Abstract
Sublethal effects of three pesticides including atrazine (triazine herbicide), DDT (organochlorinated insecticide), and chlorpyrifos (organophosphate insecticide) on acetylcholinesterase (AChE), general esterase (GE), glutathione S-transferase (GST), and cytochrome P450 monooxygenase (P450) activities were evaluated in the aquatic midge Chironomus tentans. Exposures of midges to atrazine at 30 and 150 micrograms per liter (μg/L) for 20 d (i.e., from the first- to fourth-instar larvae) enhanced P450 O-deethylation activity by 12.5- and 15.5-fold, respectively, but did not significantly change AChE, GST, and GE activities. Similar exposures to DDT at 0.01 and 0.05 μg/L did not significantly affect AChE, GE, and P450 activities; however, DDT at 0.05 μg/L enhanced GST activity toward the substrate 1-chloro-2, 4-dinitrobenzene by 33.6%. Exposures of midges to chlorpyrifos at 0.10 μg/L for 20 d reduced AChE activity by 59.8%, and GE activities toward the substrates α-naphthyl acetate and β-naphthyl acetate by 30.7 and 48.8%, respectively. The reduced GE activities appear to be due to the inhibition of several esterases, particularly the one with a slow migration, by chlorpyrifos as demonstrated by non-denaturing polyacrylamide gel electrophoresis. Furthermore, exposure of midges to chlorpyrifos at 0.10 μg/L for 20 d enhanced the P450 O-deethylation activity by 3.3-fold although no significant effect was observed at 0.02 μg/L for the same enzyme. These results provide insights into the sublethal effects of these commonly detected pesticides in aquatic environments on important enzymes in aquatic midges.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The extensive use of pesticides over the past several decades has led to their recurrent detection in many surface and ground waters (Hopkins et al. 2000; Larson et al. 1999). Atrazine is an extensively used triazine herbicide in a variety of agricultural and other broad-leaf weed and grass control practices. More than 38 million kilograms of atrazine are applied annually in the US (Gianessi and Puffer 1994; Gianessi 1998; US EPA 1994a; Solomon et al. 1996). It is a relatively persistent and mobile herbicide that is often deposited to surface water by spray drift and erosion, and reaches ground water as a result of leaching. Previous studies reported atrazine residues as high as 700 and 2300 μg/L in the ground water of 13 states and in the surface water of 31 states, respectively, during peak application periods (Thurman et al. 1992; Pratt et al. 1997). However, surface and ground water concentrations of atrazine are typically below the US EPA’s drinking water standard maximum contaminant level of 3 μg/L (Kello 1989; Thurman et al. 1992; Solomon et al. 1996; Pratt et al. 1997; Baker 1998).
Dichlorodiphenyltrichloroethane (DDT) is an organochlorinated insecticide used to control both agricultural and nonagricultural pests. Although the use of DDT, especially in agriculture, has been banned in the US due to its bioaccumulability, it continues to be used to control insect vectors of diseases in some parts of the world. Due to its extensive use, DDT is a ubiquitous pesticide recurrently transformed and redistributed in the environment (Faroon et al. 2002). It is essentially immobile in soil and, therefore, water contamination is significantly caused by runoff from erosion. Monitoring studies show that concentrations of DDT in all media have been declining throughout the US after the use of DDT was banned (Faroon et al. 2002). However, DDT concentrations ranging from 0.12 to 350 μg/L are still reported in the surface sediment of several watersheds in the central and southeastern US (Van Metre et al. 1997; Cooper et al. 2003).
Chlorpyrifos is a broad-spectrum organophosphate (OP) insecticide used for controlling insect pests on a variety of vegetable, orchard, and ornamental crops. Approximately 9 million kilograms of active ingredient are used yearly with about 26% of the total volume applied to corn (US EPA 2000). In recent years, the major non-crop uses for chlorpyrifos include indoor pest control, cattle ear tags, tick and flea products, as well as subterranean termite control. Surface water contamination is significantly caused by runoff from eroding soil and spray drift during application (US EPA 2000). For example, maximum concentrations of chlorpyrifos detected in rivers and streams in the Lake Erie basin of Ohio ranged from 0.16 to 3.84 μg/L (Richards and Baker 1993). In a cornfield spray study, mean concentrations of chlorpyrifos measured in adjacent water bodies ranged from nondetectable levels to 67 μg/L (US EPA 2000). Nevertheless, chlorpyrifos concentrations found in several major surface water monitoring studies rarely exceeded 0.40 μg/L (US EPA 2000).
