Abstract
Contamination from persistent organic pollutants is a pervasive global problem that urgently demands global concern and action. In the present study, concentrations of organochlorine (OC) pesticides and polychlorinated biphenyls (PCBs) were determined in 37 samples of female adipose tissue collected in Hong Kong hospitals. Among the pollutants analyzed, DDTs (2.79 ng/g fat), HCHs (0.72 ng/g fat), and PCBs (0.19 ng/g fat) were prominent compounds in most of the adipose tissue. p,p′-DDE and hexachlorinated biphenyls were found in all samples, whereas heptachlor epoxide and dieldrin were found only in some samples. An estimation of toxic equivalency concentration (TEQ) due to dioxin-like coplanar PCBs was also performed. The estimated TEQPCBs was 2.01 pg/g fat. This study also compared our previous results obtained from the milk samples of the same donors. Significant correlations are obtained for DDTs and HCHs between milk and adipose tissue. Detailed review of available information concerning OC pesticides and PCBs in different ecological compartments indicated that bioconcentration and biomagnification of these contaminants are common phenomena of the Pearl River Delta region, which has undergone rapid socioeconomic change in the past 20 years. It is suggested to establish a regional organization in order to coordinate the monitoring of persistent organic pollutants in the region.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Polychlorinated biphenyls (PCBs) and organochlorine (OC) pesticides are lipophilic stable toxic compounds that occur in most environmental compartments including soil, water, sediment, and biota (Loganathan and Kannan 1994). They were synthesized chemicals for different applications, e.g., DDT, synthesized in 1939, is a well-known OC insecticide that has a broad range of agricultural and nonagricultural applications worldwide. Many countries banned the use of DDT since the 1970s (WHO 1979), but it is still used in certain parts of the world to control vector-borne diseases, such as malaria. PCBs are a group of synthetic chlorinated biphenyls. Owing to their nonflammable and insulating properties, they have been used widely as coolants and lubricants in transformers, capacitors, and other electrical equipment. Many countries stopped the manufacture of PCBs beginning in the 1980s because there was evidence that PCBs built up in the environment, causing harmful effects on environmental and human health (SRC 1987).
Upon release into the environment, OC pesticides and PCBs will enter soil, water, or air. They are essentially immobile in soil due to the fact that they are strongly adsorbed onto the surface layer of soils. Likewise, as a consequence of their extremely low water solubility, they are also adsorbed onto particulates in water and settled into sediments. They can undergo long-range transport through the atmosphere in a process known as “global distillation,” where they migrate from warmer regions to colder regions through repeated cycles of volatilization from soil and water surfaces followed by deposition of these pollutants onto surfaces through dry and wet deposition processes (Ritter et al. 1995). Once PCBs and OCs pesticides enter the biological system, they tend to accumulate and biomagnify in higher trophic level organisms (Tanabe et al. 1983; Elliott et al. 1988). Because humans occupy the top position in the trophic levels, they are obviously exposed to a higher level of these contaminants from aquatic and terrestrial food chains (Travis et al. 1988; Loganathan et al. 1993). Our previous study indicated that the mean levels of p,p′-DDT (Hong Kong: 0.39; Guangzhou: 0.70 μg/g fat), p,p′-DDE (2.48; 2.85), and β-HCH (0.95; 1.11) contained in human milk samples collected from Hong Kong and Guangzhou, two large cities located in south China, were 2–15-fold higher when compared with studies conducted in UK, Germany, Sweden, Spain, and Canada (CKC Wong et al. 2002).
OC pesticides and PCBs have also been detected in human adipose tissue in some countries, including the United States (Archibeque-Eagle et al. 1997), Korea (Kang et al. 1997), Japan (Minh et al. 2000), and China (Nakata et al. 2002). Direct measurement of OCs and PCBs levels in adipose tissue of human populations is good indicator to show the extent of exposure to the chemicals, and evaluate the health hazards. The present study attempted to estimate the body burden of these pollutants in the adipose tissue of a group of mothers who have given birth, in Hong Kong. The concentrations of these contaminants in adipose tissue will be compared with those contained in the milk samples collected from the same donors, to see whether there was any correlation between the two.
Together with our intensive studies of OC pesticides and PCBs in different ecological compartments of the Pearl River Delta (Liang et al. 1999; Zhou et al. 1999; Wong CKC et al. 2000; Wong CKC et al. 2002; Wong and Poon 2002), assessment of these deadly pollutants in the region will be also addressed.
Materials and Methods
Chemicals
All solvents and reagents used were pesticide-scan grade, Bio-Beads S-X1, 200–400 mesh (Bio-Rad Laboratories) and florisil, 60–100 mesh (Mallinckrodt) were used for sample cleanup processes. Anhydrous sodium sulfate was cleaned and activated at 450°C for 4 h. Florisil was activated at 130°C for 6 h. Reference chemical standards (16 organochlorinated pesticides, PCB congeners, Aroclor 1242, 1254, and 1260) were purchased from ChemService and AccuStandard.
