Abstract
The concentration of mercury (Hg) and methylmercury (MeHg) was determined for largemouth bass (Micropterus salmoides), bluegill (Lepomis macrochirus) and black crappie (Pomoxis nigromaculatus) from Maryland reservoirs. Overall, there was a large difference in normalized bass MeHg concentration (for fish of ∼370 mm) between the reservoirs, ranging from <100 ng g−1 to almost 800 ng g−1. Furthermore, the relationship between fish weight and MeHg concentration varied substantially between lakes, and showed no geographical relationship. The concentration of Hg, MeHg and ancillary parameters were determined in the water and correlations were sought between the normalized concentration of MeHg in bass and both physical and chemical parameters of the reservoirs, as well as the concentration of MeHg in the prey of the largemouth bass. Bass MeHg concentration correlated with dissolved MeHg and dissolved organic carbon, but not with other chemical parameters. There was no relationship to physical characteristics that varied over orders of magnitude for these reservoirs. Dissolved MeHg did not correlate with any chemical or physical attributes. Overall, this study suggests that water column MeHg is a good predictor of fish concentration but that the water column MeHg cannot be predicted based on usually measured chemical and physical characteristics of fresh water bodies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mercury (Hg) contamination in freshwater fish has been recognized as a problem in Europe and North America for over three decades. Elevated concentrations of the organic form of Hg (methylmercury, MeHg) in fish is considered a health concern, because consumption of fish is the largest source of MeHg in the human diet (WHO 1990) and that of fish-eating wildlife (Wolfe et al. 1998; Wiener et al. 2002). Atmospheric transport and deposition is the major source of mercury to remote lakes, and inputs have increased as a result of anthropogenic activities (Mason et al. 1994). Studies in Maryland (Mason et al. 1997a, 1997b, 2000a) suggest that atmospheric deposition to this region is higher than in other locations in the United States. Sources of MeHg to the aquatic system have been identified to be from precipitation, in-lake methylation, and runoff from wetlands (Hultberg et al. 1994; Watras et al. 1994; Rudd 1995), but in situ production is the primary source of MeHg for most aquatic systems (Gilmour et al. 1992; Benoit et al. 2003). MeHg differs from the inorganic form in that it is more toxic, more mobile, and more readily bioaccumulated by aquatic organisms. Thus, it accounts for the majority of the total mercury (HgT) in the muscle tissue of higher trophic level (piscivorous) freshwater fish (Grieb et al. 1990; Bloom 1992; Bloom and Watras 1992).
In the United States, most advisories issued for contaminants in fish are for MeHg, and currently 45 states have issued fish advisories for Hg (USEPA 1995a, 2004). The state of Maryland issued an advisory for freshwater reservoirs in December 2001, based on the preliminary data from this study and others (MDE 2004; Gilmour 1999; Gilmour and Riedel 2000). The intention of the study detailed here was to investigate MeHg levels primarily in largemouth bass (Micropterus salmoides), and secondarily in bluegill (Lepomis macrochirus) and black crappie (Pomoxis nigromaculatus) in Maryland reservoirs and the factors influencing MeHg levels in these fish. The focus of the study was fish that were recreationally important, and thus only fish of allowable size were investigated. Largemouth bass are found in all waters of Maryland, from freshwater to brackish water. They are primarily fish-eating predators, although their diets also include invertebrates and amphibians, and bass in Maryland reservoirs appear to have a propensity for consuming crayfish, if available (Murdy et al. 1997). Water column characteristics were measured and these were related to fish concentration, as were other physical and biological parameters of the reservoirs. Such relationships allow an evaluation of the factors influencing the levels of MeHg in Maryland fish, and the factors controlling bioaccumulation.
Materials and Methods
Study Sites
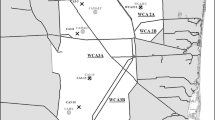
Fish were collected from 20 reservoirs in Maryland (Fig. 1) during late spring and summer of 2000 and 2001. Details on each reservoir are contained in Table 1. Information about the number of fish collected and analyzed as well as the mean length and weight of the fish is shown in Table 2. The reservoirs in eastern Maryland (Lake Lariat, Tuckahoe, St. Mary’s Lake, and Johnson’s Pond) are characterized by relatively low elevation above mean sea level, whereas the reservoirs in western Maryland (Deep Creek Lake, Broadford Lake, Savage, Piney (Frostburg), and Rocky Gap) are located on the Appalachian Plateau, approximately 400–600 m above mean sea level. The geological composition in western Maryland is primarily siltstone, limestone, shale, and coal. Precambrian metamorphic geology prevails in central Maryland, where most of the reservoirs are located (Big Pool and Potomac #4 on the Potomac River, Duckett, Clopper, Tridelphia, Piney Run, Liberty, and Prettyboy reservoirs, Loch Raven, and Conowingo Dam), and Quaternary sand, silt, clay, and peat abound the Eastern Shore (USGS 2001).
Location of the study sites in Maryland. Acronyms are used for the reservoirs in all figures as follows: DCL = Deep Creek Lake; BFL =Broadford Lake; SAV = Savage Reservoir; PINF = Piney Dam, Frostburg; RGP = Rocky Gap Park Reservoir (Lake Habeeb); BP = Big Pool, on the Potomac River; POT4 = Potomac #4, on the Potomac River; PBY = Prettyboy Reservoir; LRV = Loch Raven; DUC = Lake Duckett; CEN = Centennial Lake; TRI = Tridelphia Reservoir; LIB = Liberty Reservoir; CLO = Clopper Lake; PRN = Piney Run Reservoir; LLR = Lake Lariat; SML = St Marys Lake; CON = Conowingo Dam; TUC = Lake Tuckahoe; JHP = Johnson’s Pond
All of the impoundments are relatively old, and thus their physicochemical characteristics should resemble that of a natural lake, and there should be no “reservoir effect” on fish concentration (Bodaly et al. 1984, 1997). However, this was tested and is discussed below. Three reservoirs (Rocky Gap, Loch Raven, and Piney (Frostburg)) have been modified in the last 15 years (1988, 1986, and 1990, respectively; Table 1). The reservoirs range widely in depth (1.5 m–54.5 m), volume (3.2 × 104–3.8 × 108 m3), and in surface area (20–8960 acres) and all have inflow from rivers and outflow controlled by a dam (MD DNR 2001).
Fish Collection and Analysis
Fish were collected by electroshock techniques in early spring/summer 2000 and 2001. The target species for this study was largemouth bass (Micropterus salmoides), but because of the potential for not finding this species in all reservoirs, bluegill (Lepomis macrochirus) and black crappie (Pomoxis nigromaculatus) were also collected to aid in the comparison of inter-lake differences. All species were collected based on three size classes, with five fish in each size class, to ensure, to the degree possible, a wide distribution in fish size for all the reservoirs. All fish were analyzed individually except for the 2001 samples of bluegill and black crappie, where each size class was treated as a composite sample. Largemouth bass were found in all reservoirs and ranged in size from 283 to 541 mm in length, and weight from 224 to 2176 g. Although some reservoirs had both bluegill and black crappie, most had only one of the species. In the field, all fish were handled by gloved personnel and, after rinsing and measuring, each fish was bagged in a plastic bag. The bagged fish were further wrapped in aluminum foil, then bagged in a second bag and shipped on ice overnight to the Chesapeake Biological Laboratory (CBL). There the fish were filleted and the axial muscle (representing the portion of fish normally consumed by humans) was removed, homogenized, and frozen until further analysis. All sampling equipment was acid cleaned prior to use and in between deployments.
For MeHg analysis of the fish, approximately 1g of subsampled axial muscle was placed in a Teflon vial and digested in an alkaline digest in a VWR Scientific forced-air oven at 60°C (Bloom 1989; Horvat et al. 1993). The MeHg was then analyzed using ethylation derivitization and preconcentration using purge and trap techniques, followed by isothermal gas chromatography separation and quantification with cold vapor atomic fluorescence spectroscopy (CVAFS) (Bloom 1989; Mason et al. 2000b); see details below. Total Hg in tissues was determined on an extract obtained by overnight digestion in a sulfuric/nitric acid solution. The diluted extract was then brominated with BrCl to ensure complete digestion, followed by tin chloride reduction and purge and trap collection of the Hg on a gold column. Quantification was by CVAFS (Mason et al. 2000b).
