Abstract
After indications from the literature that nutrient concentrations may modify the toxicity of herbicides to natural periphyton communities, this study aims to provide experimental proof for atrazine. In this microcosm experiment, phosphate (P) addition did not ameliorate atrazine toxicity to periphyton. Three weeks of P addition did not increase atrazine tolerance (measured as EC50 in acute toxicity tests), whereas exposure to atrazine under conditions that were either P-limited or non-P-limited clearly reduced the development of algal biomass. Long-term exposure to atrazine induced tolerance of the community to the herbicide, and this was not influenced by P addition. Tolerance induction in this microcosm experiment has been compared with previously published field data from the same area of study and indicates that tolerance induction by atrazine may take place under atrazine exposure in streams as well as in microcosms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surface runoff in human-impacted landscapes has many implications for the ecology of running waters. A major environmental problem in agricultural streams is increased nutrient loading due to the application of fertilizers and subsequent leaching of nutrients to surface and ground water (Ekholm et al. 2000). In addition, the intensive use of pesticides can lead to the contamination of these freshwater ecosystems (Pereira and Hostettler 1993; Ronday et al. 1998). For example, atrazine, one of the most widespread herbicides worldwide, has been detected in a wide range of surface and ground waters (Graymore et al. 2001), and this led to many studies on risks to freshwater ecosystems (Huber 1993; Solomon et al. 1996; Graymore et al. 2001). The effect of atrazine on algal communities may be influenced by many environmental and biological factors (Detenbeck et al. 1996; Guasch et al. 1997; Berard et al. 1999; DeLorenzo et al. 1999; Seguin et al. 2002; Weiner et al. 2004). However, the influence of a variable nutrient supply on the effect of atrazine-like herbicides on stream periphyton communities has received only marginal attention (Barreiro and Pratt 1994; Pratt and Barreiro 1998). The aim of this work was to test the influence of phosphate availability on periphyton atrazine toxicity. A previous study carried out in various other European rivers (Guasch et al. 1998) showed that periphytic communities growing in river sites with higher background atrazine concentration, but also high nutrient loads, were more tolerant to atrazine than those communities growing in the less polluted river sites. Because this was a field study, the results suggested that atrazine and/or nutrient loads could explain part of the variability of the data set, but there was no evidence of a causal relationship. The River Keersop, a lowland stream. that is a tributary of the polluted River Dommel in The Netherlands, was included in this study, and was placed by the ordination analysis among the less polluted sites. Based on the results and hypothesis formulated in this field study, a microcosm experiment was planned in order to investigate directly the interaction between phosphate availability and atrazine toxicity on stream periphyton from the River Keersop. These communities were experimentally enriched with phosphate and/or exposed to atrazine in laboratory microcosms for several weeks in order to identify the specific effects of each treatment and the interaction between them. The objectives of this study were, first, to investigate the influence of phosphate availability on atrazine toxicity and, second, to better understand the causes of variation in the tolerance of periphyton to atrazine under ecologically relevant field conditions.
Materials and Methods
Two consecutive experiments were performed during July and September 1996 to test the influence of nutrient addition (experiment 1) and the interaction between nutrient addition and atrazine exposure (experiment 2) on the tolerance of periphyton communities to atrazine.
Periphyton Communities
Periphyton communities were obtained by allowing them to colonize the surface of sand-blasted glass discs (1.5 cm2) mounted in racks suspended in the River Keersop for 1 week. The River Keersop (51°20′N, 5°24′E) is a relatively clean lowland stream that is a tributary of the polluted River Dommel, at the border between The Netherlands and Belgium (Ivorra et al. 1999). Colonized substrata were subsequently transported from the field in cool boxes and transferred to the experimental aquaria in the laboratory.
Laboratory Experiments
Experimental units were 10-L indoor aquaria described in Ivorra et al. (2002). Each aquarium was filled with 6 L of river water (Keersop) and contained 18 polyethylene holders of 10 glass substrata (total 180). Water was stirred with paddles moving at a speed of approximately 0.1 m/sec. Water temperature was controlled by a cooling system and light was provided with fluorescent tubes. Water temperature was 12°C and a 12 h/12 h light/dark cycle (110 μmol photons/m2 sec) was applied.