Samples
Female adipose tissue samples (about 10 g) of ethnic Chinese residents living in Hong Kong were taken from their abdomen during their Cesarean operations from June 1999 to July 2000. A total of 37 samples were taken from patients age 33.9 ± 33. The samples were preserved with 2 ml of 37% formaldehyde and stored in a −20°C freezer until extraction.
Extraction
Each frozen sample was weighed in a 100-ml reagent bottle and homogenized with 10 g anhydrous sodium sulfate with a stainless steel blender for 1 min. The sample was then extracted with 50 ml 1:1 acetone and hexane in a shaking incubator at 40°C for at least 12 h. The extraction process was repeated twice. The extract was then concentrated to 5 ml using a rotary evaporator at 80°C. One fifth of the concentrated extract was used for fat content determination.
Cleanup
Gel permeation chromatography (Gilson) was used to remove the residual fat content in the extract. The remained extract was then added to a GPC glass column packed with preswollen and washed Bio-Beads S-X1 corresponding to 70 g dry material using dichloromethane as eluant (modified from USEPA Solid Waste Analysis) (USEPA 1996a). The extract was then further cleaned with a micro-florisil column with 15 ml of 1:1 hexane and dichloromethane (modified from USEPA Solid Waste Analysis) (USEPA 1996b). The cleaned extract was concentrated to 2 ml and added with GCMS internal standard.
Gas Chromatograpy–Mass Spectrometer Analysis
GC/MS analyses were performed using Agilent 6890 gas chromatography equipped with an Agilent 5970 mass spectrometer. A 30 m × 0.25 mm × 0.25 μm crosslinked with 5% phenylmethyl silicone (HP 5MS) capillary column was used for compound separation. The GC/MS was operated in the selective ion monitoring mode for chemical identification and quantification. Two different GCMS programs were used to determine PCBs and OC pesticides separately. Sixteen OC pesticides including aldrin, α-HCH, β-HCH, δ-HCH, γ-HCH, 4,4′-DDD, 4,4′-DDE, 4,4′-DDT, dieldrin, endosulfan I, endosulfan II, endosulfan sulfate, endrin, endrin aldehyde, endrin ketone, heptachlor, and heptachlor epoxide were analyzed. Results for total DDTs and HCHs were the sum of 4,4′-DDD, 4,4′-DDE and 4,4′-DDT and α-HCH, β-HCH, δ-HCH and γ-HCH, respectively. Aroclor 1242, 1254, and 1260 were used to determine retention times of total PCBs. Total PCBs detected were summed as mono, di, tri, tetra, penta, hexa, hepta, octa, nona, and deca–PCBs. Different congeners were also monitored in the samples for the study of WHO-toxic equivalency concentration (TEQ) of dioxin-like PCBs.
Quality Control
At regular intervals, solvent blanks were subjected to the entire analytical procedures to determine background interference. Reference materials and solvent spike samples were used to check extraction efficiency and recoveries from GPC and florisil cleanup (Archibeque-Eagle et al. 1996). Two reference materials (milk powder CRM 178 and 450) were analyzed with 93 ± 23% of the certified values of PCBs and DDTs. Recoveries of PCBs and DDTs were 92 ± 13% and 89 ± 9%, respectively, for the solvent spike samples.
Statistical Analysis
Statistical analyses were conducted using SPSS version 8. Concentrations of PCBs and OC pesticides were expressed as arithmetic means ± standard deviations. Descriptive statistics were used to characterize the pollutants in samples of human adipose tissue and milk. T tests were performed to assess the relationship between pollutants in the two different types of samples. Spearman rank correlation coefficients were calculated to assess statistical significance of the correlation coefficients for paired samples.
Results and Discussion
Concentrations of PCBs and OC Pesticides in Human Adipose Tissue
PCBs were detected in all adipose tissue samples, Among the 16 selected OC pesticides tested, only β-HCH and p,p′-DDE were found in all samples. Some samples also contained trace amounts of dieldrin (3 samples with 0.009 ± 0.006 μg/g fat), heptachlor epoxide (15 samples with 0.006 ± 0.002 μg/g fat), and p,p′-DDD (31 samples with 0.053 ± 0.037 μg/g fat) (Table 1).
Table 2 compares the present results with results obtained from other countries. The level of PCBs of Hong Kong samples is similar to that from China, which is relatively low compared to developed countries. It might be due to the slower pace of industrialization in Hong Kong (1970s) and China (1980s), compared to developed countries (1950s and 1960s). In addition, PCBs production was banned in the late 1970s globally.