Water Sampling and Analysis
Water sampling was conducted during midsummer 2001 and late spring/early summer 2002 and 2003 in an attempt to sample the reservoirs when they were thermally stratified. The sampling was done in accordance with EPA Method 1669 (USEPA 1998). Surface samples were collected by hand from a boat. Clean, acid-filled double-bagged 2L Teflon bottles were used for water collection. Prior to sampling, each bottle was emptied and rinsed with water downstream from the sampling location. The filled bottle was recapped, double-bagged, and stored in a cooler for transport back to the laboratory. Approximately 1 L of sample from each bottle was filtered for Hg and MeHg onto quartz fiber filters (precleaned by combustion in a furnace overnight at 600°C) using acid-cleaned glassware. All equipment used for filtering was acid washed between samples and rinsed with Q-water. Filtered and unfiltered water was stored in acid-cleaned Teflon bottles and both water samples were spiked with Optima HCl acid and stored in a refrigerator until analyses were performed.
For MeHg analysis, water samples were distilled after additions of a 50% sulfuric acid solution and a 20% potassium chloride solution (Horvat et al. 1993). The MeHg in the distillate was derivitized with sodium tetraethylborate to convert it to volatile methyl-ethyl-mercury (Bloom 1989). The volatile adduct was then purged from solution and collected onto a Tenax trap. The MeHg was thermally desorbed from the trap and analyzed by isothermal gas chromatography separation with CVAFS. Total Hg was measured in water samples after BrCl oxidation of samples (0.5 mL of 2N BrCl added; Bloom and Crecelius 1983, USEPA 1995b) and pre-reduction with hydroxalamine. The samples were then reduced with tin chloride solution, purged to remove elemental Hg to a gold trap, and the amount of Hg in samples was then determined by two-stage gold amalgamation CVAFS (Bloom and Fitzgerald 1988).
Not all the reservoirs’ waters were sampled as part of this study. For Conowingo Dam, water samples were not collected because samples had been collected by our group over a 12-month period as part of a study of Hg biogeochemistry in rivers flowing into the Chesapeake Bay (Lawson et al. 2001). The collection and analytical techniques were comparable with this study. No water samples were taken from Rocky Gap, and data for total Hg, collected by Castro et al. (2002) using similar techniques, is used in the comparison of data below. Big Pool and Potomac #4 are on the Potomac River in relatively close proximity to each other and only one set of water samples was collected for these sites. Fish from Centenial Lake were only collected in the latter part of the project and because of season, no water samples were collected from this reservoir.
The pH of the water was measured at the laboratory using standard techniques. The pH meter was calibrated using buffered solutions on each occasion. Particulate organic carbon (POC), nitrogen, and total suspended solids (TSS) were subsampled from an unfiltered water sample and collected onto a glass fiber filter (GF/F 0.7 μm). Dissolved organic carbon (DOC), dissolved nutrients, chloride, and sulfate were subsampled from filtered water and stored frozen until subsequent analysis. All ancillary parameters were measured by CBL Analytical Services.
Quality Control Procedures
Standard calibration curves with r2 of at least 0.99 were run daily and a standard addition spike was added to 1 in every 20 samples to check for matrix interferences. Laboratory duplicates and external certified standard reference materials (SRM) (digestates of IAEA SRM 142) of known MeHg and total Hg concentrations were analyzed daily to ensure the accuracy of results. Duplicate analysis of 10% of the MeHg samples yielded no significant difference, and 95% of all SRM replicates analyzed for MeHg fell within the certified ranges. Detection limits (DL) for MeHg were based on three standard deviations of sample blank measurements. The DL for MeHg was 0.01 ng/L for water and 0.1 ng/g for fish. Field and travel blanks were typically less than the detection limit. For total Hg, the DL was 0.1 ng/L for water and 0.05 ng/g for fish.
To investigate the stability of MeHg in frozen fish tissue, analysis of MeHg in one fish sample was performed 11 times during a 17-month period. Results from this analysis show that MeHg concentrations in the frozen fish sample remained stable over this period (average 20.5 ± 4.7 ng g−1 wet weight). The slope of the relationship between concentration and time was not significantly different from zero.
Statistical Analysis
Pearson correlation analysis was used to determine p values between length and weight of fish and MeHg concentration and weight. For the interlake comparison, fish data were normalized to the length of fish, using a range of 362 to 377 mm, to remove variability based on the fish size from the examination of correlations between variables. To obtain the normalized concentration, the regression equations for each reservoir shown in Fig. 6 were used to estimate the concentration in a fish of 370 mm. For those reservoirs where no relationship was evident, the concentration was estimated based on the concentration in fish within the 362–377 mm size range. The correlations between normalized fish concentration and other variables were examined using both linear regression of variables and through multivariate analysis (stepwise multilinear regression). Linear regression equations were determined using either Sigmaplot or Excel, whereas the multivariate regression was done using Quattro Pro. Comparison of regression coefficients between Sigmaplot and Excel showed that both software packages returned the same values of the regression equations.
Results and Discussion
Largemouth Bass
Information about the average length, weight, and MeHg concentration in each fish from the reservoirs is contained in Table 2. Fish length ranged from 276 to 563 mm (376 ± 54, n = 249). Mean length was highest in Lake Lariat at 438 ± 80 mm (n = 7), and lowest in Big Pool at 304 ± 16 mm (n = 5). Weight of the bass ranged from 224 to 2688 g (777 ± 391, n = 249) with the mean weight highest in Lake Lariat, 1246 ± 669 g (n = 7) and lowest in Big Pool, 322 ± 72 g (n = 5). Correlation between total length (mm) and weight (g) of largemouth bass revealed a significant positive correlation when fish from all reservoirs were combined (p < 0.01, r2 = 0.92, n = 249; slope = 6.99 g mm−1) (Fig. 2). There is some indication of a deviation from a straight line for the largest fish. Such a strong relationship between length and weight can be an indicator that the fish live under similar conditions in all reservoirs and none of the populations are under significant stress. Although this relationship does not indicate that all of the fish are growing at the same rate, growth rates for largemouth bass have been found to be relatively similar across the study reservoirs (Klotz and Johnson 2000) and are close to the statewide average (Elser 1962) (Fig. 3). On a regional basis, there are differences in the length–weight relationship. For western Maryland, the slope of the length–weight relationship (5.80 g mm−1) is lower than for the reservoirs in central Maryland (6.58 g mm−1) and coastal Maryland (7.86 g mm−1). So, overall, fish of the same length weighed more for the coastal reservoirs than for the others, and those in western Maryland reservoirs weighed the least. Such differences may be expected, given that the reservoirs in the coastal zone likely warm up more quickly and are more productive earlier in the season because of differences in climatic factors, such as elevation, amount of snow, and the mediating coastal effects on temperature. A comparable strong relationship between the weight and length of largemouth bass has been documented in other states where Hg studies have been done, such as in the northeast United States (Rose et al. 1999; Hanten et al. 1998).
Growth rate of largemouth bass in reservoirs and lakes in Maryland. Growth rate reported by Elser (1962) is an average for the state. Taken from MD DNR (2000)
Large largemouth bass are considered to be piscivorous (Lioa et al. 2002), although there was an indication of a preference for crayfish in the limited gut analysis done in this study (Sveinsdottir 2002), and thus differences in growth rate may reflect differences in food availability and type. Growth rate can potentially influence the MeHg bioaccumulation rate. For example, for a 500-mm fish, extrapolation of the growth data in Fig. 3 indicates that ages range from somewhat more than 8 years to about 11 years. This is a fairly important difference for the larger fish. For the smaller fish, the differences are less pronounced. No growth rate data are available for the reservoirs of the coastal plain. However, if the fish were growing more quickly, then it is expected that the concentration of MeHg in the tissue would be lower because bioaccumulation is more a factor of age than size, and growth dilution is an important variable in terms of fish MeHg concentration (Simoneau et al. 2005; Jackson 1991; Hudson et al. 1994). However, there was no trend between concentration and reservoir location (Table 2), and the highest concentration fish are from coastal reservoirs, which is contrary to expectation, based on the influence of growth rate on MeHg concentration.