Experiment 1 (exp 1) involved three different treatments: control and two different phosphate concentrations. Six aquaria were used: two controls, two with 300 μg P/L (P-1), and two with 600 μg P/L (P-2). Experiment 2 (exp 2 ) involved four treatments: control, phosphate, atrazine, and phosphate plus atrazine. Eight aquaria were used: two controls, two with 300 μg P/L (P), two with 100 μg/L atrazine (ATR), and two with 300 μg P/L and 100 μg/L atrazine (P + ATR).
Phosphate was added in the form of K2HPO4 (Merck, Germany). Atrazine was added from a stock solution (Riedel-de Haen, Germany). Concentrated stock solution was dissolved in acetone. The Final acetone concentration was < 0.001% (v/v) in the treated aquaria.
Treatment effects were followed weekly by analyzing chlorophyll concentration. Photosynthetic activity and tolerance induction were determined at the end of both experiments (after 3 weeks of treatment). Tolerance induction was measured in short-term tests following the pollution-induced community tolerance (PICT) approach (Paulsson 2000).
Characterization of Water Chemistry
Before and after each renewal of test solutions in the laboratory, samples were taken to determine pH and the actual phosphate and atrazine concentrations. Phosphate concentration was determined according to Murphy and Riley (1962). Concentrations of atrazine were checked by high performance liquid chromatography using a 4.6*250-mm Intersil ODS 2.5-μm analytical column. A UV detector (Applied Biosystems, model 785) operating at 220 nm was used.
Algal Biomass
Five colonized glass substrata were taken every week from each aquarium to determine chlorophyll content. Chlorophyll-a was measured photometrically after sonication for 4 min and extraction in 90% acetone (Jeffrey and Humphrey 1975).
Short-Term Atrazine Toxicity Tests
Short-term atrazine toxicity tests followed the procedure described in Nystrom et al. (2000). At the end of each experiment (after 3 weeks), 40–50 periphyton glass discs were removed from each aquarium to perform a short-term test for inhibition of photosynthetic activity. The periphyton glass discs were detached from the polyethylene holders and stored in unfiltered control water during handling. The discs were distributed among 20-ml glass vials with 2 ml of river water strained through a glass-fiber filter (GF/F). An equal volume of atrazine solution was distributed from double-concentration stock solutions (prepared with GF/F-filtered river water) in a geometric concentration series (0; 0.43; 21.6, 43.1, 216, 431, 2157, and 4314 μg/L atrazine) with five replicates for each concentration. Carbon uptake in the controls was used to characterize the photosynthetic activity of periphyton after 3 weeks of exposure to the different treatments described above. The short-term tests were performed in an incubator. Incubations were carried out in continuously shaken river water; light and temperature conditions were the same as in the aquaria. After 60 minutes of pre-exposure, 100 μl of H14CO3 solution was added to each vial to estimate photosynthetic activity, The H14CO3 solution was prepared by a 200-fold dilution of a stock solution of 2 mCi/ml (Amersham, Buckinghamshire, UK) with milli-Q water adjusted to a pH of 9 with 0.1 M NaOH. After 1 h of incubation, the incorporation was ended by adding formaldehyde at a final concentration of 2% (v/v). To drive off the remaining inorganic carbon, 200 μl of concentrated acetic acid was added and the samples were dried at 60°C under a stream of air. To enhance the release of incorporated 14C carbon from the periphyton, 1 ml dimethylsulfoxide was added. After adding 9 ml of scintillation cocktail, radioactivity in the samples was measured using a TRI-CARB 1600 TR liquid scintillation analyzer (Packard, USA). Disintegrations per minute (DPM) were obtained using a standard quench-correction program. EC50 values were calculated from DPM using a nonlinear fit procedure. The photosynthetic rate from the controls was measured as the incorporation of 14C-HCO3. C-uptake per incubated glass was calculated taking into account the incorporation of 14C, the 14C-HCO3 added, and dissolved inorganic carbon (DIC).
Data Treatment
Differences between treatments in phosphate and atrazine concentration (four replicates × two aquaria per treatment), algal biomass (five replicates × two aquaria per treatment), photosynthesis (five replicates × two aquaria per treatment), and EC50 (one estimated value × two aquaria per treatment) were examined by analysis of variance (ANOVA) using the STATISTICA program. Between-factors ANOVA of repeated-measures was applied to detect treatment effects over time (time as a repeated factor), and between-factors ANOVA was applied to detect differences between treatments for each sampling date (Winer 1971). For the first experiment, the factors included in the ANOVA were time (with three levels) and phosphate (also with three levels). For the second experiment three factors were included: time (with three levels); phosphate (with two levels); and atrazine (also with two levels). For each date, treatment effects were then analyzed post hoc by a Tukey HSD test.