Nevertheless, the level of total DDTs of the Hong Kong population is higher than in samples tested in all other countries, except China and Mexico. The high level of DDT in Mexico is understandable because DDT was widely used for malaria control, which has been banned recently (Diaz-Bamga et al. 2003). However, an unexpected high level of total DDTs is noted for the study conducted in China. This is possibly due to the fact that there may be some illegal use of DDT in China (even though it was banned in 1983), in addition to the fact that China has requested exemption from the Stockholm Convention for the production and use of DDT as an intermediate and for vector control (Wong MH et al. 2002).
Other studies also reported higher levels of DDTs in different environmental compartments of our region, e.g., marine water (Yang et al. 1997) and sediment (Hong et al. 1995). Because most of the food sources consumed in Hong Kong come from China, therefore high levels of DDTs are obtained for the Hong Kong population (CSD 1999–2000). A similar pattern is also found in the levels of total HCHs for Hong Kong and China populations.
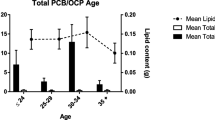
Survey year and age of donors are important factors for studying persistent organic pollutants (POPs) in the adipose tissue. Reductions of POPs contaminants have been reported in several time-trends studies (Choi et al. 2002; Loganathan et al. 1993; Lundén and Norén 1998; Robinson et al. 1990; Ott et al. 1999), Most POPs contained in adipose tissue revealed a decreasing trend reported by different countries. Because dietary intake is a major route to increase POPs level in the body (Rappe 1992), age of donors needs to be considered for studying body burden of POPs. Our previous study on human milk also showed that concentrations of HCHs, DDTs, and PCBs increased according to the increase of age of donors (Wong CKC et al. 2002).
Relationship of PCBs and OC Pesticides in Human Milk and Adipose Tissue
A total of 26 pairs of samples (human milk and adipose tissue) were used to study their relationship with POPs contamination. Table 3 lists the average values of PCBs (milk—74; adipose—177 ng/g), DDTs (milk—3270; adipose—2990 ng/g) and HCHs (milk—1011; adipose—746 ng/g), all fat basis. When comparing the sample means using t test, there were significant correlations for DDTs and HCHs between milk and adipose tissue. There is a lack of information related to the relationship of POPs in human milk and adipose tissue. Similar results were noted for two studies where positive correlations of DDTs were obtained between two types of samples (Kanja et al. 1992; Waliszewsld et al. 2001).
Using the individual pair samples, Figure 1 further shows that there are positive correlations for PCBs, DDTs, and HCHs between milk and adipose tissue, according to Spearman rank correlation coefficients, all with p < 0.01. High levels of coherence of the persistent contaminants are in line with other studies (Kanja et al. 1992; Skaare et al. 1988; Waliszewski et al. 2001). It may be concluded that residues of PCBs and OC pesticides in both human milk and adipose tissue could be used as indicators to show human accumulative exposure of these pollutants.
Contamination of OC Pesticides and PCBs in South China
Table 4 summarizes major findings of OC pesticides and PCBs in different environmental compartments (river water and sediment, marine water and sediment, soil, food, biota, and human) of the Hong Kong and Pearl River Delta region. Low concentrations of these pollutants (mostly >0.1 μg/L) were reported in both fresh and marine waters. This is not unexpected because of their lipophilic nature and low solubility in water. The concentrations of PCBs and lindane (HCH) were lower than the maximum contamination levels of 0.5 and 0.2 μg/L respectively stipulated for national primary drinking water by the USEPA (USEPA 2003). In addition, the concentrations of these pollutants in river and marine water were also lower than the Australian and New Zealand guidelines for protection (90% of species protection) of fresh and marine water quality (ANZEC 2000).
Because of their hydrophobia nature, pollutants have a stronger affinity for particulate materials than water. Therefore, the bottom sediments of river, estuarine, and coastal areas constitute the major sink for these pollutants. The concentration ranges for DDTs (0.3–1629 μg/kg dry wt), HCHs (0.1–16.7 μg/kg dry wt), and PCBs (0.2–339 μg/kg dry wt) in river and marine sediments of the Pearl River Delta were very diversified, depending on sampling locations. For example, lower concentrations were found in mangrove sediment (Liang et al. 1999, Tam and Yao 2002), when compared with estuarine sediment (Mai et al. 2002). Moreover, the highest DDT and PCB levels found in the region were close to the intervention levels established by Dutch or Canadian guidelines, indicating possible ecotoxicological risks.
When comparing the results obtained from inland rivers (Zhou et al. 1999) with Mai Po Marshes (Liang et al. 1999) in Hong Kong, it was revealed that there was an obvious bioconcentration of PCBs for tilapia (fish) collected from the polluted inland rivers (267–310 μ/4g/kg dry wt in fish, 43–461 μ/4g/kg dry wt in sediment), which were much higher than those collected from Mai Po Marshes (7.2–10.3 in fish, 2.9 in sediment). This indicates that POPs have imposed adverse effects on some local organisms thriving in contaminated sites.
