower Hg concentrations) being eaten. Snowy egrets do not have this option; thus, no relationship was found with Hg in snowy egret eggs and water storage at Lahontan Reservoir.
References
Bloom NS (1989) Definition of picogram levels of methyl mercury by aqueous phase ethylation, followed by chryogenic gas chromatography with cold vapor atomic fluorescence detection. Can J Fish Aquat Sci 46:1131–1140
Blus LJ (1984) DDE in birds’ eggs: Comparison of two methods for estimating critical levels. Wilson Bull 96:268–276
Brumbaugh WG, Krabbenhoft DP, Helsel DR, Wiener JG, Echols KR (2001) A national pilot study of mercury contamination of aquatic ecosystems along multiple gradients: bioaccumulation in fish. USGS Biological Science Report 2001–0009
Custer TW, Peterson DW Jr (1991) Growth rates of great egret, snowy egret and black-crowned night-heron chicks. Colonial Waterbirds 14:46–50
Davis WE Jr (1993) Black-crowned night-heron. In: Poole A, Gill F (eds) The birds of North America, No 74. The Academy Natural Sciences, Philadelphia
DesGranges J-L, Rodrigue J, Tardif B, Laperle M (1998) Mercury accumulation and biomagnification in ospreys (Pandion haliaetus) in the James Bay and Hudson Bay regions of Quebec. Arch Environ Contam Toxicol 35:330–341
Evers DC, Lane OP, Savoy L, Goodale W (2004) Assessing the impacts of methylmercury on piscivorous wildlife using a wildlife criterion value based on the common loon, 1998–2003. Report BRI 2004–05 to Maine Department of Environmental Protection. BioDiversity Research Institute, Gorham, ME
Gill GA, Bruland KW (1990) Mercury speciation in surface freshwater systems in California and other areas. Environ Sci Tech 24:1392–1400
Hancock J, Kushlan J (1984) The herons handbook. Harper and Row, New York
Heinz GH (1979) Methylmercury: reproductive and behavioral effects on three generations of mallard ducks. J Wildl Manage 43:394–401
Heinz GH, Hoffman DJ (2004) Mercury accumulation and loss in mallard eggs. Environ Toxicol Chem 23:222–224
Henny CJ, Hoffman DJ, Hill EF, Spalding MG, Grove RA, Keith JO (2000) Effects of mercury on fish-eating birds nesting along the mid to lower Carson River, Nevada. Unpublished report. USGS–Forest & Rangeland Ecosystem Science Center, Corvallis, OR
Henny CJ, Hill EF, Hoffman DJ, Spalding MG, Grove RA (2002) Nineteenth century mercury: hazard to wading birds and cormorants of the Carson River, Nevada. Ecotoxicology 11:213–231
Hoffman RJ, Taylor RL (1998) Mercury and suspended sediment, Carson River Basin, Nevada: loads to and from Lahontan Reservoir in flood year 1997 and deposition in reservoir prior to 1983. USGS Fact Sheet FS-001-98, Carson City, NV
Hoffman RJ, Thomas KA (2000) Methylmercury in water and bottom sediment along the Carson River system, Nevada and California, September 1998. USGS Water–Resources Investigations Report 00-4013
Hughes KD, Ewins PJ, Clark KE (1997) A comparison of mercury levels in feathers and eggs of osprey (Pandion haliaetus) in the North American Great Lakes. Arch Environ Contam Toxicol 33:441–452
Marvin-DiPasquale MC, Agee J, McGowan C, Hines M, Krabbenhoft D, Oremland RS (2000) Methylmercury formation and degredation in sediments of the Carson River system. Interim Report-II. EPA, Region IX, Superfund Division, San Francisco, CA
Newton I, Haas MB (1988) Pollutants in merlin eggs and their effects on breeding. Br Birds 81:258–269
SAS Institute (1999) SAS user’s guide: Statistics, Version 8.02. SAS Institute, Inc, Cary, NC
Smith GH (1943) The history of the Comstock Lode, 1850–1920. Nevada Bureau Mines Geol Bull 37:1–305
Stickel LF, Wiemeyer SN, Blus LJ (1973) Pesticide residues in eggs of wild birds: adjustment for loss of moisture and lipid. Bull Environ Contam Toxicol 9:193–196
Van Denburgh AS (1973) Mercury in Carson and Truckee River basins in Nevada. USGS Open-File Report 73–352
Wayne DM, Warwick JJ, Lechler PJ, Gill GA, Lyons WB (1996) Mercury contamination on the Carson River, Nevada: a preliminary study of the impact of mining wastes. Water Air Soil Pollut 92:391–408
Wolford JW, Boag DA (1971) Food habits of black-crowned night-herons in southern Alberta. Auk 88:435–437
Acknowledgments
We thank K. Penner and J. Sizemore, Nevada State Parks for assisting in field aspects of the study at Lahontan Reservoir. P. Bradley and associates, Nevada Department of Wildlife, Elko, assisted with field data collection at the reference site. A. Paul and K. Thomas, USGS, Carson City, kindly provided water data for the Carson River system. W. Praskins (San Francisco, CA) and S. Taylor (Research Triangle Park, NC) coordinated the study for EPA, which partially funded this study. A review by M. Marvin-DiPasquale, USGS, Menlo Park, CA improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henny, C.J., Hill, E.F., Grove, R.A. et al. Mercury and Drought Along the Lower Carson River, Nevada: I. Snowy Egret and Black-Crowned Night-Heron Annual Exposure to Mercury, 1997–2006. Arch Environ Contam Toxicol 53, 269–280 (2007). https://doi.org/10.1007/s00244-006-0163-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-006-0163-7