Although pesticides, such as atrazine, DDT, or chlorpyrifos, may be deposited into aquatic environments at relatively high concentrations during the peak application periods of spring and early summer, the transient nature of pesticide concentrations in aquatic systems dictates that many aquatic organisms are rarely exposed to elevated concentrations of pesticides for continuous periods of time. However, biochemical or physiological impairments may occur when organisms are exposed to a pesticide or its metabolites at sublethal concentrations for extended periods of time. This study examined the sublethal effects of atrazine, DDT, and chlorpyrifos on the OP target enzyme acetylcholinesterase (AChE), and three major detoxification enzyme systems including general esterases (GE), glutathione S-transferases (GST), and cytochrome P450 monooxygenases (P450) in the aquatic midge Chironomus tentans following 20-d exposures. Results from this study are expected to provide insights into the sublethal effects of these commonly detected pesticides in aquatic environments on important enzymes in aquatic midges.
Materials and Methods
Test Organism
The aquatic midges C. tentans were taken from the colonies cultured in the Department of Entomology at Kansas State University according to the US Environmental Protection Agency standard operating procedures for static cultures (US EPA 1994b), with slight modifications. Instead of separating each generation from the egg masses, the midges were reared in mixed-age brood cultures.
Chemicals
Acetone (American Chemical Society certified) was purchased from Fisher Scientific (Pittsburgh, PA). Acetylthiocholine iodide (ATC), α-naphthol, α-naphthyl acetate (α-NA), β-naphthol, β-naphthyl acetate (β-NA), reduced β-nicotinamide adenine dinucleotide phosphate (β-NADPH), bicinchoninic acid solution (BCA), 1-chloro-2, 4-dinitrobenzene (CDNB), 5,5′ -dithio-bis (2-nitrobenzoic acid) (DTNB), 7-ethoxycoumarin (7-EC), fast blue B salt (O-dianisidine, tetrazotized), fast garnet GBC salt (2-methyl-4 [(2-methylphenyl)-azo] benzenediazonium salt), glutathione, glutathione reductase, sodium dodecyl sulfate (SDS), umbelliferone (7-hydroxycoumarin), and Triton X-100 were purchased from Sigma Chemical Company (St. Louis, MO). Chlorpyrifos [O,O-diethyl O-3,5,6-trichloro-2-pyridylphosphorothioate] (99.5% pure), atrazine [6-chloro-N-ethyl-N′ -(1-methylethyl)-1,3,5-triazine-2,4-diamine] (99%), and DDT (19% of o,p′ -DDT, 74.2% of p,p′ -DDT) were purchased from Chem Service (West Chester, PA). Acrylamide and bovine serum albumin (BSA) were purchased from Bio-Rad Laboratories (Hercules, CA). 3,4-Dichloronitrobenzene (DCNB) was purchased from Aldrich Chemical Co. (Milwaukee, WI).
Chronic Exposure to Individual Pesticides
The pesticide bioassays were performed using protocols described by US EPA (1994b), with some modifications. Briefly, 10 male and 15 female adult midges were collected from the mixed-age brood cultures and transferred to a net cage (28 × 28 × 29.5 cm). A dish containing reconstituted water was placed into the cage for egg deposition. Egg masses were collected after 24 h and transferred to Petri dishes containing reconstituted water. The egg masses were incubated for 48 h in a growth chamber (Percival Scientific, Boone, IA, USA) at 25 ± 1°C with a 16:8-h light:dark photoperiod (maximum light intensity: 80 μmol/m2/s).