Results
Concentrations of Dissolved Compounds
The background concentrations (means ± SD) of phosphate were slightly lower (74 ± 23 μg P/L) and more stable during exp 1 than during exp 2 (114 ± 106 μg P/L). Stream water pH (7.7 ± 0.3) and conductivity (481 ± 33 μS/cm) were similar in both periods. Total inorganic carbon (TIC) was 22 mg/L, total organic carbon 8 mg/L, DIC 21 mg/L, and dissolved organic carbon 7 mg/L.
A good agreement between nominal and actual phosphate concentration was always obtained after water renewal (Table 1 and Figure 1). Phosphate concentrations declined after 3–4 days in all the aquaria. Consequently, phosphate concentrations before water renewals were 20–45% of the nominal values. Changes in pH were also observed (Table 1). Atrazine concentration after water renewal was 46% above nominal concentration and showed small changes over time. The remaining atrazine concentration before water renewal was always above 80% of the measured initial concentration (Table 1).
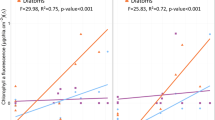
Phosphate concentration after water renewal in experiment 1 and experiment 2. Values are means (standard deviation) of seven samples taken throughout the experiment (upper graphs). Algal biomass (measured as chlorophyll concentration) and photosynthesis (measured as the incorporation of 14C-HCO3). Values are means (standard error) of 10 replicates obtained from two aquaria after 3 weeks of treatment. Different letters indicate significant differences between treatments analyzed post hoc by one- and two-way analysis of variance.
Periphyton Communities
In exp 1, controls and P-enriched aquaria differed in their algal growth. When the experiment started (day 0), the chlorophyll concentration was 0.29 (± 0.12) μg/cm2. Temporal changes in chlorophyll concentration were not significant in the controls (Table 2) but showed a marked (three- to fourfold) increase over time in both P treatments (ANOVA, factor time: F = 116.89, p < 0.00001; factor phosphate: F = 60.2, p < 0.00001). Post-hoc comparison between treatments for each sampling date gave the following results. Chlorophyll was not enhanced by P-addition after 1 week of treatment, but it was higher in both P-treatments than in the controls from week 2 onwards (week 2: factor phosphate: F = 37.7, p < 0.00001; week 3: factor phosphate: F = 32.3, p < 0.00001). Treatment effects on photosynthesis were similar to the effects on algal biomass (Table 2 and Figure 1). After 3 weeks of exposure, P-addition also caused a significant enhancement of photosynthesis (factor phosphate: F = 48.5, p < 0.00001). Algal biomass and carbon uptake were between three and four times higher in both P-treatments than in the controls (Fig. 1). EC50 values obtained by dose–response curves ranged between 216 and 308 μg/L atrazine and did not respond to P-addition (Fig. 2).
Effective atrazine concentration (EC50) obtained from short-term tests (2 h) of atrazine (eight concentrations) versus the photosynthesis of periphyton (measured as the incorporation of 14C-HCO3) after 3 weeks of treatment. Black and white bars correspond to periphyton communities from different aquaria. Different letters indicate significant differences between treatments obtained by analysis of variance.
When exp 2 started (day 0), the chlorophyll concentration was 0.30 (± 0.07) μg/cm2, The chlorophyll concentration increased in all the aquaria over time. It did not change in response to P-addition, and the increase was smaller in the aquaria with atrazine (Table 2). This was shown by the results of the ANOVA (factor time: F = 87.8, p < 0.00001; factor phosphate: F = 4.63, p = 0.03 and factor atrazine: F = 65.6, p < 0.00001). A post-hoc comparison showed that the effect of atrazine was significant from week 1 onwards (week 1: F = 21.8; p < 0.001; week 2: F = 25.2; p < 0.00001; week 3: F = 46; p < 0.0001). After 3 weeks of exposure, atrazine treatment caused a 40% reduction of algal biomass in comparison with the nonexposed communities (Figure 2), At the same time, a significant enhancement of C uptake in response to P-treatment was detected (factor phosphate: F = 8.44, p = 0.006) (Table 2, Figure 1). Atrazine reduced carbon uptake in the aquaria with P supply (P and P + ATR treatments), but the effects of atrazine were not significant in the aquaria with no P supply (control and ATR treatment, Figure 2). EC50 values for short-term atrazine exposure obtained from periphyton communities growing in aquaria without atrazine (Control and P treatment) did not differ significantly (293 and 235 μg/L, respectively) and were of the same magnitude as in experiment 1 (Figure 2). The long-term addition of atrazine reduced short-term toxicity (increased the EC50) independently of P-supply (factor atrazine; F = 17.7; p = 0.014).
