Abstract
Mixed halogenated dimethyl bipyrroles (HDBPs), which are thought to be produced naturally, were quantified in whale and dolphin products marketed for human consumption in Japan. The major component of HDBPs was 3,3′,4,4′-tetrabromo-5,5′-dichloro-1,1′-dimethyl -2,2′-bipyrrole (Br4Cl2-DBP), accounting for 85% of the total of five HDBPs detected, followed by Br3Cl2-DBP. Mean concentrations of HDBPs ranged from 0.27 μg/g lipid (n = 31) in minke whale (Balaenoptera acutorostrata) from the northwest Pacific Ocean to 11.8 μg/g lipid (n = 33) in bottlenose dolphin (Tursiops trucatus) from the southwest Japanese coastal water. At higher levels, HDBPs made up 37% of the total organohalogen body burden in Dall’s porpoise (Phocoenoides dalli), whereas the contribution was less than 8.9% in minke whales. In two data subsets from Baird’s beaked whale (Berardius bairdii), the products from the Pacific Ocean contained significantly higher concentrations of HDBPs than those from the Sea of Japan. Furthermore, the geographical distribution of HDBPs did not resemble those of ubiquitous anthropogenic organochlorines, such as polychlorinated biphenyl (PCBs). Higher concentration ratios of ΣHDBP/ΣPCB and different patterns of HDBP congeners were observed in whale products from the Asia-Pacific as compared to non-Pacific Ocean mammals reported previously. These results support the hypothesis that HDBPs and anthropogenic organochlorines have different sources and that the consumption of HDBPs by Japanese individuals could be an exposure/health risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Studies of pollutant impact on the marine environment have assumed that organic contaminants are anthropogenic in origin. In recent years, however, new bioaccumulative organohalogen compounds, proposed to be of natural origin, have been detected in marine biota from different sites throughout the world. These compounds comprised mixed halogenated dimethylbipyrroles (HDBPs) (Tittlemier et al. 1999, 2002), a heptachloromethylbipyrrole (Vetter et al. 2000; Vetter 2002), a mixed halogenated monoterpene (Vetter et al. 2001a), and polybrominated phenoxy anisoles (Vetter et al. 2001b; Marsh et al. 2004; Teuten et al. 2005). Increasing interest has focused on HDBPs, first determined in seabird eggs from Canada in 1999 (Tittlemier et al. 1999), the major component of which is 1,1′-dimethyl-3,3′4,4′-tetrabromo-5,5′-dichloro-2,2′-bipyrrole (Br4Cl2-DBP). The global distribution of HDBPs has been determined in marine mammals such as Dall’s porpoise (Phocoenoides dalli) and harbor seals (Phoca vitulina) (Tittlemier et al. 2002) as well as canned fish composites in Canadian markets at comparable levels to anthropogenic compounds, such as polychlorinated biphenyls (PCBs) (Tittlemier 2004). Radiocarbon analysis strongly suggested that HDBPs are produced from a recent source of carbon and are thus likely to have a biogenic origin, as opposed to most industrially produced organohalogens (Reddy et al. 2002, 2004). Although the geographical source of HDBPs has been poorly understood, these compounds were more abundant in marine samples from the North Pacific Ocean rather than the Atlantic Ocean, and HDBPs were not detected in herring gull eggs obtained from the freshwater environment of the Great Lakes (Tittlemier et al. 1999).
Many pinnipeds and odontocetes occupy the upper levels of marine food webs and thus accumulate relatively high levels of organohalogens, such as PCBs, DDTs, and chlordanes (CHLs) (Tanabe et al. 1983; Muir et al. 1988; Aono et al. 1997; Prudente et al. 1997). The blubber of these marine mammals is a good sample matrix for examination of the widespread distribution of persistent organohalogens (Mössner and Ballschmiter 1997). In Japan, small cetaceans, i.e., odontocetes (beaked whales, dolphins, and porpoises), and mystecetes (mainly minke whales) have been hunted locally and their products are sold for human consumption (Dalebout et al. 2004). In our recent survey, the highly contaminant levels of organochlorines were observed in whale products (Simmonds et al. 2002) and several unidentified organohalogens with similar lipophilicity to PCBs were isolated from whale products.