MeHg concentrations in the fish fillet samples ranged from 9.0 to 2077 ng g−1 wet weight (mean = 325 ± 283 ng g−1, n = 249) (Fig. 4; Table 2). These values are similar to the documented range of MeHg in largemouth bass in Massachusetts (Rose et al. 1999), Maine (Stafford and Haines 1997), and in Connecticut (Hanten et al. 1998; Ward and Neumann 1999). A significant statistical difference was observed between the reservoirs containing the average highest and lowest MeHg in the largemouth bass (p < 0.001). Mean MeHg concentrations in the fish were highest in Lake Lariat, 1044 ± 580 ng g−1 wet weight (n = 7) and lowest in fish from Centennial, 98.5 ± 59.1 ng g−1 wet weight (n = 7) (Table 2). Variability in fish size between reservoirs can account for some of this difference. However, the estimated normalized concentration for each reservoir, for fish of 370 mm (362–377 mm (∼4–5-year-old fish); Fig. 5), demonstrates that there are important differences between reservoirs that cannot be accounted for by size. Note that all reservoirs are not in Fig. 5 because of limitations in numbers of fish and the ability to estimate fish concentration for the size range. Based on the normalized concentrations, the highest largemouth bass concentrations were in fish from two coastal reservoirs (Lake Lariat and St. Mary’s Lake) and in two western reservoirs (Piney (Frostburg) and Savage reservoirs). The MeHg concentrations in fish in the central Maryland reservoirs were overall the lowest, although low fish concentrations were found in reservoirs from all the regions.
Standardized concentrations of methylmercury in largemouth bass from all the reservoirs. The standardized length is based on concentration–length regression data for fish within the 362–377 mm size range (average 370 mm) for each reservoir. If no regression line was obtained, concentrations were estimated from the available data. Acronyms are the same as in Fig. 1.
There was a strong correlation between fish MeHg concentration and length and weight for all largemouth bass (Fig. 4; for length, r2 = 0.26; for weight, r2 =0.28; p < 0.01). A positive correlation with size and concentration of MeHg in fish has been documented in many other instances for largemouth bass (Rose et al. 1999; Hanten et al. 1998; Horwitz et al. 1995), and for other predator fish species (e.g., Stafford and Haines 1997; Ward and Neumann 1999). The relationship between weight and total MeHg concentration in largemouth bass in each reservoir is illustrated in Fig. 6. Similar relationships were found for length (not shown). Fifteen reservoirs showed a significant correlation (p < 0.05) between the concentration and size variables. For the other reservoirs, data were either too limited, showed no trend, or had high variability. The rate of accumulation of MeHg into the fish (shown as regression slopes) (ranging from 0.09 to 0.96 (ng g−1) g−1 for weight) show that accumulation rates vary substantially between reservoirs—overall by an order of magnitude—even for those in close proximity.
Individual reservoir plots of methylmercury content versus fish weight for largemouth bass. Graphs (a)–(c) represent reservoirs from western Maryland. DCL = Deep Creek Lake; BFL = Broadford Lake; SAV = Savage; PINF = Piney Dam, Frostburg; RGP = Rocky Gap; BP = Big Pool; and POT4 = Potomac #4. Graphs d)-f) are for reservoirs within central Maryland. PBY = Prettyboy Reservoir; LRV = Loch Raven; DUC = Duckett; CEN = Centennial; TRI = Tridelphia; and LIB = Liberty. Graphs (g)–(i) are for reservoirs within both central and coastal Maryland. CLO = Clopper; PRN = Piney Run Reservoir; CON = Conowingo Dam; TUC = Tuckahoe; JHP = Johnson’s Pond; LLR = Lake Lariat; and SML = St Mary’s Lake. Note the different scales for graph (i).
Within a region, such as in western Maryland, reservoirs had different regression slopes covering the range of values. Deep Creek, Broadford, and Piney (Frostburg), which are geographically close (Fig. 1), had similar slopes on a weight basis (0.39, 0.21, and 0.41 (ng g−1) g−1, respectively; Fig. 6a and 6b), whereas Savage, which is also in the vicinity of these reservoirs, had a relatively steeper regression slope of 0.86 (ng g−1) g−1. In comparison, Big Pool, on the Potomac River, and Rocky Gap, which are located east of the other four reservoirs (Fig. 1), showed no relationship between weight and fish MeHg concentration, and the fish showed little change in concentration with size (Fig. 6c). Concentrations were also low compared to the other reservoirs. Similar results have been documented by others (Simonin et al. 1994; Rose et al. 1999; Sonesten 2001), where regression slopes between weight and MeHg concentration in tissue of largemouth bass, brown bullhead, and perch in a variety of lakes were found to be unequal.
The variability of slopes implies individual trends in each of the reservoirs and reflects the effects of chemical and/or physical characteristics of the reservoir on the MeHg accumulation by the fish. Additionally, biological factors such as growth rate, age, size, physiology, and diet might also influence the accumulation and final concentration of MeHg in largemouth bass. As seen in Fig. 6, the regression lines may not intercept the y-axis at 0, suggesting a change in slope with age, or a nonlinear relationship overall. This would be expected if largemouth bass change their food preferences with age (i.e., change their rate of MeHg uptake). Typically, fish feed at higher trophic levels when older, thus increasing their MeHg intake as they age. Also, their rate of growth decreases with age (Fig. 3) and as a consequence, the effect of growth dilution on MeHg concentration decreases. The largemouth bass in this study are those that have reached harvestable length (circa 305 mm = 4 years on average, Klotz and Johnson 2000), and it is reasonable to approximate the slope of the relationships for these older fish with a linear equation. This approach is validated by comparison of the estimated MeHg concentrations in smaller largemouth bass from three reservoirs (Piney (Frostburg), Deep Creek, and Rocky Gap) from Castro et al. 2002; only total Hg was measured, and it has been assumed here that 90% of HgT in the fish muscle is MeHg) with the data collected in this study (Fig. 7). This comparison demonstrates that MeHg concentrations are relatively low in small fish, but also shows that the rate of increase is fairly linear for these reservoirs. Indeed, if anything, the regression slope is decreasing somewhat with increasing fish weight.
Comparison of the data collected under this project with those collected by others (Castro et al. 2002), which mostly involved smaller fish, for three reservoirs in western Maryland. DCL = Deep Creek Lake; PINF = Piney, Frostburg; and RGP = Rocky Gap Park.
The relationships in Fig. 7 for the various reservoirs corroborate the analysis based on the larger fish that the rate of increase for the fish concentration in Piney (Frostburg) with weight is much larger than that for Rocky Gap, with the rate of accumulation being intermediate for fish in Deep Creek. Changes and differences in diet alone could account for these differences between lakes, although other factors are also likely important. Although stomach analysis was done in an observational fashion in this study, the results, and other anecdotal evidence, suggest that largemouth bass in Maryland consume crayfish as a large fraction of their prey (70% of identified prey; Sveinsdottir 2002). These omnivorous invertebrates are “lower” in the food chain than small fish, as demonstrated in a stream study in western Maryland (Mason et al. 2000b) where the crayfish had a lower bioaccumulation factor and a lower %MeHg in tissue than either predatory insects or small fish, such as dace and sucker. Thus, a bass feeding predominantly on crayfish should have a lower burden than one feeding exclusively on small fish, all else being equal. Thus, given the caveat that a one-time analysis of food in fish stomachs gives no real indication of feeding preferences, it is possible that differences in food availability between lakes may be one factor accounting for the differences in both the concentration and the rate of accumulation with size.