To assess the sublethal effects of atrazine, DDT, and chlorpyrifos on various enzyme activities, newly hatched first-instar C. tentans larvae (within 4 h) were exposed to two concentrations of each pesticide for 20 d. The concentrations of pesticides were 30 and 150 μg/L for atrazine, 0.01 and 0.05 μg/L for DDT, and 0.02 and 0.1 μg/L for chlorpyrifos. These pesticide concentrations are environmentally relevant (see the Introduction) and were chosen based on our previous bioassays (Rakontondravelo 2004). Neither atrazine nor DDT at these concentrations affected larval survivorship in 20-d bioassays. Chlorpyrifos at 0.02 μg/L did not affect the larval survivorship, whereas at 0.10 μg/L it reduced larval survivorship by 67% in 20 d. Each pesticide was delivered by adding 10 μl of pesticide solution to 300 ml reconstituted water containing 15 midges and 10 ml clean sand in a glass beaker. The same procedure was used to treat midges with corresponding concentrations of acetone in water (33 μl/L) as controls. The bioassays were repeated six times for each pesticide concentration and control. The treated midges were maintained in the growth chamber at 25 ± 1°C with a 16:8-h light:dark photoperiod. During the pesticide exposures, water was replenished every 72 h with new oxygenated water containing a corresponding pesticide at the same concentration and slurry of Tetra-fin flake fish food was added daily. The midge larvae reached the fourth-instar stage after 20 d. All surviving midge larvae were collected from each beaker as a sample and used for various enzyme assays.
Acetylcholinesterase (AChE) Assay
AChE activity in fourth-instar C. tentans was determined according to the method of Ellman et al. (1961) as modified by Zhu et al. (1996) using ATC as a substrate. Each sample was homogenized in ice-cold 0.1 M phosphate buffer (pH 7.5) containing 0.3% (v/v) Triton X-100 at the rate of one midge larva per 100 μl homogenizing buffer. The homogenates were centrifuged at 15,000g for 15 min at 4°C and the supernatants were used as enzyme sources. AChE activity was determined using an enzyme kinetic microplate reader (Molecular Devices, Menlo Park, CA, USA) at 405 nm.
General Esterase (GE) Assay
GE activity was determined in fourth-instar C. tentans larvae according to the method described by Zhu and Gao (1998). Each sample was homogenized in ice-cold 0.1 M phosphate buffer (pH 7.5) containing 0.3% (v/v) Triton X-100. After the homogenates were centrifuged at 15,000g for 15 min at 4°C, the supernatants were used as the enzyme source for measuring GE activities with α-NA or β-NA as substrates. The absorbance was read using a V max enzyme kinetic microplate reader at 600 and 560 nm for α-NA and β-NA, respectively.
Electrophoretic Analysis of General Esterases (GE)
Nondenaturing polyacrylamide gel electrophoresis (PAGE) was performed to separate GE in a Mini-Protean II vertical electrophoresis apparatus (Bio-Rad, Hercules, CA) using a 4% stacking gel and 7.5% separating gel. The enzyme was prepared as described above for GE assays. The gel electrophoresis and GE staining procedures were essentially the same as described by Zhu and Gao (1998) using α-NA or β-NA as substrates and fast garnet GBC salt as chromogenic agent.
Glutathione S-Transferase (GST) Assay
GST activity in fourth-instar C. tentans larvae was determined according to the method of Zhu et al. (2000) using CDNB and DCNB as substrates. The conjugation of glutathione towards CDNB or DCNB was determined by recording the change in absorbance at 340 nm for CDNB and 344 nm for DCNB for 1 min with 10-sec intervals using an Ultrospec 3000 UV/visible spectrophotometer (Pharmacia Biotech, Ltd., Cambridge, UK). Nonenzymatic controls were performed in parallel in order to correct for nonenzymatic conjugation.
Cytochrome P450 Monooxygenase (P450) Assay
P450 activity was determined based on the method of Stumpf and Nauen (2001) with some modifications (Anderson and Zhu 2004) using 7-ethoxycoumarin (7-EC) as a substrate. The fluorescence of umbelliferone was measured with a FLX800TBIDE microplate fluorescence reader (Bio-Tek Instruments, Inc., Winooski, VT, USA) at 465 nm while exciting at 390 nm.