The present study was performed to investigate whether natural organohalogens, such as HDBPs, appear in all whale products from commercial markets in Japan. For this purpose, whale products were collected, classified genetically into species, and analyzed for HDBPs together with PCBs and organochlorine pesticides. The quantitative results were compared between odontocetes and mystecetes, between Baird’s beaked whales from the Sea of Japan and the offshore Pacific water, and between the Asia-Pacific water samples (this study) and non-Pacific Ocean samples reported recently. This is the first report of the occurrence of HDBPs in whale products from the Japanese marine environment.
Materials and Methods
Sampling
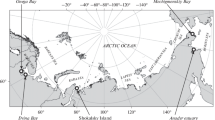
Whale blubber and meat were collected from retail outlets across Japan from 2001 to 2003. Purchases were conducted synoptically to control for potential differences in seasonal availability of local bycatch or the scientific program (JARPN II) (Simmonds et al. 2002; Dalebout et al. 2004). Products were identified to species based on phylogenetic analyses of mtDNA control region sequences as described by Dalebout et al. (2004). Fresh meat and blubber (n = 238 in total) were derived from mystecetes and odontocetes. The mystecetes included minke whales (n = 71), Bryde’s whales (n = 8), and sei whales (n = 4), whereas the odontocetes included bottlenose dolphins (Tursiops trucatus, n = 33), short-finned pilot whales (Globicephala macrorhynchus, n = 30), striped dolphins (Stenella coeruleoalba, n = 25), rough-toothed dolphins (Steno bredanensis, n = 3), pantropical spotted dolphins (Stenella attenuata, n = 3), and false killer whales (Pseudorca crassidens, n = 4) from Okinawa (southern coastal water of Japan) or Wakayama (off Kii Peninsula), and Dall’s porpoises (Phocoenoides dalli, n = 16) from Miyagi (off Sanriku), and Baird’s beaked whales (Berardius bairdii, n = 35) from Hakodate (the Sea of Japan) and Miyagi (off Sanriku) (Figure 1). Products identified as North Pacific minke whales were further identified to O-stock, which forms in offshore Pacific waters (n = 33), and J-stock, which mainly occupies the Sea of Japan (n = 48) (Dalebout et al. 2002). Some of the products that were difficult to discriminate genetically among striped dolphin, common dolphin, and bottlenose dolphin were tentatively grouped into striped dolphin products.
Chemicals
Two HDBP congeners, Br4Cl2-DBP and 3,3′,4,4′,5,5′-hexabromo-1,1′-dimethyl-2,2′-bipyrrole (Br6-DBP), were synthesized according to the method of Gribble et al. (1999). The spectroscopic data for both HDBPs were identical in all respects to the data reported previously (Gribble et al. 1999). Br3Cl2-, Br3Cl3-, and Br5Cl-DBPs were obtained as byproducts during the chlorination or bromination process of 1,1′-dimethyl-2,2′-bipyrrole, which were characterized by gas chromatography/mass spectrometry (GC/MS). 2,3,4,5,6,3′,4′,5′-Octachlorobiphenyl (CB205) was used as an internal standard (IS) for quantification of HDBPs in this study.
Sample Clean-up
The procedure was performed according to a modification of our previous method (Simmonds et al. 2002). Accurately weighed samples (2–10 g) were cut into small species and mixed with a 10-fold amount of anhydrous sodium sulfate. The mixtures spiked with an IS solution of CB205 were wet-packed with n-hexane/dichloromethane (1:1) into a glass column (2 cm, i.d.). The filtered extracts were concentrated and the lipid contents were determined gravimetrically. The lipids were then removed by gel permeation chromatography (Bio-Beads, SX-3, Bio-Rad Laboratories), with elution with n-hexane/dichloromethane (1:1) for organohalogen residues. The eluate containing organohalogens was concentrated to dryness and dissolved in n-hexane (1 mL), which was applied to an activated silica gel S-1 column (1 g, Wako Pure Chemical Industries Ltd., Japan), with elution with n-hexane (12 mL) for HDBPs, together with PCBs, DDTs, or CHLs. The eluate was reduced to 500 μL and subjected to GC/MS.