Biomagnification of OC pesticides and PCBs is also observed in the Pearl River Delta, with concentrations of PCBs. DDTs, and HCHs much higher in whales than in mussels and fish observed in the region. Biomagnifications of POPs are also recorded in other countries (e.g., Lake Michigan, USA–Trowbridge and Swackhamer 2002; Svalbard, Canada – Borga et al. 2001; Sweden–Berglund et al. 2000). It is commonly found that concentrations of OC pollutants in whales are more than 10-fold higher than that in humans. This may be due to the differences in diets. In general, concentrations of OC in aquatic food > meat > dairy products > vegetable > cereals (Abad et al. 2002; Focant et al. 2002; Kurunthachalam et al. 2001; Nakata et al. 2002; Tsutsumi et al. 2001; Zhang et al. 1999). Fish and other aquatic organisms are the major food source for dolphins and whales; therefore, the bioaccumulation of OC would be higher. Some studies also show a positive relationship of fish consumption and body burden of OC (Norén 1983; Grimvallet et al. 1997; Wong CKC et al. 2002).
Detailed examination of PCB congeners in the adipose tissue of the present results show that concentrations of marker PCBs (28, 52, 101, 118, 138, 153, and 180) are summed up with the value of 159 ± 97 ng/g of fat. Moreover, PCBs 87, 101, 105, 118, 138, 153, 180, 187, and 190 are the predominant congeners, which are similar to the study of specific PCB composition data in Arcolor 1254 and 1260 (Frame et al. 1996). Their composition is >90% of the total PCBs in the human adipose tissue. Some of the above major PCBs are dioxin-like PCBs. The dioxin-like PCBs, including four non-ortho (PCB-77, PCB-81, PCB-126, and PCB-169) and eight mono-ortho (PCB-105, PCB-114, PCB-118, PCB-123, PCB-156, PCB-157, PCB-167 and PCB-189) substituted congeners that have been shown in experimental systems to exert a number of responses similar to those observed for 2,3,7,8-TCDD. There is evidence to suggest that there is a common mechanism of action of TCDD and these dioxin-like PCBs in biological systems, based on their binding to an intercellular protein, the Ah-receptor (Safe 1992, 1995). Table 5 is an estimation of toxic equivalency concentration (TEQ) of dioxin-like PCBs found in present study. The calculation is based on Van den Berg (Van den Berg et al. 1997). However, there is a major deficit in the estimation, because concentrations of non-ortho PCBs were below method detection limits, whereas PCB-126 and PCB-169 have a relatively greater toxic equivalent factor compared to other dioxin-like PCBs. As a result, the calculated TEQ would be underestimated and should be represented more precisely as WHO- mono-ortho-TEQPCBs. Be that as it may, the present estimated TEQ of 2.011 pg/g is much lower than that obtained from Japanese human adipose tissue (15.3 pg/g) (Choi et al. 2002). It is not unexpected because the actual concentration of PCBs in Japanese adipose tissue (1.71 μg/g fat) was 10-folder higher than results obtained in this study (0.19 μg/g fat) (Minh et al. 2000). In addition, the TEQPCBs obtained in present study are comparable to that obtained in human milk samples from Hong Kong (Soechitrain et al. 2003; Malisch and van Leeuwen 2003).
There is a severe lack of information concerning POPs in various ecological compartments in the Pearl River Delta region, which has undergone a very rapid socioeconomic change in the past 20 years. In order to provide more basic data for ecotoxicological and human health risk assessment of the region, a regional organization should be established for setting up a monitoring network using standardized methodologies, with vigorous quality assurance and quality control The more reliable data generated could be used to more accurately assess the fates and effects, including the transboundary movement of POPs in the region.