Of the reservoirs in central Maryland (Fig. 1), Loch Raven, Prettyboy, and Duckett had similar regression slopes (0.47, 0.43, and 0.39 (ng g−1) g−1, respectively), as shown in Fig. 6d and 6e, and these are similar to those of Deep Creek and Piney (Frostburg), and to St. Mary’s Lake, in southern Maryland (slope = 0.41 (ng g−1) g−1; Fig. 6f). Liberty and Piney Run both had relatively low regression slopes (0.15 and 0.11 (ng g−1) g−1), whereas there was no relationship for fish in Clopper. Overall, however, MeHg concentrations for fish in Clopper were similar to Piney Run. Only small fish were obtained from Centennial reservoir (Fig. 6e). Tridelphia had an intermediate slope (Fig. 6f).
Johnson’s Pond and Tuckahoe, both on Maryland’s Eastern Shore, had similar regression slopes (0.30 and 0.28 (ng g−1) g−1, respectively) (Fig. 6h). Largemouth bass in Lake Lariat had a considerably larger bioaccumulation rate than the other coastal plain reservoirs (0.76 (ng g−1) g−1; Fig. 6h), similar to that of Savage (Fig. 6b). Finally, bass in CON had a rate of increase of 0.09 (ng g−1) g−1, the lowest for the coastal reservoirs.
The differences in the accumulation rates of MeHg in largemouth bass across the 20 reservoirs might be caused by many variables, such as the chemistry of the water, condition of the environmental surroundings and physical characteristics of the reservoirs (Bodaly et al. 1993). There is no sense from the relationships shown above that this is entirely dependent on the reservoir location. The four highest slopes were from reservoirs located throughout the state (in decreasing order: Savage, Lake Lariat, Loch Raven, and Prettyboy reservoirs). At the opposite end, the four lowest significant slopes were from, in increasing order: Conowingo Dam, Piney Run, Liberty, and Broadford reservoirs. Five reservoirs had no significant relationships but this is likely the function of either too little data, or too little spread in fish size.
Mercury in Bluegill and Black Crappie
The size of bluegill and black crappie analyzed were limited to those of harvestable size. For bluegill, the minimum size caught was 125 mm; for black crappie, 150 mm. Maximum and minimum weight of fish caught were, respectively, 236 g and 40 g for bluegill, and 340 g and 60 g for black crappie. The average size and concentration of MeHg in these fish are shown in Table 3 and in Fig. 8. The MeHg in the majority of these fish was less than 300 ng g−1 wet weight. The difference in the fish MeHg concentration between reservoirs was similar in trend to that of the largemouth bass. Indeed, there was a significant correlation for both bluegill and crappie between their average concentration and that of the bass (r2 = 0.64 (n = 13; p < 0.01) and 0.61 (n = 7; p < 0.05), respectively), and the slope of the relationship was similar for both (slope ∼ 0.2). Bluegill feed mostly on macroinvertebrates (Olson et al. 2003), although small fish may form part of the diet. For black crappie, a recent study in a lake in Iowa indicated that these fish were feeding mostly on amphipods and insect larvae, with amphipods forming the majority of their diet (Liao et al. 2002). For those reservoirs that had both bluegill and black crappie, there was generally a consistent trend in that reservoirs with high MeHg concentration in bass also had high concentration in both of the other species (Fig. 8), and with the concentration in bluegill and black crappie being relatively similar, except in Clopper Lake.
For bluegill, there was a significant correlation between weight and MeHg concentration (r2 = 0.26; n = 94; p < 0.01; slope = 0.70 (ng g−1) g−1), based on the 2000 data for all the fish, as found for the bass. The slope of the weight/fish MeHg concentration relationship for all the bluegill is within a factor of two of the average for all largemouth bass (0.38 ng g−1) g−1). However, there did not appear to be a strong relationship between weight and MeHg concentration in bluegill in the individual reservoirs. Indeed, for the bluegills, only three of the seven reservoirs sampled in 2000 had a linear significant correlation (Broadford, r2 = 0.52; Rocky Gap, r2 = 0.61 and Johnson’s Pond, r2 = 0.47). This may be due to the smaller size range in fish, or may reflect the more varied diet of these fish compared to the bass as bluegill, feeding mostly on invertebrates, which likely comprise both benthic and pelagic species, likely show a larger variation in MeHg burden.
For crappie, there was no significant trend between weight and MeHg concentration and little indication of an increasing concentration with size. This may be a function of the more limited dataset but also may reflect the fact that the diet of these fish does not appear to change dramatically with age, with large fish still preferring a diet of amphipods and other invertebrates (Olson et al. 2003). For both species, the % MeHg in the muscle tissue was relatively high, between 80 and 85% of the total Hg, on average. The relatively high % MeHg, and the similarity in the slopes of the weight/concentration relationships, may suggest that the bluegill and the largemouth bass have a more similar diet than may have been anticipated from expected feeding preferences. This likely reflects a relatively high proportion of crayfish in the bass diet for the fish in these reservoirs.
Mercury, Methylmercury, and Ancillary Parameters in Water
Overall, TSS in the reservoirs were relatively low but variable (Table 4). The average TSS concentration was 7.8 ± 10.0 mg L−1 (n = 37) and was lowest in Piney (Frostburg) (0.9 ± 0.3 mg L−1, n = 2) and highest in Loch Raven (27.9 ± 19.9 mg L−1, n = 3). These values are in agreement with TSS concentrations observed in samples from other Maryland streams (Mason 2000) but lower than the Chesapeake Bay and the major rivers (Lawson et al. 2001, Lawson and Mason 2001, Mason et al. 1999). Concentrations of total mercury (HgT) and MeHg in the dissolved fraction of the surface water samples are shown in Table 5. Average HgT concentrations ranged from 0.4 ± 0.1 ng L−1 (Rocky Gap; Castro et al. 2002) to 18.1 ± 0.43 ng L−1 (Duckett). There was a significant range in concentration with the highest concentrations being found in the reservoirs from the middle of the state. Such levels of HgT are much higher than have been found by others for freshwater systems (Driscoll et al. 1995; Hudson et al. 1994; Mason and Sullivan 1997; Sorenson et al. 1990), but the reproducibility of results over two years for Duckett suggest that the data represent a consistent and not a transient signal. Relatively low HgT concentrations were found in western Maryland, and dissolved Hg was <1.5 ng L−1 for all these reservoirs. Most of the reservoirs with high HgT (>5 ng L−1) had relatively low dissolved Hg suggesting that the Hg was mostly attached to particulate matter, as would be expected for these relatively eutrophic systems. However, for Duckett and Clopper, which had the highest HgT concentrations, most of the Hg was in the dissolved fraction (Table 5). The source of the high Hg in these two reservoirs is not known and must be relatively local as Tridelphia, which is on the Patuxent River above Duckett, has HgT concentrations a factor of 2-3 lower and dissolved Hg <3 ng L−1. The reservoirs with the highest HgT do not have high normalized MeHg concentrations in the largemouth bass, suggesting no relationship between HgT and fish concentration (Table 6), as found in many other studies (e.g., Driscoll et al. 1995). This is also true for dissolved Hg, where no relationship exists, even when the two extremely high concentrations are considered outliers.
The total MeHg concentrations observed in the water samples were generally lower than 0.4 ng L−1, on average. For Lake Lariat, low values were found in 2002 and much higher concentrations (>1 ng L−1) were found in 2003, suggesting high variability. Variability was also apparent in the other reservoirs that were sampled more than once, although it was less extreme. Concentrations similar to those found here have been found in surface waters of many freshwater systems (e.g., Watras et al. 1995a, Lawson et al. 2001). Dissolved MeHg concentrations were above 0.25 ng / L−1 in Piney (Frostburg) and Deep Creek in western Maryland, and in Piney Run and Lake Lariat. Overall, a large fraction of the total MeHg was in the dissolved phase (Table 5). There was a significant correlation between normalized fish concentration and dissolved MeHg for the reservoirs (Table 6; average values used for MeHg in water).