Protein Assay
The concentration of total protein in each sample preparation was determined based on the method of Smith et al. (1985) using bovine serum albumin as a standard. The measurement was carried out using the microplate reader (Molecular Devices) at 560 nm.
Statistical Analysis
The differences in enzyme activity for each pesticide were statistically compared using Fisher’s least significant difference (LSD) multiple comparison test (SAS Institute 1996).
Results
Effect of Pesticides on Acetylcholinesterase (AChE) Activity
There was no significant difference in AChE activity in C. tentans larvae exposed to atrazine at either 30 or 150 μg/L for 20 d as compared with the solvent (i.e., acetone) controls (Table 1). Similarly, the concentration of DDT at either 0.01 or 0.05 μg/L had no significant effect on the AChE activity in the midges as compared with either of the controls. However, chlorpyrifos at 0.10 μg/L reduced AChE activity by 59.8% as compared with the solvent control.
Effect of Pesticides on General Esterase (GE) Activity
There was no significant difference in GE activity towards α-NA and β-NA in midge larvae exposed to atrazine at either 30 or 150 μg/L for 20 d as compared with either water-only or solvent controls (Table 2). Likewise, DDT at either 0.01 or 0.05 μg/L had no significant effect on GE activity towards either α-NA or β-NA as compared with the solvent control. However, chlorpyrifos at 0.02 μg/L reduced GE activity towards β-NA by 17.1% and at 0.10 μg/L reduced the enzyme activity towards α-NA and β-NA by 30.7 and 48.8%, respectively, as compared with solvent controls.
Electrophoretic Analysis of Pesticide Effects on General Esterases (GE)
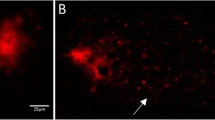
The nondenaturing PAGE revealed multiple esterases in fourth-instar C. tentans larvae with the capability of hydrolyzing both α-NA and β-NA substrates (Figure. 1). However, neither atrazine (30 and 150 μg/L) nor DDT (0.01 and 0.05 μg/L) showed any significant effect on GE activity or banding pattern in midges. In contrast, chlorpyrifos at 0.02 μg/L significantly reduced the activity of at least one esterase and at 0.10 μg/L completely inhibited the esterase activity as shown on the gel. Nevertheless, no significant differences in GE activity or banding pattern were found between the two substrates in all the pesticide treatments (gel picture for α-NA not shown).
General esterase (GE) profiles in fourth-instar larvae of C. tentans exposed to three pesticides for 20 d. GE were separated by nondenaturing polyacrylamide gel electrophoresis and visualized using β-naphthyl acetate (β-NA) as a substrate and fast garnet GBC salt as a chromogenic agent. Arrow indicates a particular esterase inhibited by chlorpyrifos in a concentration-dependent manner. CK - Acetone: control without solvent (acetone) in water; CK+ Acetone: control with acetone (33 μl/L) in water
Effect of Pesticides on Glutathione S-Transferase (GST) Activity
GST activities towards CDNB and DCNB in midge larvae exposed to atrazine (30 and 150 μg/L) or chlorpyrifos (0.02 and 0.10 μg/L) were not significantly different from their corresponding solvent controls (p > 0.05, Table 3). However, DDT at 0.05 μg/L enhanced GST activity towards CDNB by 33.6% as compared with the solvent control.
Effect of Pesticides on Cytochrome P450 Monooxygenase (P450) Activity
Exposures of the midge larvae to DDT at 0.01 and 0.05 μg/L or chlorpyrifos at 0.02 μg/L for 20 d did not result in significant changes in the P450 O-deethylation activity as compared with the water-only and solvent controls (p > 0.05, Table 4). However, when the midge larvae were exposed to chlorpyrifos at 0.10 μg/L, the enzyme activity was increased by 3.3-fold as compared with that of the solvent control. Furthermore, the enzyme activity in midge larvae exposed to atrazine at 30 and 150 μg/L was enhanced by 12.5- and 15.5-fold, respectively, as compared with the solvent controls (Table 4).