References
E Abad JJ Llerena J Saulo J Caixach J Rivera (2002) ArticleTitleStudy on PCDDs/PCDFs and co-PCBs content in food samples from Catalonia (Spain) Chemosphere 46 1435–1441 Occurrence Handle10.1016/S0045-6535(01)00247-8 Occurrence Handle12002473
ANZEC, Australian and New Zealand Environment and Conservation Council (2000) Australian and New Zealand guidelines for fresh and marine water quality. National Water Quality Management Strategy, Agriculture and Resource Management Council of Australia and New Zealand
S Archibeque-Eagle JD Tessari DT Winn (1996) ArticleTitleQuality assurance/quality control procedures for chlorinated hydrocarbons in human breast adipose tissue J Toxicol Environ Health 49 589–598 Occurrence Handle10.1080/009841096160646 Occurrence Handle8977626
S Archibeque-Eagle JD Tessari DT Winn (1997) ArticleTitleComparison of organochlorine pesticides and polychlorinated biphenyl residues in human breast adipose tissue and serum J Toxicol Environ Health 52 285–293 Occurrence Handle10.1080/009841097159584 Occurrence Handle9354175
O Berglund P Larsson G Ewald L Okla (2000) ArticleTitleBioaccumulation and differential partitioning of polychlorinated biphenyls in freshwater, plankton food webs Can J Fish Aqua Sci 57 1160–1168 Occurrence Handle10.1139/cjfas-57-6-1160
K Borga GW Gabrielsen JU Skaare (2001) ArticleTitleBiomagnification of organochlorines along Barents sea food chain Environ Pollut 113 187–198 Occurrence Handle10.1016/S0269-7491(00)00171-8 Occurrence Handle11383336
HM Chan KM Chan M Dickman (1999) ArticleTitleOrganochlorines in Hong Kong fish Mar Pollut Bull 39 346–351 Occurrence Handle10.1016/S0025-326X(99)00011-9
JW Choi Y Miyabara S Hashimoto M Morita (2002) ArticleTitleComparison of PCDD/F and coplanar PCB concentrations in Japanese human adipose tissue collected in 1970–1971, 1994–1996 and 2000 Chemosphere 47 591–597 Occurrence Handle10.1016/S0045-6535(02)00008-5 Occurrence Handle12047070
DW Connell RSS Wu BJ Richardson K Leung PSK Lam PA Connell (1998) ArticleTitleFate and risk evaluation of persistent organic contaminants and related compounds in Victoria Harbour, Hong Kong Chemosphere 36 2019–2030 Occurrence Handle10.1016/S0045-6535(97)10087-X Occurrence Handle9532729
CSD, Census and Statistics Department (1999–2000) Hong Kong trade statistics — import, Census and Statistics Department, Hong Kong
F Diaz-Barriga V Borja-Aburto S Waliszewski L Yanez (2003) DDT in Mexico. In The handbook of environmental chemistry vol. 3, Part O — persistent organic pollutants Springer-Verlag Berlin, Heideberg 372–399
JE Elliott RJ Norstrom JA Keith (1988) ArticleTitleOrganochlorines and eggshell thinning in northern gannets (Sula bassanus) from eastern Canada, 1968—1984 Environ Pollut 52 81–102 Occurrence Handle10.1016/0269-7491(88)90083-8 Occurrence Handle15092609
EPD, Environmental Protection Department (1998) Marine water quality in Hong–1997. Water Policy and Planning Group, EPD, Hong Kong Government
JF Focant G Eppe C Pirard AC Massart JE Andre E Pauw ParticleDe (2002) ArticleTitleLevels and cogener distributions of PCDDs, PCDFs and non-ortho PCBs in Belgian foodstuffs assessment of dietary intake Chemosphere 48 167–179 Occurrence Handle10.1016/S0045-6535(02)00104-2 Occurrence Handle12117051
GM Frame JW Cochran SS Boewadt (1996) ArticleTitleComplete PCB congener distributions for 17 Aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis J High Resol Chromatogr 19 657–668 Occurrence Handle10.1002/jhrc.1240191202
E Grimvall L Rylander P Nilsson-Ehle U Nilsson U Stromberg L Hagmar C Ostman (1997) ArticleTitleMonitoring of polychlorinated biphenyls in human blood plasma: methodological developments and influence of age, lactation and fish consumption Arch Environ Contam Toxicol 32 329–336 Occurrence Handle10.1007/s002449900193 Occurrence Handle9096084
H Hong L Xu L Zhang JC Chen YS Wong TSM Wan (1995) ArticleTitleEnvironmental fate and chemistry of organic pollutants in the sediment of Xiamen and Victoria harbours Mar Pollut Bull 31 229–236 Occurrence Handle10.1016/0025-326X(95)00115-4
HMH Ip (1990) ArticleTitleChlorinated pesticides in foodstuffs in Hong Kong Arch Environ Contam Toxicol 19 291–296 Occurrence Handle10.1007/BF01056099 Occurrence Handle2322022