Identification and Quantification
Analyses of HDBPs and organochlorines were performed using a gas chromatograph (Agilent 6980N) equipped with a mass-selective detector (5973i) in electron-ionization and selected ion monitoring mode (EI-SIM). An HP-5 column (30 m × 0.25 mm, 0.25-μm-thick stationary phase, J&W Scientific Inc., Folsom, CA) was installed in the GC. In the full-scan EI mode, m/z 50 to 650 were recorded. Helium was used as a carrier gas at a constant flow rate of 1.0 mL/min. The injector and transferline temperatures were 250°C and 280°C, respectively. The GC oven program was as follows: After injection at 70°C (1.5 min), the temperature was increased at 20°C/min to 230°C (2 min), then at 4°C /min to 280°C (20 min). Total run time was 35 min. In the SIM mode of HDBPs, the channels (m/z values) of M++4 and M++6 ions for Br2Cl2-, Br3Cl2-, Br3Cl3-, Br4Cl2-, Br5Cl-, and Br6-DBPs were monitored. The Br4Cl2- and Br6-DBPs were quantified by the relative response factor to the IS. The quantification of the other congeners was based on the assumption that their responses were the same as for Br4Cl2-DBP. The concentration of total PCBs was summed for 62 PCB isomers (BP-MS, Wellington Laboratories Inc., Canada). Concentrations of total DDTs were calculated as the sum of p,p′-DDT, p,p′-DDE, and p,p′-DDD, and total concentrations of CHLs were calculated as the sum of trans- and cis-chlordanes, trans- and cis-nonachlors, and oxychlordane. These organochlorines were quantified by GC/MS in EI/SIM mode. Recoveries throughout the procedure were 91 ± 5% for CB153, 93 ± 6% for CB205 (IS), 91 ± 5% for Br4Cl2-DBP, and 83 ± 6% for Br6-DBP (n = 5, respectively). The limits of detection were 50 pg/g lipid for organochlorines and 10 p/g lipid for HDBPs. The calibration curve was linear between 2 and 50 ng/mL for Br4Cl2-DBP. Procedural blanks were analyzed simultaneously with every batch of 10 samples to check interference or contamination from solvents and glassware.
Results and Discussion
Identification of HDBPs
Figure 2 shows the GC/EI-MS total ion chromatogram of halogenated pollutants in the blubber of a false killer whale from the southwest coastal water of Japan (off Okinawa). In full scan mass spectra, five HDBP congeners were detected in samples, together with anthropogenic organochlorines (e.g., 2,2′,4,4′,5,5′-hexachlorobiphenyl (CB153), p,p′-DDE, and trans-nonachlor). The major HDBP congener, which eluted from the HP-5 column between 2,2′,3,4,4′,5,5′-heptachlorobiphenyl (CB180) and PCB205 (IS), was identified as 3,3′,4,4′-tetrabromo-5,5′-dichloro-1,1′-dimethyl-2,2′-bipyrrole (Br4Cl2-DBP) by comparison with authentic reference standards. A novel signal (retention time, 13.4 min) was identified as Br3Cl2-DBP (molecular ion: m/z 462, major fragment ions: m/z 291, 306, and 344) with an unknown halogen substitution pattern on full scan EI mass spectra (Figure 3). The other congeners, Br3Cl3-, Br5Cl-, and Br6-DBPs, which were reported previously (Tittlemier et al. 2002), were also detected at lower ion abundances than Br4Cl2-DBPs. Thus, Br4Cl2-DBP was found to be one of the most abundant congeners in whale products collected from the Pacific coastal water. Their occurrence in an assortment of biota indicated a widespread distribution of HDBPs in the Japanese Pacific environment. As with the other halogenated compounds also proposed to be produced naturally, two abundant signals were observed and characterized by EI-MS and retention times compared to authentic standards. One was 2,3,3′,4,4′,5,5′-heptachloro-1′-methyl-1,2′-bipyrrole (molecular ion of m/z 384, labeled as Q1 in Figure 2), which has recently been identified in marine mammals from Australia, Africa, and the Antarctic (Vetter and Jun 2003). The others were tetrabrominated phenoxyanisoles (m/z 512), which have been isolated from marine sponges (Dysidea sp.) and accumulated at the parts-per-million levels in marine wildlife (e.g., bottlenose dolphins) from the Australia region (Vetter et al. 2002).