Discussion
Pesticidetarget and detoxification enzymes are often examined for potential effects of pesticides on target and nontarget organisms. Some of these enzymes are also frequently used as biomarkers of pesticide exposures in various organisms because the activity of these enzymes in the organisms could change in response to pesticide exposures (Johnson 1993). To date, most studies have focused on the responses of enzymes to acute pesticide exposures, and relatively few reports have assessed changes of enzyme activity in the organisms chronically exposed to sublethal concentrations of pesticides. This study examined the responses of several important enzymes (AChE, GEs, GSTs, and P450) in aquatic midge larvae exposed to sublethal concentrations of three pesticides (atrazine, chlorpyrifos, and DDT) for 20 d.
It has been well established that AChE plays an integral role in cholinergic nerve transmission and is the target site of inhibition by OP and carbamate insecticides. The assessment of AChE activity is a common diagnostic tool for evaluating the exposure of an organism to various pesticides, particularly OP and carbamate insecticides (Hyne and Maher 2003). Our study demonstrated a significant decrease of AChE activity (59.8%) in C. tentans larvae chronically (20 d) exposed to chlorpyrifos at an environmentally relevant concentration (0.10 μg/L). Such an inhibition was associated with 67% mortality of the midge larvae after 20 d (Rakotondravelo 2004). In contrast to the results of Jin-Clark et al. (2002) showing approximately 30% inhibition of AChE in fourth-instar C. tentans exposed to chlorpyrifos at 0.25 μg/L for 48 h, our current study indicates that chronic exposures of C. tentans larvae to chlorpyrifos even at a lower concentration can result in a more pronounced inhibition to AChE as compared with acute exposures to the same insecticide. Such an increase of AChE inhibition in an organism could lead to increased mortality as demonstrated by Ibrahim et al. (1998). These researchers confirmed the inhibition of AChE activity in other aquatic midges by several OPs in a concentration-dependent manner, and described that a 20 to 30% inhibition of AChE activity by an OP accounts for as much as 30 to 40% mortality in the midge C. riparius.
Our study did not find any significant effect on AChE activity in C. tentans larvae exposed to atrazine at either 30 or 150 μg/L for 20 d. Anderson and Zhu (2004) reported that an acute exposure of atrazine as high as 1000 μg/L did not significantly alter AChE activity in fourth-instar C. tentans. Anderson and Lydy (2002) also demonstrated that exposure to atrazine at 200 μg/L had no effect on AChE activity in the aquatic amphipod Hyalella azteca. Based on the previous and current studies, it is logical to state that both acute and chronic exposures of atrazine to C. tentans larvae and perhaps other aquatic organisms will not significantly affect AChE. Our study also did not show any significant effect of DDT at either 0.01 or 0.05 μg/L on AChE activity in C. tentans. These results agree with those of previous studies showing no significant effects of other organochlorinated insecticides, such as lindane, on AChE activity in C. riparius exposed to concentrations 10,000-fold higher than those used in this study (Ibrahim et al. 1998). However, these findings should not be surprising because AChE is not a target enzyme for either triazine herbicides or organochlorinated insecticides.
As expected, our results in evaluating the effects of atrazine, chlorpyrifos, and DDT on GE activity showed similar results as those on AChE in C. tentans larvae. Chlorpyrifos at 0.10 μg/L suppressed GE activity in C. tentans by 30.7% towards α-NA and by 48.8% towards β-NA following 20-d exposures (Table 2). As revealed by PAGE, exposure of C. tentans larvae to chlorpyrifos at 0.02 μg/L for 20 d significantly reduced the activity of an esterase with slow migration whereas chlorpyrifos at 0.10 μg/L completely suppressed the same esterase relative to the solvent control (Figure. 1). However, neither atrazine nor DDT at the test concentrations had a significant effect on GE activity in C. tentans for α-NA or β-NA. Because the mechanism employed by GE to detoxify via hydrolysis or sequestration constitutes one of the most important defenses against exposure to pesticides such as OPs, inhibition to GE by OPs may minimize similar inhibition to AChE in a competitive manner (Zhu and Brindley 1992). Thus, GE may serve as a reservoir for OPs to reduce the inhibition of AChE and, therefore, reduce the mortality of the organism.