HMH Ip DJH Phillips (1989) ArticleTitleOrganochlorine chemicals in human breast milk in Hong Kong Arch Environ Contam Toxicol 18 490–494 Occurrence Handle10.1007/BF01055014 Occurrence Handle2774666
A Kamarianos EG Iosifidou C Batzios IE Psomas S Kilikidis (1997) ArticleTitleResidues of organochlorine pesticides and PCBs in human adipose tissue in Greece Fresenius Environ Bull 6 383–389
YS Kang M Matsuda M Kawano T Wakimoto BY Min (1997) ArticleTitleOrganochlorine pesticides, polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and dibenzofurans in human adipose tissue from western Kyungnam, Korea, Chemosphere 35 2107–2117 Occurrence Handle10.1016/S0045-6535(97)00289-0 Occurrence Handle9375352
LW Kanja JU Skaare SBO Ojwang CK Maitai (1992) ArticleTitleA comparison of organochlorine pesticide residues in maternal adipose tissue, maternal blood, cord blood and human milk from mother/infant pairs Arch Environ Contam Toxicol 22 21–24 Occurrence Handle10.1007/BF00213297 Occurrence Handle1554250
SK Kurunthachalam K Kurunthachalam NP Odathurai PSS Vellakovil N Junko M Shigeki (2001) ArticleTitlePolychlorinated dibenzo-p-dioxins, dibenzofurans, and polychlorinated biphenyls in human tissues, meat, fish and wildlife samples from India Environ Sci Technol 35 3488–3455 Occurrence Handle10.1021/es010579g Occurrence Handle11563651
Y Liang MH Wong RBE Shutes DM Revitt (1999) ArticleTitleEcological risk assessment of polychlorinated biphenyl contamination in the Mai Po marshes nature reserve, Hong Kong Water Res 33 1337–1346 Occurrence Handle10.1016/S0043-1354(98)00353-4
BG Loganathan K Kannan (1994) ArticleTitleGlobal organochlorine contamination trends: an overview Ambio 23 187–191
BG Loganathan S Tanabe Y Hidaka M Kawano H Hidaka R Tatsukawa (1993) ArticleTitleTemporal trends of persistent organochlorine residues in human adipose tissue from Japan, 1928–1985 Environ Pollut 81 31–39 Occurrence Handle10.1016/0269-7491(93)90025-J Occurrence Handle15091834
RA Lordo KT Dinh JG Schwemberger (1996) ArticleTitleSemivolatile organic compounds in adipose tissue: estimated averages for the US population and selected subpopulations Am J Pub Health 86 1253–1259 Occurrence Handle8806377
A Lundén K Norén (1998) ArticleTitlePolychlorinated naphthalenes and other organochlorine contaminants in Swedish human milk, 1987–1992 Arch Environ Contam 34 414–423 Occurrence Handle10.1007/s002449900338
BX Mai JM Fu GY Sheng YH Kang Z Lin G Zhang YS Min EY Zeng (2002) ArticleTitleChlorinated and polycyclic aromatic hydrocarbons in riverine and estuarine sediments from Pearl River Delta, China Environ Pollut 117 457–474 Occurrence Handle10.1016/S0269-7491(01)00193-2 Occurrence Handle11911529
Malisch R, van Leeuwen FXR (2003) Results of the third round of the WHO-Coordinated Exposure Study on the Levels of PCBs, PCDDs and PCDFs in Human Milk. DIOXIN 2003, World Health Organization
M Mariottini C Guerranti S Aurigi I Corsi S Focardi (2002) ArticleTitlePesticides and polychlorinated biphenyls residues in human adipose tissue Bull Environ Contam Toxicol 68 72–78 Occurrence Handle11731834
TB Minh M Watanabe H Nakata S Tanabe TA Jefferson (1999) ArticleTitleContamination by persistent organochlorines in small cetaceans from Hong Kong coastal waters Mar Pollut Bull 39 383–392 Occurrence Handle10.1016/S0025-326X(99)00066-1
TB Minh M Watanabe S Tababe T Yamada J Hata S Watanabe (2000) ArticleTitleOccurrence of Tris (4-chlorophenyl) methane, Tris (4-chlorophenyl) methanol and some other persistence organochlorines in Japanese human adipose tissues Environ Health Perspect 108 599–603 Occurrence Handle10903611
I Monirith D Ueno S Takahashi H Nakata A Sudaryanto A Subramanian S Karuppiah A Ismail M Muchtar J Zheng BJ Richardson M Prudente ND Hue TS Tana AV Tkalin S Tanabe (2003) ArticleTitleAsia-Pacific mussel watch: monitoring contamination of persistent organochlorine compounds in coastal waters of Asian countries Mar Pollut Bull 46 281–300 Occurrence Handle10.1016/S0025-326X(02)00400-9 Occurrence Handle12604061
H Nakata M Kawazoe K Arizono S Abe T Kitano H Shimada W Li X Ding (2002) ArticleTitleOrganochlorine pesticides and polychlorinated biphenyl residues in foodstuffs and human tissue from China: Status of contamination, historical trend and human dietary exposure Arch Environ Contam Toxicol 43 473–480 Occurrence Handle10.1007/s00244-002-1254-8 Occurrence Handle12399919