Concentration of HDBPs and PCBs
Table 1 shows the concentrations of HDBPs and PCBs, and the ratios of Br4Cl2-DBP/CB153 in the products of various species from the Japanese coastal water. The results indicated a wide concentration range of HDBPs across all species and sampling locations studied. HDBPs were present at higher concentrations in odontocetes (toothed whales) than in mystecetes (baleen whales), at a similar contamination trend to PCBs (Minh et al. 2000; Kajiwara et al. 2002). The lower distribution of HDBPs in mystecetes may reflect the feeding habits of low trophic levels. In addition, metabolism may also affect interspecies differences in HDBP concentrations. For mystecetes, the HDBP concentrations were about fivefold higher in minke whales (0.27 μg/g lipid, n = 31) than in the other baleen whales (0.01–0.06 μg/g, n = 12). For odontocetes from the Pacific coastal water in Japan, the mean concentrations (lipid basis) of total HDBPs were in the order: pantropical spotted dolphin (13.3 μg/g, n = 3) > bottlenose dolphin (11.8 μg/g, n = 33) > striped dolphin (9.00 μg/g, n = 25) > Dall’s porpoise (2.86 μg/g, n = 16). The highest concentration of HDBPs among all products was found in bottlenose dolphin blubber (40.7 μg/g) from southern Japanese coastal water (off Okinawa). The bioaccumulation levels of HDBPs in the present study resembled those in previous studies (Tittlemier et al. 2002), in which the concentration of total HDBPs was the highest (mean, 2.54 μg/g lipid) in Dall’s porpoise (Phocoenoides dalli) from the northwestern Pacific Ocean. In addition, it was noted that the HDBPs were distributed at comparable levels to PCBs in the same individual whales and dolphins from the Japanese environment (Table 1).
Geographical Differences in HDBP Distribution
Although blubber products from Baird’s beaked whales from the Pacific coast (off Sanriku) contained higher levels of HDBPs, those from the Sea of Japan contained HDBPs at 60-fold lower levels, despite the higher levels of PCB contamination in the same individual (Table 1). This finding indicates the marked geographical distribution of HDBPs between the Sea of Japan and the Pacific, although there were no significant differences in the levels of HDBPs between O- and J-stock minke whales inhabiting the offshore Pacific waters and the Sea of Japan, respectively (Table 1). The HDBP levels in J-stock minke whales showed large variations, which may have been due to the extensive movement of J-stock minke whales along the Pacific coast of Japan during some parts of the year (Dalebout et al. 2002). In general, there are likely to be confounding factors, such as species-, age-, and sex-specific differences in feeding ecology, biotransformation capabilities, sample condition, and interindividual variability, which contribute to the observed variations in HDBP concentrations. However, the variations in HDBP concentrations within the same species appear to be strongly governed by habitat, and the present study suggests that the Sea of Japan is not the ecological source of HDBPs. Such geographical differences have been noted previously in the Canadian environment, where HDBPs were shown to be distributed in the North Pacific coast rather than in the Atlantic coast, and not detected in herring gull eggs obtained from the freshwater environment of the Great Lakes. Taken together, the geographical distribution of HDBPs does not resemble that of anthropogenic organohalogens.
HDBP Congener Patterns
The relative compositions (%) of each HDBP congener to the total HDBPs detected in six whale species products are shown in Figure 4. Among the HDBPs, Br4Cl2-DBP was the most predominant congener, accounting for 70–96% (average 85%) of the total HDBPs. The percentage of Br4Cl2-DBP was slightly higher in Baird’s beaked whales than in Dall’s porpoises. The significant contribution of Br4Cl2-DBP in this study coincides with recent data from dolphins in the North Pacific (Tittlemier et al. 2002). The second-highest contributor was Br3Cl2-DBP (5–16%), which may be a dehalogenated metabolite of Br4Cl2-DBP and/or Br3Cl3-DBP in whale bodies. On the other hand, the contribution of Br6-DBP was less than 1%, although Br6-DBP has been reported to be relatively abundant (about 12% contribution) in the blubber of dolphins from New Zealand and the Bering Sea (Tittlemier et al. 2002). The geographical differences in HDBP congener patterns between Asian and New Zealand/Bering Sea dolphins may be due to the different feeding habits of mammals or different biosynthesis process of HDBPs in each ecosystem.