Our study showed that DDT at 0.05 μg/L increased GST activity by approximately 33.6% towards CDNB in C. tentans following a 20-d exposure as compared with the solvent control (Table 3). Thus, our results agree well with those of Plapp and Casida (1970), and Hayaoka and Deuterman (1982) showing elevated GST activity in many different species following DDT exposures. Our study, however, did not show any significant effect of atrazine or chlorpyrifos on GST activity towards either CDNB or DCNB in midge larvae. Our results agree with those of Egaas et al. (1993) showing no effect on GST activity by atrazine in insects, but are different from those of Hodge et al. (2000) showing an inhibitory effect on GST by chlorpyrifos. The GSTs represent an important family of detoxification enzymes that catalyze the conjugation of a wide range of molecules with the tripeptide glutathione. Pharmacologically, DDT is a relatively suitable substrate to be conjugated by GST due to its hydrophobicity. Thus, increased GST activity in DDT-treated midge larvae may represent a substrate (i.e., DDT)-induced phenomenon. However, it is unknown as to whether such an increased GST activity was due to an increased expression of GST gene(s).
Our study revealed that atrazine can significantly increase P450 activity in C. tentans larvae (Table 4). After the midge larvae were exposed to atrazine at 30 and 150 μg/L for 20 d, the enzyme activity was increased by 12.5- and 15.5-fold, respectively, as compared with the acetone control. Furthermore, P450 activity was also increased by 3.3-fold when the midge larvae were exposed to chlorpyrifos at 0.10 μg/L for 20 d. However, DDT at 0.01 and 0.05 μg/L did not show a significant effect on P450 activity in midge larvae. Previous studies have demonstrated that atrazine is capable of increasing P450 activity (Anderson and Zhu 2004) and P450 gene expression (Londono et al. 2004) in C. tentans larvae following acute exposures. Apparently, atrazine-induced P450 activity is likely caused by an up-regulation of P450 gene expression as observed by Londono et al. (2004).
The induction of P450 by atrazine may affect the toxicity of other pesticides co-existing in the environment. In C. tentans, for example, the induction of P450 by atrazine was found to increase the toxicities of several OPs including dimethoate, disulfoton, and demeton-S-methyl possibly by enhancing the oxidative activation of these compounds to O-analog or sulfoxide analogs with increased anticholinesterase activity (Anderson and Zhu 2004). In contrast, the induction of P450 by atrazine was found to reduce the toxicity of the OP omethaote possibly by enhancing oxidative metabolic detoxification since omethaote does not require oxidative activation. In addition, our results on increased P450 activity in chlorpyrifos-treated midges agree with that of Anderson and Zhu (2004) showing a considerable increase in P450 activity in C. tentans exposed to the OP dimethoate. Such an increase in P450 activity may affect the midge’s susceptibility to other toxic contaminants in water either by activating a less toxic parent compound to a more toxic metabolite or by detoxifying a toxic molecule to a less toxic metabolite in the organism as demonstrated by Anderson and Zhu (2004).
Conclusions
Our study revealed that sublethal exposures of midges to atrazine dramatically enhanced P450 activity but did not significantly affect AChE, GST, and GE activities. Sublethal exposures of midges to DDT did not appear to significantly affect AChE, GE, and P450 activities, but can enhance GST activity. Furthermore, exposures of midges to chlorpyrifos can significantly reduce AChE and GE activities. These results provide insights into the sublethal effect of these commonly detected pesticides in an aquatic environment on important enzymes in aquatic midges. Some of these pesticidetarget and detoxification enzymes may be further evaluated to serve as potential biomarkers in order to examine the exposures of non-target aquatic organisms to sublethal concentrations of pesticides in aquatic environments.