Discussion
Interaction Between Phosphate and Atrazine Toxicity
This microcosm study shows that the sensitivity to atrazine in periphyton communities limited by P does not differ from that in nutrient-replete communities.
The enhancement of photosynthesis or the increase in algal biomass after P-addition is indicative of P-limitation. In the first experiment, P-addition caused an increase in photosynthesis and algal biomass about four times higher than in the controls. In the second experiment, P-limitation was also detected, because photosynthesis was clearly enhanced after P-addition. However, the effects were small and were not observed as increased algal biomass. On this occasion, background phosphate concentration and initial algal biomass were slightly higher than in the first experiment, indicating that phosphate limitation was less evident.
P-supply could not reduce the inhibitory effect of atrazine on periphyton and did not significantly influence tolerance induction by atrazine exposure. When periphyton was exposed to atrazine together with P-supply (P + ATR treatment), there was no reduction in the toxic effects on algal biomass, which were of the same magnitude as those observed under background P-concentration: 40% reduction in algal biomass in both the P + ATR and the ATR treatments (Figure 1).
In contrast with the results obtained from other phytotoxic compounds such as copper and zinc, which clearly demonstrate that P addition reduces metal toxicity (Ivorra et al. 2002; Paulsson et al. 2002; Guasch et al. 2004), there is no evidence in the literature supporting the existence of an antagonism between phosphate and the effect of herbicides such as atrazine. This difference is probably related to their different modes of action (Guasch et al. 2003). Several studies indicate that herbicides inhibiting photosynthesis (e.g., atrazine) are the most toxic to carbon uptake, whereas herbicides interfering with other cell metabolic processes (e.g., zinc, copper) are the most toxic to phosphate and ammonium uptake. In the case of atrazine, Brown and Lean (1995) reported effects on carbon uptake at atrazine levels between two and three orders of magnitude lower than those required to reduce the uptake of phosphate and ammonia. Similar conclusions may be derived from the results obtained by Barreiro and Pratt (1994) in a mesocosm study with diquat. In this investigation, diquat inhibited photosynthesis but did not cause significant changes on the alkaline phosphatase activity of the community, in contrast with the marked influence that zinc or copper may cause on this metabolic endpoint (Paulsson et al. 2002).
In contrast with the negative interaction between phosphate and zinc or copper toxicity described above, a positive interaction or an absence of interaction between phosphate and other phytotoxic compounds has been observed at different levels of biological organization. Mohapatra and Mohanty (1992) investigated the differential effect of dimethoate on Anabaena doliolum with change in nutrient status. Herbicide toxicity was reduced by the addition of N2, and this was attributed to the mode of action of dimethoate inhibiting nitrogen fixation. However, phosphate addition increased the toxic effect of the herbicide, and this was associated with an additional stress caused by phosphate. Similarly, Shabana et al. (2001) found that with increasing relative concentration of phosphate (N:P < 1), the effect of the herbicide pendimethalin on the green algae Protosiphon botryoides increased. Battah et al. (2001) found that the addition of phosphate did not influence the inhibition of Anabaena variabilis growth by thiobencarb.
Investigations performed at the community level produce results that are in agreement with those obtained with cultures. Detenbeck et al. (1996) observed that the effect of atrazine on the algal communities growing in wetland mesocosms was higher with the addition of phosphate (positive interaction), whereas Barreiro and Pratt (1994) and Pratt and Barreiro (1998) exposed periphyton communities to diquat under different nutrient regimes and obtained similar effects on the structure and function of periphyton (no interaction).