Ancillary data from the reservoirs are shown in Table 4. pH was similar across the reservoirs, with no reservoirs having low pHs such as found in some lakes in the Midwestern United States. Overall, the reservoirs can be considered to be circumneutral, with an overall average pH of 7.5 ± 2.0 (n = 38). Statistically, no significant difference in pH was found among the Maryland regions. Dissolved organic carbon (DOC) concentrations were also relatively consistent across the reservoirs. Average DOC concentration was 3.7 ± 2.0 mg L−1, ranging from 1.95 mg L−1 in Prettyboy to 6.51 mg L−1 in St. Mary’s Lake (Table 4). No significant difference in DOC concentrations was observed across regions. However, values in western Maryland were generally low, whereas DOC concentrations in southern Maryland reservoirs were the highest. These DOC concentrations are fairly low, however, compared to values obtained in lakes in Wisconsin (average about 7 mg L−1, Watras et al. 1995b) but similar to concentrations found in Maryland streams (Mason 2000). Even given the low range in DOC between these reservoirs, there was a correlation with fish MeHg (Table 6), as has been found by others (Driscoll et al. 1995). Particulate carbon (POC) was found to be relatively low across all of the reservoirs (1.1 ± 0.8 mg L−1, n = 25), and in four of the reservoirs concentrations were at or below detection limits.
Nitrate and phosphate concentrations were also consistent in the reservoirs (Table 4). Ammonia concentrations ranged from 0.01 mg L−1 in Piney (Frostburg) to 0.36 mg L−1 in Big Pool, with an average of 0.05 ± 0.05 mg L−1 (n = 38). Sulfate concentrations in the reservoirs showed more variability and ranged from 3.4 ± 0.4 mg L−1 in Prettyboy (n = 3) to 36.8 ± 1.6 mg L−1 (Potomac #4, n = 2), with an average concentration of 9.5 ± 2.8 mg L−1 (n = 38). Comparable values have been documented in various aquatic systems such as in New York, northern Wisconsin, and Massachusetts (Simonin et al. 1994; Watras et al. 1995a; Rose et al. 1999) and the values appear to be within the normal ranges of freshwater systems. The sulfate levels found suggest that the sulfate-reducing bacteria, the principal methylators of Hg in aquatic systems, are not sulfate-limited in any of the reservoirs. In support of this, there was no relationship between sulfate and MeHg concentration in fish (Table 6).
Chloride concentrations (Cl), in contrast to the other ancillary measurements, ranged widely across the reservoirs. Average concentration across all of the reservoirs was 33.8 ± 28.6 mg L−1 (n = 38). Values in Liberty were lowest (1.7 ± 0.1 mg L−1, n = 3), whereas average values for Clopper were 135.8 ± 6.9 mg L−1 (n = 3). These values are relatively high compared to lakes in northern Wisconsin (0.1–4.0 mg L−1, Watras et al. 1995a). However, similarly elevated values were found in lakes in Massachusetts (1–35 mg L−1, Rose et al. 1999). Although several factors could be responsible for the high amount of Cl in the reservoirs, the most likely source is salt that is used for melting of snow in winter. A lack of correlation between Cl and nutrients suggests that Cl is not being derived from detergents and water-softening agents. There has been a general increase in the Cl concentration in the reservoirs surrounding Baltimore in the last two decades (B. Stacks, personal communication, 2002), and this is likely related to increases in population densities around the reservoirs. Population density around Loch Raven, for example, (1.8 people/acre) is significantly higher than for Liberty (0.7 people/acre; MD DNR, 2001), which is also bigger and deeper, and both population and relative size could account for the dramatic differences in Cl concentrations between the two reservoirs. Overall, however, Cl concentration did not correlate with lake size parameters, or other water quality parameters (Table 6).
Interestingly, sulfate concentration appeared to be a function of surface area, volume, and watershed/lake area likely implicating the importance of atmospheric deposition as a source of sulfate to these reservoirs (Table 6). However, there was no relationship between sulfate concentration and either MeHg in water or MeHg in fish. Many studies have found relationships between fish concentration and water column parameters for lakes (Lindquist et al. 1991; Johnston et al. 1991; Joslin 1994; Lange et al. 1993). For the Maryland reservoirs, however, the concentration of MeHg in bass did not correlate with the concentrations of nutrients or major ions, or with pH. Similarly, Rose et al. (1999) found no relationship between bass concentration and the water quality variables they measured. In this study, the variability in these parameters across the reservoirs was relatively small, and thus a lack of correlation does not necessarily mean that these variables are not important in some instances.
The strongest relationships for water column variables were between filtered MeHg and DOC concentrations and normalized fish MeHg concentration (Table 6). The relationship with MeHg in water would be expected if differences in food chain dynamics, and in prey items for bass, are relatively small between lakes. Such a relationship therefore implies that the food chain dynamics are not the overwhelming factor controlling fish concentration. The concentration of filtered MeHg is not strongly related, however, with any of the other variables (Table 6), suggesting that neither reservoir size nor sulfate, DOC, or other water column variables are important parameters. The concentration of filtered Hg in the reservoirs was also not a function of any of the other variables except for Cl concentration, suggesting that it is unrelated to reservoir size or to any other water column parameter considered important in MeHg dynamics.
Relationships Between Fish Tissue Concentrations, Diet, and Other Variables
As mentioned above, food sources for the largemouth bass may differ between the reservoirs. Because aquatic organisms low in the food chain generally contain lower concentrations of MeHg than higher trophic level organisms, fish feeding on lower trophic level animals consequently bioaccumulate less MeHg into their tissues during their lifetime than fish feeding higher in the food chain (Lindquist et al. 1991; MacCrimmon et al. 1983; Mason et al. 2000b). In an effort to assess food effects, in the first 2 years of the project, 106 largemouth bass were sampled for gut content. Only half of the fish examined contained food remnants in their gut (Sveinsdottir 2002). Of these, 70% was identified to be crayfish, 17% sunfish, and less than 1% channel catfish. Note must be taken that the fish were captured at the same time in both years (late spring, early summer) and gut content might not be representative of the food sources largemouth bass consume during the whole year. In addition, sunfish and other soft tissue organism are more easily digested than crayfish, and that might explain the prevalence of crayfish remnants in the stomachs over that of other fish. Furthermore, largemouth bass are known to be opportunistic feeders and have been found to eat both terrestrial and aquatic organisms. However, gut content analysis can give an idea of the MeHg concentration in the diet of largemouth bass in the reservoirs that were studied.
To assess the importance of differences in diet, crayfish and small forage fish were collected from a number of the reservoirs (n = 8). The average length of crayfish collected was 38.3 ± 51.9 mm (ranging from 7.0 to 138 mm) and the average MeHg concentration was 22.1 ± 13.5 ng g−1 wet weight (ranging from 7.24 to 44.58 ng g−1). The average length of the forage fish collected (small bluegill, black crappie, golden shiner, yellow perch, white sucker, and pumpkinseed) was 59.5 ± 72.0 mm (range 3.2–216 mm) and the average MeHg concentration was 42.7 ± 23.9 ng g−1 wet weight (range 9.4–108.2 ng g−1), higher than for crayfish, as expected, based on their higher trophic position (Mason et al. 2000b). The mean HgT concentration in the forage fish was 73.6 ng g−1 wet weight (range 9.1–164.9 ng g−1), and, on average, they contained 58% MeHg. The relationship between the average MeHg concentrations of small fish, which could be consumed as prey, and the average normalized MeHg concentration of largemouth bass (362–377 mm) in each of the reservoirs was weak and not statistically significant (r2 = 0.24; n = 8). In addition, there was not a significant correlation with crayfish concentration (r2 = 0.17; n = 7).