References
Anderson TD, Lydy MJ (2002) Increased toxicity to invertebrates associated with a mixture of atrazine and organophosphate insecticides. Environ Toxicol Chem 21:1507–1514
Anderson TD, Zhu KY (2004) Synergistic and antagonistic effects of atrazine on the toxicity of organophosphorodithioate and organophosphorothioate insecticides to Chironomus tentans (Diptera: Chironomidae). Pestic Biochem Physiol 80:54–64
Baker DB (1998) Sediment, nutrient, and pesticide transport in selected Great Lakes tributaries. Great Lakes National Program Office, US Environmental Protection Agency, Region 5
Cooper C, Smith Jr. S, Moore M (2003) Surface water, ground water and sediment quality in three Oxbow lake watersheds in the Mississippi Delta agricultural region: Pesticides. Int J Ecol Environ Sci 29:171–184
Egaas E, Skaare JU, Svendsen NO, Sandvik M, Falls JG, Dauterman WC, Collier TK, Netland J (1993) A comparative study of effects of atrazine on xenobiotic metabolizing enzymes in fish and insect, and in in vitro phase II atrazine metabolism in some fish, insects, mammals, and one plant species. Comp Biochem Physiol 106:141–149
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Faroon O, Harris MO, Llados F, Swarts S, Sage G, Citra M, Gefell D (2002) Toxicological profile for DDT, DDE, and DDD. US Department of Health and Human Services, Atlanta, GA
Gianessi LP (1998) Benefits of triazine herbicides. In: Ballantine LG, McFarland JE, Hackett DS (eds) Triazine herbicides risk assessment. American Chemical Society, Washington, DC. pp 1–8
Gianessi LP, Puffer C (1994) Herbicide use in the United States, resources for the future. US Government Printing Office, Washington, DC
Hayaoka T, Dauterman C (1982) Induction of glutathione S-transferase by phenobarbital and pesticides in various house fly strains and its effect on toxicity. Pestic Biochem Physiol 7:113–119
Hodge S, Longley M, Booth L, Heppelthwaite V, O’Halloran K (2000) An evaluation of glutathione S-transferase activity in Tasmanian lacewing (Micromus tasmaniae) as a biomarker of organophosphate contamination. Bull Environ Contam Toxicol 65:8–15
Hopkins EH, Hippe DJ, Frick EA, Buell GR (2000) Organophosphorus pesticide occurrence and distribution in surface and ground water of the United States, 1992-1997. US Geological Survey Open-File Report 00-187. US Geological Survey, Reston, VA
Hyne RV, Maher WA (2003) Invertebrate biomarkers: Links to toxicosis that predict population decline. Ecotoxicol Environ Saf 54:366–374
Ibrahim H, Kheir R, Helmi S, Lewis J, Crane M (1998) Effects of organophosphates, carbamates, pyrethroid and organochlorine pesticides, and a heavy metal on survival and cholinesterase activity of Chironomus riparius Meingen. Bull Environ Contam Toxicol 60:448–455
Jin-Clark Y, Lydy MJ, Zhu KY (2002) Effects of atrazine and cyanazine on chlorpyrifos toxicity in Chironomus tentans (Diptera: Chironomidae). Environ Toxicol Chem 21:598–603
Johnson (1993) Mechanisms of and biomarkers for acute and delayed neuropathic effects of organophosphorus esters. In: Travis CC (ed) Use of biomarkers in assessing health and environmental impacts of chemical pollutants. NATO ASI Series, Plenum Press, New York, pp 169–182
Kello D (1989) WHO drinking water quality guidelines for selected herbicides. Food Addit Contam 6:579–585
Larson SJ, Gilliom RJ, Capel PD (1999) Pesticides in streams of the United States: Initial results from the National Water-Quality Assessment program. US Geological Survey Water Resources Investigations Report 98-4222, US Geological Survey, Sacramento, CA
Londono DK, Siegfried BD, Lydy MJ (2004) Atrazine induction of a family 4 cytochrome P450 gene in Chironomus tentans (Diptera: Chironomidae). Chemosphere 56:701–706
Plapp FW, Casida J (1970) Induction by DDT and dieldrin of insecticide metabolism by house fly enzymes. J Econ Entomol 63:1091–1092