Tolerance Induction
Atrazine exposure induced an increase in community tolerance (determined by short-term toxicity tests) and this induction was not influenced by P-supply. Induction of atrazine tolerance after a few weeks of exposure has also been described for periphyton and phytoplankton (Nystrom et al. 2000; Seguin et al. 2002), but the role of nutrients in tolerance induction has not previously been addressed. In our microcosms, 3 weeks of exposure to atrazine reduced the algal biomass, but this reduction was relatively small (less than 50%) and the photosynthetic rate of the community was not inhibited (Table 2), indicating that a part of the community could overcome the inhibitory effect of this toxicant. These results indicate that the process of tolerance induction to atrazine does not require P-supply, supporting the view that P-enrichment will not influence atrazine toxicity in the long term.
Comparison Between Experimental and Field Data on the Sensitivity of Stream Periphyton to Atrazine
The results of atrazine toxicity obtained in this laboratory experiment were compared with atrazine toxicity measurements on stream periphyton communities with the aim of supporting the hypothesis that atrazine pre-exposure may induce tolerance in the field and that nutrient availability will not interfere with this tolerance induction. These field observations were part of a larger study on European streams (Guasch et al. 1998), and only local observations in the same river system (nonshaded sites of the R. Meuse) are reported here for comparison. The EC50 values measured in these stream periphyton communities (377 ± 113 μg atrazine/L) are in the same range as those obtained in our microcosm experiments (392 ± 173 μg atrazine/L). In addition, periphyton communities growing in river sites with higher background atrazine concentration, but also high nutrient loads, had lower sensitivity to atrazine than those communities growing in pristine river sites. Background atrazine concentration was always low (< 0.5 μg atrazine/L, but the presence of traces of atrazine in the river water was considered as an indication of episodes of higher concentration that were not recorded because of the limited sampling. The River Keersop’s periphyton community presented one of the lowest EC50 values (278 μg atrazine/L), as did the less polluted rivers (302 μg atrazine/L in the River Geul and 327 μg atrazine/L in the River Neerpelt). At the opposite end of the scale were periphyton communities growing in the most polluted rivers (i.e., the River Keizsesveer and the River Eijsden) presenting traces of atrazine (01–0.2 μg atrazine/L) and higher EC50 values (427 and 552 μg atrazine/L, respectively).
In this experiment, periphyton communities exposed for 3 weeks at 130 μg atrazine/L became twice as tolerant to this herbicide (EC50 = 631 ± 50 μg atrazine/L) as the nonexposed communities (EC50 = 296 ± 20 μg atrazine/L). However, the addition of phosphate alone did not cause any significant change in the EC50: 338 ± 76 and 268 ± 35 μg atrazine/L in the control and P-treatments, respectively. We can therefore expect to detect pre-exposure to atrazine by tolerance induction in the fluvial periphyton community with no major influence of nutrient supply.
References
Barreiro R, Pratt JR (1994) Interaction of toxicants and communities: the role of nutrients. Environ Toxicol Chem 13:361–368
Battah MG, Shabana EF, Kobbia IA, Heladle HM (2001) Differential effects of thiobencarb toxicity on growth and photosynthesis of Anabaena variabilis with changes in phosphate level. Ecotoxicol Environ Saf 49:235–239
Berard A, LeboulangerC, Pelte T (1999) Tolerance of Oscillatoria limnetica Lemmermann to atrazine in natural phytoplankton populations and in pure culture: influence of season and temperature. Arch Environ Contam Toxicol 37:472–479
Brown LS, Lean DRS (1995) Toxicity of selected pesticides to lake phytoplankton measured using photosynthesis inhibition compared to maximal uptake rates of phosphate and ammonium. Environ Toxicol Chem 14:93–98
DeLorenzo ME, Lauth J, Pennington PL, Scott GI, Ross PE (1999) Atrazine effects on the microbial food web in tidal creek mesocosms. Aquat Toxicol 46:241–251
Detenbeck NE, Hermanutz R, Alien K, Swift MC (1996) Fate and effects of the herbicide atrazine in flow-through wetland mesocosms. Environ Toxicol Chem 158:937–946