In addition to the differences in diet, various physical factors of the reservoirs and their surroundings could also influence MeHg accumulation in fish by changing the input of Hg and MeHg into the system and the residence time of the water. Reservoir size is one potential variable influencing bioaccumulation. If methylation of Hg occurs mainly in sediments, then there is a negative relationship, albeit complex, between reservoir size and the ratio of sediment area/water column volume. Thus, larger reservoirs may have less MeHg in the water column. Reservoir volume and/or depth will affect the rate at which the lakes heat up in summer, which may impact the overall methylation potential. Also, depth may impact stratification and the development of seasonal anoxia. Bodaly et al. (1993) found a relationship between reservoir size and Hg in fish in Canada. In this study, reservoirs varied by nearly three orders of magnitude in area, in volume by more than two orders of magnitude, and also varied markedly in depth (Table 1). Conowingo Dam is a relatively large reservoir (3.82 × 108 m3), whereas Lake Lariat is a small impoundment (1.88 × 106 m3). Volume and surface area were strongly related, and these parameters were also related to watershed/lake volume (Table 6). However, there was not a significant relationship for any of these variables with normalized fish MeHg concentration (Table 6), although the association did not appear to be totally random. In addition, filtered MeHg was not a function of reservoir physical characteristics (Table 6). Average water retention time for each reservoir, which was estimated from average flow at the outlet and the nominal reservoir volume (Table 1), did not show any relationship with MeHg water concentration or fish MeHg. Retention times varied over a wide range from days for shallow systems such as Tuckahoe to more than a year for the largest, deepest reservoirs (Deep Creek and Savage). However, some large reservoirs, such as Conowingo Dam, had a relatively short retention time. Overall, most reservoirs had retention times of months to a year (Table 1). In contrast to this study, Hanten et al. (1998) compared artificial and natural water bodies in Connecticut and found for the impoundments that largemouth bass Hg content correlated with lake retention time and with watershed/lake area.
The reservoirs sampled in this study varied widely in age from those built in the 1920s and 1930s—Deep Creek Lake, Broadford, Piney (Frostburg), Prettyboy, Loch Raven, and Conowingo Dam—to Piney Run, which was completed in 1990. Additionally, Potomac #4, a dam constructed initially on the Potomac River in 1869, was altered in 1994, and Piney (Frostburg) was also altered in the 1990s. There has been a noted “reservoir effect” where MeHg levels in fish from recently flooded reservoirs have been shown to be elevated compared to prior to flooding (Bodaly et al. 1984, 1997). However, such an effect lasts approximately 25 years. Thus, most of the reservoirs in this study should be unaffected because they are more than 25 years old. Indeed, there was no correlation between reservoir age and fish MeHg concentration.
Stepwise multivariate regression analysis was used to investigate further whether the relationship between fish MeHg concentration and dissolved MeHg would be better explained by the inclusion of additional variables. However, based on stepwise regression analysis, inclusion of DOC, and additional variables that appeared to show the strongest association with fish MeHg, did not improve the predictive power above that of the relationship with dissolved MeHg concentration alone. As illustrated by the value of the regression statistic, the relationship between water MeHg and that of fish does not explain a high proportion of the variability (∼25%).
Few studies investigating the influences on fish MeHg concentration have also measured MeHg in the water column, so the association found above demonstrates that, for these systems, the factors influencing MeHg concentrations in water are those that determine, to a large extent, the concentration of MeHg in fish. DOC is an important variable, for a number of reasons. First, elevated DOC concentrations impact the rate of uptake of MeHg at the base of the food chain, by complexing the Hg and MeHg in a form that is less bioavailable for uptake than when bound to inorganic ligands (Mason et al. 1996; Mason 2001). Based on the DOC concentrations measured here, it is estimated that the majority of the MeHg in the dissolved fraction would be associated with DOC (Hudson et al. 1994). However, if the presence of DOC was reducing uptake, then the relationship between DOC and fish MeHg should be negative, which it is not (Table 6). Such an inverse relationship between DOC and fish MeHg has been found by others (Driscoll et al. 1995; Hudson et al. 1994; Watras et al. 1994), and likely reflect the larger range in DOC concentrations found in these studies.
However, DOC can also complex the Hg and MeHg in solution and prevent its adsorption and removal by particulate matter. However, this would likely result in a correlation between DOC and dissolved MeHg, which did not exist for this dataset (Table 6). Finally, the MeHg is either produced within the lake, or provided via watershed inputs, and, in some systems, the watershed input, and the amount of wetlands in the watershed where methylation is enhanced, has been correlated with MeHg in water or in fish (Hultberg et al. 1994; Hurley et al. 1995; Lindquist et al. 1991; Benoit et al. 2003). However, most of the reservoirs in this study do not have significant wetlands associated with them; although data are not available for all the reservoirs, for those where data are available, the percent wetland is mostly <1% of the watershed area. Thus, the impact of methylation within wetlands is small for the reservoirs in this study. Therefore, the positive relationship between MeHg in fish and DOC likely represents the importance of DOC in transporting MeHg from the sites of methylation to the sites of bioaccumulation, and because of the relatively small range in DOC concentration across the reservoirs, there is no substantial impact of this variability on the relatively bioavailabilty of MeHg at the base of the food chain.
In summary, therefore, the factors controlling water column MeHg are complex, and clearly cannot be ascribed to being entirely due to any of the physical and chemical variables measured in this study (Table 6). It would be expected that increased sulfate concentration could stimulate Hg methylation, but the range in concentrations found here are relatively high compared to other systems where an impact of sulfate on MeHg in fish was observed (Hudson et al. 1994; Watras et al. 1994). Thus, it appears that factors controlling the transport of the MeHg from the site of methylation, or specific differences in sediment Hg concentration and/or the methylation rate, that were not measured in this study, could be the primary factors influencing the concentration of MeHg in the water.
Bioaccumulation Factors
The US EPA (EPA 2001) has devised water quality criteria based on fish MeHg concentration and has derived bioaccumulation factors (BAF) for various trophic level fish to allow the estimation of water column MeHg concentrations from fish data, because in many situations it is not possible to measure dissolved MeHg concentrations. The average value derived for trophic level 4 (piscivorous) fish is log BAF = 6.43; for trophic level 3, it is 5.83. The BAFs for the reservoirs in this study are listed in Table 7. The largemouth bass have an average log BAF of 6.33 ± 0.29, whereas the bluegill and crappie have lower but similar values: 5.76 ± 0.32 and 5.75 ± 0.39, respectively. Thus, the values obtained from this study are similar to the average values proposed by EPA. Overall, the variability (relative percent difference) between sites is relatively high, from ∼60% for the bass to ∼80% for the crappie. Thus, this suggests that the use of an average, or generic, BAF value will lead to substantial error in the estimation of water MeHg concentrations using such a factor, and will not discern the variability that obviously exists between ecosystems. The log BAF value obtained here for largemouth bass is similar to that of Paller et al. (2004) (log BAF = 6.5), but the sunfish BAF found in that study was higher (log BAF = 6.2). Clearly, the bluegill in the Maryland reservoirs is feeding on lower trophic level food than those in the Savannah River, the study site of Paller et al. (2004). That bluegill and crappie are trophic level three is consistent with what is known about their feeding behavior.
In summary, the relatively strong relationship between MeHg in largemouth bass and its weight supports the notion that MeHg constantly accumulates in fish with increasing size, as has been documented by numerous studies (e.g., Rose et al. 1999; Hanten et al. 1998; Horwitz et al. 1995; Ward and Neumann 1999; Hueter et al. 1995; Stafford and Haines 1997; Somers and Jackson 1993). The variability observed between regression slopes of the reservoirs indicates that there are other factors controlling the MeHg accumulation in largemouth bass and its prey than merely their diet, as discussed above. Primarily, the concentration of MeHg in the water and the DOC concentration appear to be the most important variables across all reservoirs. However, a variety of both physical and chemical characteristics of the reservoirs can be responsible for the variation of MeHg uptake in fish, and the specific variables of importance will differ for different locations, depending on the variability within the region of study. Such factors may include geological influences (bedrock and sediments), chemical variability (e.g., water quality and mercury biogeochemistry) and physical variability (e.g., lake and watershed size, lake depth, wetland size) (Rose et al. 1999; Wren and MacCrimmon 1983; Sonesten 2001; Simonin et al. 1994; Rudd 1995).
However, the correlation between MeHg concentration in fish and water suggests that measurements of water column MeHg should be made in studies of fish bioaccumulation and variability across ecosystems to further determine the importance of this variable. Furthermore, the use of fish MeHg concentrations and generic BAF values to determine whether an ecosystem exceeds a particular water quality criterion is obviously of limited usefulness. Overall, there is clearly not one specific parameter that determines fish MeHg concentration, and thus it is difficult to extrapolate across regions and ecosystem types to predict MeHg concentrations in fish from easily measured environmental variables.