K Norén (1983) ArticleTitleLevels of organochlorine contaminations inhuman milk in relation to the dietary habits of the mothers Acta Paaediatr Scand 72 811–816
M Ott K Failing U Lang SH Schurbring HJ Gent S Georgii H Brunn (1999) ArticleTitleContamination of human milk in middle Hesse, Germany–a cross-sectional study on the changing levels of chlorinated pesticides, PCB congeners and recent levels of nitro musks Chemosphere 38 293–297 Occurrence Handle10.1016/S0045-6535(98)00165-9 Occurrence Handle10901656
ECM Parsons HM Chan R Kinoshita (1999) ArticleTitleTrace metal and organochlorine concentrations in a Pygmy Bryde’s whale (Balaenoptera edeni) from the South China Sea Mar Pollut Bull 38 51–55 Occurrence Handle10.1016/S0025-326X(98)00092-7
A Pauwels A Covaci J Weyler L Delbeke M Dhont P Suttler ParticleDe D Hooghe PJC Schepens (2000) ArticleTitleComparison of persistent organic pollutants residues in serum and adipose tissue in a female population in Belgium. 1996–1998 Arch Environ Contam Toxicol 39 265–270 Occurrence Handle10.1007/s002440010104 Occurrence Handle10871430
C Rappe (1992) ArticleTitleDietary exposure and human levels of PCDDs and PCDFs Chemosphere 25 231–234 Occurrence Handle10.1016/0045-6535(92)90521-R
BJ Richardson GJ Zheng (1999) ArticleTitleChlorinated hydrocarbon contaminants in Hong Kong surfacial sediments Chemosphere 6 913–923 Occurrence Handle10.1016/S0045-6535(99)00041-7
Ritter L, Solomon KR, Forget J, Stemeroff M, O’Leart C (1995) Persistent organic pollutants assessment report. Inter-Organization Programme for the Sound Management of Chemical, The International Programme on Chemical Safety (IPCS)
PE Robinson GA Mack J Remmers R Levy L Mohadjer (1990) ArticleTitleTrends of PCBs, hexachlorobenzene and β-benzene hexachloride levels in the adipose tissue of the US population Environ Res 53 175–192 Occurrence Handle10.1016/S0013-9351(05)80118-5 Occurrence Handle1701383
S Safe (1992) ArticleTitlePCBs, PCDD/F and related compound: environmental and mechanistic considerations which support the development of toxic equivalency factors CRC Crit Rev Toxicol 21 51–88
S Safe (1995) ArticleTitleDevelopment and validation of the toxic equivalency (TEF) approach for the risk assessment of PCBs Organohalogen Comp 22 131–141
JU Skaare JM Tuveng HA Sande (1988) ArticleTitleOrganochlorine pesticides and polychlorinated biphenyls in maternal adipose tissue, blood, milk and cord blood from mothers and their infants living in Norway Arch Environ Contain Toxicol 17 55–63 Occurrence Handle10.1007/BF01055154
A Smeds P Saukko (2001) ArticleTitleIdentification and quantification of polychlorinated biphenyls and some endocrine disrupting pesticides in human adipose tissue from Finland Chemosphere 44 1463–1471 Occurrence Handle10.1016/S0045-6535(00)00313-1 Occurrence Handle11513126
SD Soechitram SM Chan EAS Nelson A Brouwer PJJ Sauer (2003) ArticleTitleComparison of dioxin and PCB concentrations in human breast milk samples from Hong Kong and the Netherlands Food Add Contam 20 65–69 Occurrence Handle10.1080/0265203021000031528
InstitutionalAuthorNameSRC, Syracuse Research Corporation (1987) Toxicological profiles for selected PCBs. Agency for Toxic Substance and Disease Registry US Public Health Service Washington, DC
NFY Tam MWY Yao (2002) ArticleTitleConcentrations of PCBs in coastal mangrove sediments of Hong Kong Mar Pollut Bull 44 642–651 Occurrence Handle10.1016/S0025-326X(01)00306-X Occurrence Handle12222887
S Tanabe T Mori R Tatsukawa N Miyazaki (1983) ArticleTitleGlobal pollution of marine mammals of PCBs, DDTs and HCHs (BHCs) Chemosphere 12 1269–1275 Occurrence Handle10.1016/0045-6535(83)90132-7
CC Travis H Hattermer-Frey AD Arms (1988) ArticleTitleRelationship between dietary intake of organic chemicals and their concentrations in human adipose tissue and breast milk Arch Environ Contam Toxicol 17 473–478 Occurrence Handle10.1007/BF01055512 Occurrence Handle3044283
AG Trowbridge DL Swackhamer (2002) ArticleTitlePreferential biomagnifications of aryl hydrocarbon hydroxylase-inducing polychlorinated biphenyl congeners in the Lake Michigan, USA, lower food web Environ Toxicol Chem 21 334–341 Occurrence Handle10.1897/1551-5028(2002)021<0334:PBOAHH>2.0.CO;2 Occurrence Handle11833802