Ekholm P, Kallio K, Salo S, Pietilanen OP, Rekolainen S, Laine Y, Joukola M (2000) Relationship between catchment characteristics and nutrient concentrations in an agricultural river system. Water Res 15:3709–3716
Graymore M, Stagnitti F, Allison G (2001) Impacts of atrazine in aquatic ecosystems. Environment Int 26:483–495
Guasch H, Muñoz I, Rosés N, Sabater S (1997) Changes in atrazine toxicity through succession of stream periphyton communities. J Appl Phycol 9:137–146
Guasch H, Ivorra N, Lehmann V, Paulsson M, Real M, Sabater S (1998) Community composition and sensitivity of periphyton to atrazine in flowing waters: the role of environmental factors. J Appl Phycol 10:203–213
Guasch H, Admiraal W, Sabater S (2003) Contrasting effects of organic and inorganic toxicants on freshwater periphyton. Aquat Toxicol 64:165–175
Guasch H, Navarro E, Serra A, Sabater S (2004) Phosphate limitation influences the sensitivity to copper in periphytic algae. Freshwat Biol 49:463–473
Huber W (1993) Ecotoxicological relevance of atrazine in aquatic systems. Environ Toxicol Chem 12:1865–1881
Ivorra N, HettelaarJ, Tubbing GMJ, Kraak MHS, Sabater S, Admiraal W (1999) Translocation of microbenthic algal assemblages used for in situ analysis of metal pollution in rivers. Arch Environ Contam Toxicol 37:19–28
Ivorra N, Hettelaar J, Kraak MHS, Sabater S, Admiraal W (2002) Responses of biofilms to combined nutrient and metal exposure. Environ Toxicol Chem 21:626–632
Jeffrey SW, Humphrey GF (1975) New spectophotometric equations for determining cholophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Bioch Physiol Pflanz 167:191–194
Mohapatra PK, Mohanty RC (1992) Differential effect of dimethoate toxicity to Anabaena doliolum with change in nutrient status. Bull Environ Contam Toxicol 48:223–229
Murphy J, Riley JP (1962) A modified single solution for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nyström B, Paulsson M, Almgren K, Blank H (2000) Evaluation of the capacity for developement of astrazine tolerance in peripyton from a Swedish freshwater site as determined by inhibition of photosynthesis and sulfolipid synthesis. Env Toxicol Chem 19:1324–1331
Paulsson M (2000) Variability in lotic periphyton communities tolerance to zinc and atrazine, in relation to bioavailability. PhD, Göteborg University, 213 pp
Paulsson M, Mansson V, Blanck H (2002) Effects of zinc on the phosphorus availability to periphyton from the Göta Älv. Aquat Toxicol 56:103–113
Pereira WE, Hostettler FD (1993) Nonpoint source contamination of the Mississippi River and its tributaries by herbicides. Environ Sci Technol 27:542–1552
Pratt JR, Barreiro R (1998) Influence of trophic status on the effect of a herbicide: a microcosm study. Arch Environ Contam Toxicol 35:404–411
Ronday R, Aalderink GH, Crum SJH (1998) Application methods of pesticides to aquatic mesocosms in order to simulate effects of spray drift. Water Res 32:147–153
Seguin F, Le Bihan F, Leboulanger C, Bérard A (2002) A risk assessment of pollution: induction of atrazine tolerance in phytoplankton communities in freshwater outdoor mesocosms, using chlorophyll fluorescence as an endpoint. Water Res 36:3227–3236
Shabana EF, Battah MG, Kobbia IA, Eladen HM (2001) Effect of pendimethalin on growth and photosynthetic activity of Protosiphon botryoides in different nutrient states. Ecotoxicol Environ Saf 49:106–110
Solomon K, Baker D, Richards R, Dixon K, KIaine S, La Point T (1994) Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem 15:31–76
Winer BJ (1971) Statistical principles in experimental design. MaGraw Hill, New York, 907 pp
Weiner JA, DeLorenzo ME, Fulton MH (2004) Relationship between uptake capacity and differential toxicity of the herbicide atrazine in selected microalgal species. Aquatic Toxicol 68:121–128
Acknowledgments
This research was performed within the framework of the EC (1996/99) project “Microbenthic Communities in European Rivers Used to Assess Effects of Land-Derived Toxicants” ENV4-CT96-0298 and the Spanish Ministry of Science and Technology project “Dynamics of toxic substances in river ecosystems: effects on river biofilms and consequences for water quality” REN2003-0772.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guasch, H., Lehmann, V., van Beusekom, B. et al. Influence of Phosphate on the Response of Periphyton to Atrazine Exposure. Arch Environ Contam Toxicol 52, 32–37 (2007). https://doi.org/10.1007/s00244-005-0186-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-005-0186-5