References
Benoit JM, Gilmour CC, Heyes A, Mason RP, Miller CL (2003) Geochemical and biological controls over mercury production and degradation in aquatic systems. In: Cai Y, Brouds OC (eds), Biogeochemistry of environmentally important trace elements. ACS Symposium Series 835, American Chemical Society, Washington, DC, pp 262–297
Bloom NS (1989) Determination of picogram levels of methylmercury by aqueous phase ethylation, followed by cryogenic gas chromatography with cold vapor atomic fluorescence detection. Can J Fish Aqua Sci 46:1131–1140
Bloom NS (1992) On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aqua Sci 49:1010–1017
Bloom NS, Crecelius EA (1983) Determination of mercury in seawater and subnanogram per liter levels. Mar Chem 14:49–59
Bloom NS, Fitzgerald WF (1988) Determination of volatile species at the picogram level by low temperature gas chromatography with cold vapor atomic fluorescence detection. Anal Chim Acta 208:151–161
Bloom NS, Watras CJ (1992) Mercury and methylmercury in individual zooplankton: Implications for bioaccumulation. Limnol Oceanogr 37:1313–1318
Bodaly RA, Hecky RE, Fudge RJP (1984) Increases in fish mercury levels in lakes flooded by the Churchill River diversion, northern Manitoba. Can J Fish Aquat Sci 41:682–691
Bodaly RA, Rudd JWM, Fudge RJP (1993) Mercury concentrations in fish related to size of remote Canadian Shield lakes. Can J Fish Aquat Sci 50:980–987
Bodaly RA, St Louis VL, Paterson MJ, Fudge RJP, Hall BD, Rosenburg DM, Rudd JWM (1997) Bioaccumulation of mercury in the aquatic food chain in newly flooded areas. In: Sigel A, Sigel H (eds), Mercury and its effects on environment and biology, metal ions in biological systems, vol 34. Marcel Dekker, New York, pp 259–287
Castro MS, McLaughlin EN, Davis SL, Morgan R (2002) Total mercury concentrations in lakes and fish in western Maryland. Arch Environ Contam Toxicol 42:545–562
Driscoll CT, Blette V, Yan C, Schofield CL, Munson R, Holsapple J (1995) The role of dissolved organic carbon in the chemistry and bioavailability of mercury in remote Adirondack Lakes. Water Air Soil Poll 80:499–508
Elser HJ (1962) Growth rates of Maryland’s freshwater fish. Natural Resource Institute, University of Maryland, ref. no. 62-13
Gilmour CC (1999) A preliminary survey of size-specific mercury concentrations in game fish from Maryland fresh and estuarine waters. Chesapeake Bay and Watershed Programs: Monitoring and non-tidal assessment. CBWP-MANTA-AD-98-9
Gilmour CC, Henry EA, Mitchell R (1992) Sulfate stimulation of mercury methylation in freshwater sediments. Environ Sci Technol 26:2281–2287
Gilmour CC, Riedel GS (2000) A survey of size-specific mercury concentrations in game fish from Maryland fresh and estuarine waters. Arch Environ Contam Toxicol 39:53–59
Grieb TM, Driscoll CT, Gloss SP, Schofield CL, Bowie GL, Porcella DB (1990) Factors affecting mercury accumulation in fish in the upper Michigan peninsula. Environ Toxicol Chem 9:919–930
Hanten RP, Neumann RM, Ward SM, Carley RJ, Perkins CR, Pirrie R (1998) Relationships between concentrations of mercury in largemouth bass and physical and chemical characteristics of Connecticut lakes. Trans Am Fish Soc 127:807–818
Horvat M, Liang L, Bloom NS (1993) Comparison of distillation with other current isolation methods for the determination of methylmercury compounds in low level environmental samples. Anal Chim Acta 282:153–168
Horwitz RJ, Ruppel B, Wisniewski S, Kiry P, Her M, Gilmour CC (1995) Mercury concentrations in freshwater fishes in New Jersey. Water Air Soil Poll 80:885–888
Hudson RJM, Gherini SA, Watras CJ, Porcella DP (1994) Modeling the biogeochemical cycle of mercury in lakes: The mercury cycling model (MCM) and its application to the MTL study lakes. In: Watras CJ, Huckabee JW (eds) Mercury pollution: Integration and synthesis. Lewis Publishers, Boca Raton, pp 473–526
Hueter RE, Fong WG, Henderson G, French MF, Manire CA (1995) Methylmercury concentration in shark muscle by size and distribution of sharks in Florida coastal waters. Water Air Soil Pollut 80:893–899
Hultberg H, Iverfeldt Å, Lee YH (1994) Methylmercury input/output and accumulation in forested catchments and critical loads for lakes in Southwestern Sweden. In: Watras CJ, Huckabee JW (eds) Mercury pollution: Integration and synthesis. Lewis Publishers, Boca Raton, pp 313–341
Hurley JA, Benoit JM, Babiarz CL, Shafer MM, Andren A, Sullivan JR, Hammond R, Webb DA (0000) Influence of watershed characteristics on mercury levels in Wisconsin rivers. Environ Sci Technol 29:1867–1875
Jackson TA (1991) Biological and environmental-control of mercury accumulation by fish in lakes and reservoirs of northern Manitoba, Canada. Can J Fish Aqua Sci 48:2449–2470
Johnston TA, Bodaly RA, Mathias JA (1991) Predicting fish mercury levels from physical characteristics of boreal reservoirs. Can J Fish Aquat Sci 48:1468–1480
Joslin JD (1994) Regional differences in mercury levels in aquatic ecosystems: A discussion of possible causal factors with implications for the Tennessee River system and northern hemisphere. Environ Manage 18:559–567
Klotz A, Johnson J (2000) Performance report, annual (2000) and Five Year (1996–2000), USFWS federal aid grant F-48-R10, survey and management of Maryland freshwater fisheries resources, Maryland DNR Fisheries Service, Freshwater Fisheries Division
Lange TR, Royals HE, Connor LL (1993) Influence of water chemistry on mercury concentration in largemouth bass from Florida lakes. Trans Am Fish Soc 122:74–84
Lawson NM, Mason RP, Laporte J-M (2001) The fate and transport of mercury, methylmercury, and other trace metals in Chesapeake Bay tributaries. Water Res 35:501–515
Lawson NM, Mason RP (2001) Concentration of mercury, methylmercury, cadmium, led, arsenic, and selenium in the rain and stream water of two contrasting watersheds in Western Maryland. Water Res 35:4039–4052
Liao H, Peirce CL, Larscheid JG (2002) Diet dynamics of the adult piscivorous fish community in Spirit Lake, Iowa, USA 1995–1997. Ecol Fresh Fish 11:178–189
Lindquist O, and others (1991) Mercury in the Swedish environment—recent research on causes, consequences and corrective methods. Water Air Soil Poll 55:143–177
MacCrimmon HR, Wren CD, Gots BL (1983) Mercury uptake by lake trout, Salelinus namaycush, relative to age, growth, and diet in Tadenac Lake with comparative data from other PreCambrian Shield lakes. Can J Fish Aquat Sci 40:114–120
Maryland Department of Natural Resources (MD DNR) (2001) Annual (2000) and Final (1996–2000) performance report. Survey and management of Maryland’s fishery resources. Maryland Department of Natural Resources, Fisheries Service, Freshwater Fisheries Division
Maryland Department of the Environment (MDE) (2004) Fish consumption advisories for Maryland lakes, impoundments and non-tidal rivers. http://www.Marylande.state.Maryland.us/programs/landprograms/hazardouswaste/mercury/fishmercury.asp
Mason RP (2000) An investigation of the influence of water quality parameters on mercury, methylmercury, arsenic, selenium and cadmium concentrations in fish of Maryland streams. Maryland Department of Natural Resources, Chesapeake Bay Research and Monitoring Division, Report # CBWP-MANTA-AD-02-1