T Tsutsumi T Yanagi M Nakamura Y Kono H Uchibe T Iida T Hori R Nakagawa K Tobiishi R Matsuda K Sasaki M Toyoda (2001) ArticleTitleUpdate of daily intake of PCDDs, PCDFs, and dioxin-like PCBs from food in Japan Chemosphere 45 1129–1137 Occurrence Handle10.1016/S0045-6535(01)00151-5 Occurrence Handle11695626
InstitutionalAuthorNameUSEPA (1996a) Florisil cleanup. Solid waste analysis SW-846 United States Environmental Protection Agency USA
InstitutionalAuthorNameUSEPA 3640 (1996b) Gel permeation chromatography. Solid waste analysis SW-846 United States Environmental Protection Agency USA
USEPA (2003) National primary drinking water standards. USA
M Berg ParticleVan den L Birnbaum ATC Bosveld B Brunstroem P Cook M Feeley JP Giesy A Hanberg R Hasegawa SW Kennedy T Kubiak JC Larsen FXR Leeuwen ParticleVan (1997) ArticleTitleToxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife Environ Health Perspect 106 775–792
SM Waliszewski AA Aguirre RM Infanzon CS Silva J Siliceo (2001) ArticleTitleOrganochlorine pesticide levels in maternal adipose tissue, maternal blood serum, umbilical blood serum and milk from inhabitants of Veracruz, Mexico Arch Environ Contam Toxicol 40 432–438 Occurrence Handle10.1007/s002440010194 Occurrence Handle11443377
InstitutionalAuthorNameWHO, World Health Organization (1979) Environmental health criteria, no. 9 DDT and its derivations WHO Geneva, Switzerland
CKC Wong HY Yeung RYH Cheung KKL Yung MH Wong (2000) ArticleTitleEcotoxicological assessment of persistent organic and heavy metal contamination in Hong Kong coastal sediment Arch Environ Contam Toxicol 38 486–493 Occurrence Handle10.1007/s002449910064 Occurrence Handle10787100
CKC Wong KM Leung BHT Poon CY Lan MH Wong (2002) ArticleTitleOrganochlorine hydrocarbons in human breast milk collected in Hong Kong and Guangzhou Arch Environ Contam Toxicol 43 364–372 Occurrence Handle10.1007/s00244-002-1210-7 Occurrence Handle12202934
MH Wong BHT Poon (2002) Sources, fates and effects of persistent organic pollutants in China, with emphasis on the Pearl River Delta H Fiedler (Eds) persistent organic pollutants Springer Heidelberg 355–370
Wong MH, Choi K, Grosheva E, Sakai S, Shibata Y, Suzuki N, Wang J, Zhou H, Leung A (2002) Regionally based assessment of persistent toxic substances. Regional report. Central and North East Asia. Global Environment Facility/United Nations Environment Program
YH Yang GY Sheng JM Fu YS Min (1997) ArticleTitleOrganochlorinated compounds in waters of the Pearl River Delta region Environ Monit Assess 44 569–575 Occurrence Handle10.1023/A:1005743932046
G Zhang YS Min BX Mai GY Sheng JM Fu ZS Wang (1999) ArticleTitleTime trend of BHCs and DDTs in a sedimentary core in Macao Estuary, Southern China Mar Pollut Bull 39 326–330 Occurrence Handle10.1016/S0025-326X(99)00010-7
Z Zhang M Dai H Hong JL Zhou G Yu (2002) ArticleTitleDissolved insecticides and polychlorinated biphenyls in the Pearl River Estuary and South China Sea J Environ Monit 4 922–928 Occurrence Handle10.1039/b206891p Occurrence Handle12509046
Zhou HY, Wong MH (2003) Screening of organochlorines in freshwater fish collected from the Pearl River Delta, People’s Republic of China. Arch Environ Contam Toxicol 46:106–113
HY Zhou RYH Cheung MH Wong (1999) ArticleTitleResidues of organochlorines in sediments and tilapia collected from inland water systems of Hong Kong Arch Environ Contam Toxicol 36 424–431 Occurrence Handle10227862
Acknowledgments
The authors would like to thank Hong Kong Baptist Hospital, Canossa Hospital, Caritas Hospital, Hong Kong Sanitarium Hospital and Union Hospital for the provision of samples and Angela Wong for technical support, This work was supported by Group Research–Central Allocation of the Research Grants Council, University Grants Committee of Hong Kong (No. HKBU-2/00 C).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poon, B.H.T., Leung, C.K.M., Wong, C.K.C. et al. Polychlorinated Biphenyls and Organochlorine Pesticides in Human Adipose Tissue and Breast Milk Collected in Hong Kong. Arch Environ Contam Toxicol 49, 274–282 (2005). https://doi.org/10.1007/s00244-004-0111-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-004-0111-3