Percentage Contribution of HDBPs to Organohalogen Body Burden
Figure 5 shows the percentage contributions of PCBs, DDTs, CHLs, and HDBPs in total halogenated pollutant body burden. The percentage of HDBPs was the highest in Dall’s porpoise (35.4%), followed by the striped dolphin (28.9%) and the lowest in the minke whale (O-stock, 8.2%) among the major species products from the Pacific. The variation may have been due to interspecies differences in the metabolic capacities of HDBPs as well as ecological difference in their bioaccumulation. For both J- and O-stock minke whales, as well as Bryde’s and sei whales, HDBPs did not constitute a large portion of the organohalogen body burdens. The lower levels of HDBPs in baleen whales may be because they feed on krill, whereas odontocetes eat fish or squid. On the other hand, for Dall’s porpoises from the northern Japanese coastal water, HDBPs were the most abundant among the organohalogens, and for bottlenose dolphins and striped dolphins, HDBPs were the third most abundant after PCBs and DDTs. Recent observations indicated that the HDBP contribution in Dall’s porpoise from the North Pacific reached 11% of total mass of quantified organochlorine body burden (Tittlemier et al. 2002). Although the actual contribution of HDBPs to the total organohalogens may be reduced, when other organohalogens, such as HCHs and HCBs were calculated, HDBPs were shown to contribute significantly to the total organohalogen body burden in all odontocete products investigated.
Relative contribution of HDBPs to the total quantified organohalogen body burden for whale products from the northwestern North Pacific Ocean. For Baird’s beaked whales, the data were based on the population from the Pacific Ocean. PCBs are given as the sum of 62 isomers; DDTs are given as the sum of p,p′-DDE, p,p′-DDT, and p,p′-DDD. CHLs are given as the sum of trans- and cis-nonachlors, trans- and cis-chlordanes, and oxychlordane
Comparison of HDBP Distribution Between the Asia-Pacific and Non-Pacific Mammals
Studies monitoring anthropogenic organohalogens in marine mammals have indicated that the highest levels of contaminants are present in dolphin and porpoise samples from the Northern Hemisphere (Minh et al. 2000; Kajiwara et al. 2002). Because CB153 and Br4Cl2-DBP were found to be good markers of the relative distribution of semivolatile anthropogenic and natural organohalogen compounds (Tittlemier et al. 2002), the ratios of Br4Cl2-DBP/CB153 in all samples were compared to recent data including the non-Pacific samples (Figure 6). The ratios of HDBPs/CB153 were higher in the order: Dall’s porpoise (9.4) > striped dolphin (6.5) > Baird’s beaked whale (4.3) > bottlenose dolphin (3.3) > pilot whale (3.2) > minke whale (1.3). Tittlemier et al. (2002) reported that the ratio was 2.0 (range, 1.0–4.1) in Dall’s porpoises from the North Pacific Ocean and 1.2 (0.13–7.11) in Fraser’s and spinner dolphins from the Mindanao Sea, the Philippines, whereas the ratio in bottlenose dolphins from the Atlantic Ocean was only 0.004. Our results and those of a previous study (Tittlemier et al. 2002) indicate that the HDBP levels and the ratios from the Asian coastal water are significantly higher than in similar species from other locations. Furthermore, the distribution of HDBPs was different from that observed for CB153, indicating that HDBPs have a different source from PCBs and related anthropogenic compounds, and that the geographical source of HDBPs is most likely in the northern Pacific or the Asia-Pacific region.