Mason RP (2001) The bioaccumulation of mercury, methylmercury and other toxic elements into pelagic and benthic organisms. In: Newman MC, Robert MH, Hale RC (eds) Coastal and estuarine risk assessment. CRC/Lewis Pub, pp 127–149
Mason RP, Fitzgerald WF, Morel FMM (1994) The biogeochemical cycling of elemental mercury: anthropogenic influences. Geochim Cosmo Acta 58:3191–3198
Mason RP, Reinfelder JR, Morel FMM (1996) Uptake, toxicity and trophic transfer of mercury in a coastal diatom. Environ Sci Technol 30:1835–1845
Mason RP, Sullivan KA (1997) Mercury in Lake Michigan. Environ Sci Technol 31:942–947
Mason RP, Lawson NM, Sullivan KA (1997a) Atmospheric deposition to the Chesapeake Bay watershed: Regional and local sources. Atmos Environ 31:3531–3540
Mason RP, Lawson NM, Sullivan KA (1997b) The concentration, speciation and sources of mercury in Chesapeake Bay precipitation. Atmos Environ 31:3541–3550
Mason RP, Lawson NM, Lawrence AL, Lee JG, Leaner JJ, Sheu GR (1999) Mercury in the Chesapeake Bay. Mar Chem 65:77–96
Mason RP, Lawson NM, Sheu G-R (2000a) Annual and seasonal trends in mercury deposition in Maryland. Atmos Environ 34:1691–1701
Mason RP, Laporte J-M, Andres S (2000b) Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium, and cadmium by freshwater invertebrates and fish. Arch Environ Contam Toxicol 38:283–297
McMurty MJ, Wales DL, Sceider WA, Beggs GL, Dimond PE (1989) Relationship of mercury concentrations in lake trout Salvelinus namaycush and smallmouth bass Micropterus dolimieu to the physical and chemical characteristics of Ontario lakes. Can J Fish Aquat Sci 46:426–434
Murdy EO, Birdsong RS, Musick JA (1997) Fishes of the Chesapeake Bay. Smithsonian Institution Press, Washington and London, pp 216–244
Olson NW, Paukert CP, Willis DW, Klammer JA (2003) Prey selection and diets of bluegill Lepomis macrochirus with differing population characteristics in two Nebraska natural lakes. Fish Manage Ecol 10:31–40
Paller MH, Bowers JA, Littrell JW, Guanlao AV (2004) Influences on mercury bioaccumulation factors for the Savannah River. Arch Environ Contam Toxicol 46:236–243
Rose J, Hutcheson MS, West CR, Pancorbo O, Hulme K, Cooperman A, DeCesare G, Isaac R, Screpetis A (1999) Fish mercury distribution in Massachusetts, USA Lakes. Environ Toxicol Chem 18:1370–1379
Rudd JWM (1995) Sources of methylmercury to freshwater ecosystems: A review. Water Air Soil Poll 80:697–713
Simoneau M, Lucotte M, Garceau S, Laliberte D, (2005) Fish growth rates modulate mercury concentrations in walleye (Sander vitreus) from eastern Canadian lake. Environ Res 98:73–82
Simonin HA, Gloss SP, Driscoll CT, Schofield CL, Kretser WA, Karcher RW, Symula J (1994) Mercury in yellow perch from Adirondack drainage lakes (New York, U.S.). In: Watras CJ, Huckabee JW (eds) Mercury Pollution-Integration and Synthesis. Lewis Publications, Boca Raton, Florida, pp 457–469
Somers KM, Jackson DA (1993) Adjusting mercury concentration for fish-size covariation: a multivariate alternative to bivariate regression. Can J Fish Aqua Sci 50:2388–2396
Sonesten L, (2001) Mercury content in roach (Rutilus rutilus L.) in circumneutral lakes—effects of catchment area and water chemistry. Environ Poll 112:471–481
Sorenson JA, Glass GE, Schmidt KW, Huber JK, Rapp GR (1990) Airborne mercury deposition and watershed characteristics in relation to mercury concentrations in water, sediment, plankton, and fish in eighty northern Minnesota lakes. Environ Sci Tech 24:17169–1727
Stafford CP, Haines TA (1997) Mercury concentrations in Maine sport fishes. Trans Am Fish Soc 126:144–152
Sveinsdottir AY (2002) Methylmercury in largemouth bass (Micropterus salmoides) and forage fish from Maryland reservoirs and factors influencing uptake. Masters Thesis. University of Maryland, College Park, Maryland
U.S. Environmental Protection Agency (USEPA) (1995a). National listing of fish consumption advisories. USEPA, EPA-823-F-95-004, Office of Water, Washington, DC
USEPA (1995b) Method 1631: Mercury in water by oxidation, purge and trap and cold vapor atomic fluorescence spectrometry. Office of Water, Washington, DC
USEPA (1998) EPA methods and guidance for analysis of water: CD ROM. Government Institutes, Washington, DC
USEPA (2001). Fish tissue criterion for methylmercury to protect human health document, EPA-823-R-01-001, Office of Water, Washington, DC. http://www.epa.gov/waterscience/criteria/methylmercury/document.html
USEPA (2004) Fish consumption advice webpage. http://www.epa.gov/waterscience/fishadvice/advice.html
U.S. Geological Service (USGS) (Feb. 2001) http://www.Maryland.water.usgs.gov/
Ward SM, Neumann RM (1999) Seasonal variations in concentrations of mercury in axial muscle tissue of largemouth bass. N Am J Fish Manage 19:89–96
Watras CJ, Morrison KA, Bloom NS (1995a) Chemical correlates of Hg and methyl-Hg in Northern Wisconsin lake waters under ice-cover. Water Air Soil Pollut 84:253–267
Watras CJ, Morrison KA Host JD (1995b) Concentration of mercury species in relationship to other site-specific factors in the surface waters of northern Wisconsin lakes. Limnol Oceanogr 40:556–565
Watras CJ, Bloom NS, Hudson RJM, Gherini S, Munson R, Claas SA, Morrison KA, Hurley J, Wiener JG, Fitzgerald WF, Mason RP, Vandal G, Powell D, Rada R, Rislov L, Winfrey M, Elder J, Krabbenhoft D, Andren AW, Babiarz C, Porcella DB, Huckabee JW (1994) Sources and fates of mercury and methylmercury in Wisconsin lakes. In: Watras CJ, Huckabee JW (eds) Mercury pollution: Integration and synthesis. Lewis Publishers, Boca Raton, pp 153–177
Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer AM (2002) Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr (eds) Handbook of ecotoxicology, 2nd ed. CRC Press, Boca Raton, Florida, p 409
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) The effects of mercury on wildlife: A comprehensive review. Environ Toxicol Chem 17:146
World Health Organization (WHO) (1990) Environmental health criteria 101: Mercury I. Geneva. 144 pp
Wren CD, MacCrimmon HR (1983) Mercury levels in sunfish, Lepomis gibbosus, relative to pH and other environmental variables of Precambrian Shield lakes. Can J Fish Aquat Sci 40:1737–1744
Acknowledgments
This research was funded by grant MA01-001-002 from the Maryland Department of Natural Resources (MD DNR) through the Resource Assessment Service. We would like to thank Paul Miller, Steve Early, and Bill Hodges with the MD DNR for their help in providing various reservoir data. Also, we would like to acknowledge the help of all other MD DNR personnel for their help and contributions in the collection of fish and water samples from the reservoirs. We would like to thank all members of the Mason laboratory for their help with water and fish analyses as well as with field work, and Brenda Yates for help with the manuscript typing. This work was part of the MS thesis of Auja Sveinsdottir. This is Contribution # 3883 of the University of Maryland Center for Environmental Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sveinsdottir, A.Y., Mason, R.P. Factors Controlling Mercury and Methylmercury Concentrations in Largemouth Bass (Micropterus salmoides) and Other Fish from Maryland Reservoirs. Arch Environ Contam Toxicol 49, 528–545 (2005). https://doi.org/10.1007/s00244-004-0221-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-004-0221-y