Pratt JR, Melendez AE, Barreiro R, Bowers NJ (1997) Predicting the ecological effects of herbicides. Ecol Appl 7:1117–1124
Rakotondravelo ML (2004) Sublethal effects of three pesticides on the aquatic midge, Chironomus tentans (Diptera: Chironomidae). M.S. thesis, Kansas State University, Manhattan, KS
Richards RP, Baker DB (1993) Pesticide concentration patterns in agricultural drainage networks in the Lake Erie basin. Environ Toxicol Chem 12:13–26
SAS Institute (1996) SAS®/STAT User’s Guide: Vers 6.12, Cary, NC
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Solomon KR, Baker DB, Richards RP, Dixon KR, Klaine SJ, LaPoint TW, Kendall RJ, Weisskopf CP, Giddings JM, Giesy JP, Hall LW, Williams WM (1996) Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem 15:31–76
Stumpf N, Nauen R (2001) Cross-resistance, inheritance, and biochemistry of mitochondrial electron transport inhibitor-acaricide resistance in Tetranychus urticae (Acari: Tetranychidae) J Econ Entomol 94:1557–1583
Thurman EM, Goolsby DA, Meyer MT, Mills MS, Pomes ML, Koplin DW (1992) A reconnaissance study of herbicides and their metabolites in surface water of the Midwestern United States using immunoassay and gas chromatography/mass spectroscopy. Environ Sci Technol 26:2440–2447
US EPA (1994a) Pesticide industry sales and usage: 1992 and 1993 market estimates. EPA-733-K-94-001, Office of Prevention, Pesticides, and Toxic Substances, US Environmental Protection Agency, Duluth, MN
US EPA (1994b) Methods for measuring the toxicity and bioaccumulation of sediment-associated contaminants with freshwater invertebrates. EPA-600-R—94-024, Office of Research and Development, Duluth, MN
US EPA (2000) Reregistration eligibility science chapter for chlorpyrifos: Fate and environmental risk assessment chapter. US Environmental Protection Agency, Washington, DC
Van Metre PC, Callender E, Fuller CC (1997) Historical trends in organochlorine compounds in river basins identified using sediment cores from reservoirs. Environ Sci Technol 31:2339–2344
Zhu KY, Brindley (1992) Significance of carboxylesterases and insensitive acetylcholinesterase in conferring organophosphate resistance in Lygus hesperus populations. Pestic Biochem Physiol 43:223–231
Zhu KY, Gao J-R (1998) Kinetic properties and variability of esterase in organophosphate-susceptible and -resistant greenbugs, Schizaphis graminum (Homoptera: Aphididae). Pestic Biochem Physiol 62:135–145
Zhu KY, Gao J-R, Starkey SR (2000) Organophosphate resistance mediated by alterations of acetylcholinesterase in a resistant clone of the greenbug, Schizaphis graminum (Homoptera: Aphididae). Pestic Biochem Physiol 68:138–147
Zhu KY, Lee SH, Clark JM (1996) Validation of a point mutation of acetylcholinesterase associated with azinophosmethyl resistance and reduced fitness in Colorado potato beetle by polymerase chain reaction coupled to enzyme inhibition assay. Pestic Biochem Physiol 57:100–108
Acknowledgments
We thank Sharon R. Starkey for her technical assistance and Yoonseong Park for reviewing an earlier version of the manuscript. This research was partially supported by the USDA NRI program through Southern Illinois University-Carbondale and the Kansas Agricultural Experiment Station, Kansas State University, to K.Y.Z. and a Fulbright scholarship to M.R. This article is contribution No. 06-55-J from the Kansas Agricultural Experiment Station. The Chironomus tentans voucher specimens (110) are located in the Museum of Entomological and Prairie Arthropod Research, Kansas State University, Manhattan, Kansas, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rakotondravelo, M.L., Anderson, T., Charlton, R.E. et al. Sublethal Effects of Three Pesticides on Activities of Selected Target and Detoxification Enzymes in the Aquatic Midge, Chironomus tentans (Diptera: Chironomidae). Arch Environ Contam Toxicol 51, 360–366 (2006). https://doi.org/10.1007/s00244-005-0227-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-005-0227-0