Comparison of the HDBPs/CB153 ratios between the Asia-Pacific and non-Pacific samples. *Data from Tittlemier et al. (2002)
Potential Sources of HDBPs
Reddy et al. (2002) extracted Br4Cl2-DBP from mammal blubbers and determined its radiocarbon (14C). The presence of detectable 14C in Br4Cl2-DBP isolated from mammals strongly suggested that it should be a biogenic source, and the majority of industrially synthesized organohalogens do not contain any measureable 14C. Naturally occurring HDBPs may be produced by a marine bacterium (e.g., Pseudoalteromonas), because it was shown to produce a structurally similar compound, 3,4,5,3′,4′,5′-hexabromo-2,2′-bipyrrole (Andersen et al. 1974; Faulkner 1980). Mixed halogenation is rare for industrial synthesis of semivolatile organohalogens but common for natural marine products (Gribble et al. 1999), and the geographic distribution was dissimilar from that of PCBs. Furthermore, it is possible that Br4Cl2-DBP results from a similar biosynthetic pathway of 2,2′-bipyrroles, followed by halogenation of the rings and methylation of the pyrrolic nitrogens. Pyrroles are highly activated for halogenation and methylation (Neidleman and Geigert 1986).
In conclusion, HDBPs and some anthropogenic organochlorines had different distribution between the Sea of Japan and the Pacific. Although the toxicological significance of HDBPs remains unknown, the consumption of HDBPs by Japanese individuals could be an exposure/health risk. Thus, more investigation is needed of their bioaccumulation process in marine food webs in order to better understand the potential source(s).
References
Andersen RJ, Wolfe MS, Faulkner DJ (1974) Autotoxic antibiotic production by a marine chromobacterium. Mar Biol 27:281–285
Aono S, Tanabe S, Fujise Y, Kato H, Tatsukawa R (1997) Persistent organochlorines in minke whale (Baleanoptera acutorostrata) and their prey species from the Antarctic and North Pacific. Environ Pollut 98:81–89
Dalebout ML, Lento GM, Cipriano F, Funahashi N, Baker CS (2002) How many protected minke whales are sold in Japan and Korea? A census by microsatellite DNA profiling. Animal Conserv 5:143–152
Dalebout ML, Laverty S, Funahashi N, Ma YU, Endo T, Haraguchi K, Olavarria C, Baker CS (2004) Molecular identification of small cetacean species from Japanese and Korean markets, 1993–2003. For consideration by the Scientific Committee of the International Whaling Commission SC/56/BC5
Faulkner DJ (1980) The handbook of environmental chemistry, part A. Springer, Berlin, pp 229–254
Gribble GW, Blank DH, Jasinski JP (1999) Synthesis and identification of two halogenated bipyrroles present in seabird eggs. Chem Commun 2195–2196
Kajiwara N, Watanabe M, Tanabe S, Nakamatsu K, Amano M, Miyazaki N (2002) Specific accumulation and temporal trends of organochlorine contaminants in Dall’s porpoises (Phocoenoides dalli) from Japanese coastal waters. Mar Pollut Bull 44:1089–1099
Marsh G, Athanasiadou M, Bergman Å, Asplund L (2004) Identification of hydroxylated and methoxylated polybrominated diphenyl ethers in Baltic Sea salmon (Salmo salar) blood. Environ Sci Technol 38:10–18
Minh TB, Nakata H, Watanabe M, Tanabe S, Miyazaki N, Jefferson TA, Purudente M, Subramanian A (2000) Isomer-specific accumulation and toxic assessment of polychlorinated biphenyls, including coplanar congeners, in cetaceans from the North Pacific and Asian coastal waters. Arch Environ Contam Toxicol 39:398–410
Mössner S, Ballschmiter K (1997) Marine mammals as global pollution indicators for organochlorines. Chemosphere 34:1285–1296
Muir DCG, Norstrom RJ, Simon M (1988) Organochlorine contaminants in arctic marine food chain: accumulation of specific polychlorinated biphenyls and chlordane-related compounds. Environ Sci Technol 22:1071–1079
Neidleman SL, Geigert J (1986) Biohalogenation: principles, basic roles, and applications. Halsted Press, New York
Prudente M, Tanabe S, Watanabe M, Subramanian A, Miyazaki N, Suarez P, Tatsukawa R (1997) Organochlorine contamination in some odontoceti species from the North Pacific and Indian Ocean. Mar Environ Res 44:415–427
Reddy CM, Xu L, Eglinton TI, Boon JP, Faulkner DJ (2002) Radiocarbon content of synthetic and natural semi-volatile halogenated organic compounds. Environ Pollut 120:163–168
Reddy CM, Xu L, O’Neil GW, Nelson RK, Eglinton TI, Faulkner DJ, Norstrom R, Ross PS, Tittlemier SA (2004) Radiocarbon evidence for a naturally produced, bioaccumulating halogenated organic compound. Environ Sci Technol 38:1992–1997
Simmonds MP, Haraguchi K, Endo T, Cipriano F, Palumbi SR, Troisi GM (2002) Human health significance of organochlorine and mercury contaminants in Japanese whale meat. J Toxicol Environ Health 65:1211–1235
Tanabe S, Mori T, Tatsukawa R (1983) Global pollution of marine mammals by PCBs, DDTs and HCHs (BHCs). Chemosphere 12:1269–1275
Teuten EL, Xu L, Reddy CM (2005) Two abundant bioaccumulated halogenated compounds are natural products. Science 307:917–920
Tittlemier SA, Simon M, Jarman WM, Elliot JE, Norstrom RJ (1999) Identification of a novel C10H6N2Br4Cl2 heterocyclic compound in seabird eggs. A bioaccumulating marine natural product? Environ Sci Technol 33:26–33
Tittlemier S, Borrell A, Duffe J, Duignan PJ, Fair P, Hall A, Hoekstra P, Kovacs KM, Krahn MM, Lebeuf M, Lydersen C, Muir D, O’Hara T, Olsson M, Pranschke J, Ross P, Siebert U, Stern G, Tanabe S, Norstrom R (2002) Global distribution of halogenated dimethyl bipyrroles in marine mammal blubber. Arch Environ Contam Toxicol 43:244–255
Tittlemier SA (2004) Dietary exposure to a group of naturally produced organohalogens (halogenated dimethyl bipyrroles) via consumption of fish and seafood. J Agric Food Chem 52:2010–2015
Vetter W, Alder L, Kallenborn R, Schlabach M (2000) Determination of Q1, an unknown organochlorine contaminant, in human milk, Antarctic air, and further environmental samples. Environ Pollut 110:401–409
Vetter W, Hiebl J, Oldham NJ (2001a) Determination and mass spectrometric investigation of a new mixed halogenated persistent component in fish and seal. Environ Sci Technol 35:4157–4162
Vetter W, Scholz E, Gaus C, Müller JF, Haynes D (2001b) Anthropogenic and natural organohalogen compounds in blubber of dolphins and dugongs (Dugong dugon) from Northeastern Australia. Arch Environ Contam Toxicol 41:221–231
Vetter W (2002) Environmental occurrence of Q1, a C9H3Cl7N2 compound, that has been identified as a natural bioaccumulative organochlorine. Chemosphere 46:1477–1483
Vetter W, Stoll E, Garson MJ, Fahey SJ, Gaus C, Müller JF (2002) Sponge halogenated natural products found at parts-per-million levels in marine mammals. Environ Toxicol Chem 21:2014–2019
Vetter W, Jun W (2003) Non-polar halogenated natural products bioaccumulated in marine samples. II. Brominated and mixed halogenated compounds. Chemosphere 52:423–431
Acknowledgments
The authors wish to thank Dr. S. Baker, University of Auckland, New Zealand for his helpful identification of species. This work was supported by Grants-in-Aid from the International Fund for Animal Welfare (IFAW), and from the Japan Society for the Promotion of Science (B17404006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haraguchi, K., Hisamichi, Y. & Endo, T. Bioaccumulation of Naturally Occurring Mixed Halogenated Dimethylbipyrroles in Whale and Dolphin Products on the Japanese Market. Arch Environ Contam Toxicol 51, 135–141 (2006). https://doi.org/10.1007/s00244-005-1140-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-005-1140-2