Abstract
Selenium has been found at elevated concentrations in water, sediments, and aquatic biota in the Elk River (British Columbia, Canada) and some of its tributaries downstream of several coal mines. Selenium water concentrations in those areas exceed Canadian and British Columbia guidelines and are above levels at which adverse effects to fish and waterfowl could occur. We compared selenium concentrations in the eggs of two riverine waterbirds, American dippers and spotted sandpipers, with measures of productivity: the number of eggs laid, egg hatchability, and nestling survival. In American dippers, the mean egg selenium concentration from the exposed areas, 1.10 ± SE 0.059 μg/g wet weight, was indistinguishable from the reference areas, 0.96 ± SE 0.059 μg/g wet weight. For spotted sandpipers, the mean egg selenium concentration in the exposed areas, 2.2 ± 0.5 μg/g wet weight, was significantly higher than in the reference areas, 1.2 ± 0.14 μg/g wet weight, but less than reported thresholds for waterfowl and other shorebirds. There were no significant differences in egg hatchability between dippers in reference and exposed areas, but reduced hatchability was apparent for sandpipers in exposed locations. Despite the slightly reduced hatchability in sandpipers, overall productivity was higher than regional norms for both species; thus, selenium did not affect the number of young recruited to local populations. We did not observe teratogenic effects in either species, although none was expected at these concentrations. Despite moderately high selenium concentrations in the water, mean egg selenium concentrations were less than predicted from uptake models. We hypothesise that the relatively low uptake of selenium into the eggs of the two waterbirds in this study is likely due to their lotic environment’s low biological transformation and uptake rates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
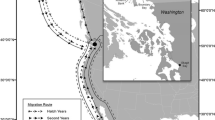
The Elk River in British Columbia, Canada (Figure 1) is renowned for its native west-slope cutthroat trout (Oncorhynchus clarki lewisi), which supports a vigorous sport fishing industry. It is also a tributary of the Kootenay River Valley, which is among British Columbia’s most important waterfowl habitats (Hayes et al. 1993). The discovery of elevated selenium concentrations in the Elk River below several coal mines (McDonald and Strosher 1998) raised concern because selenium can be toxic to birds and fish at relatively low concentrations.
Selenium concentrations as high as 107 ± SD 14 μg/L have been recorded in tributary streams below active mining areas (EVS Environment Consultants 2003a). In the Elk River itself above the coal mines, and in tributaries above mining areas, selenium concentrations are generally <1.0 μg/L (rarely up to about 5.0 μg/L in creeks that pass through coal-bearing strata), whereas those below the coal mines were as high as 7.4 μg/L (EVS Environment Consultants 2003b; Kennedy et al. 2000; McDonald and Strosher 1998, 2000). Waterborne selenium concentrations less than 1.0 μg/L have been reported to pose no risk to waterbirds, whereas those more than about 5.0 μg/L pose a high risk to birds (Adams et al. 1998; Lemly 1993, 1996). The British Columbia water quality guideline to protect aquatic life is 2.0 μg/L (Nagpal and Howell 2001), and the Canadian “no effect” guideline is 1.0 μg/L (Environment Canada/Health Canada 1995).
Selenium that has bioaccumulated into tissues can have a wide variety of toxic effects to birds, including effects on metabolism, growth, and reproduction. Selenium in the eggs of wild water birds is often strongly correlated with the concentrations in the water where they live (Adams et al. 1998; Ohlendorf et al. 1993; Skorupa 1998b). It affects reproduction in birds through mortality of the egg or hatchling and through teratogenesis. Teratogenic deformities, which occur at selenium concentrations above those that begin to affect hatchability and nestling survival, can include anophthalmy (no eyes), spinal and bill deformations, defects in internal organs, and histological abnormalities. Selenium can cause teratogenic effects and mortality in embryos and chicks even when adults seem unaffected. The purpose of this study was to determine whether productivity in two waterbirds, American dippers (Cinclus mexicanus) and spotted sandpipers (Actitis macularia), was the same in selenium-contaminated waters receiving coal mine runoff as in unpolluted reference areas.
Materials and Methods
Species Selection
American dippers (hereafter, “dippers”) and spotted sandpipers (hereafter, “sandpipers”) were selected for this study because they are present throughout the study area and feed on aquatic invertebrates.
Dippers are passerine birds (song birds) that live along swiftly moving streams and feed mainly on aquatic invertebrates; in essence, they occupy the same habitat as trout. Dippers build nests of moss on rock faces or ledges above the water, typically near or behind a waterfall. The young remain in or near the nest and are provisioned by both parents until fledged. Trace metals and metalloids in streams can be taken up by benthic invertebrates and then transferred to higher trophic-level organisms (Clements 1992; Dallinger and Kautzky 1985). Because of this, American dippers and their European counterparts (Cinclus cinclus) have been used as indicators of water quality (Acchuleta 1996; Logie et al. 1996; Strom et al. 2002; Tyler and Ormerod 1992). Dippers tend to be absent in polluted streams (Sorace et al. 2002). Morrissey et al. (2004a) found that, in another British Columbia river system, most (85%) of dippers nest in smaller, tributary streams at high elevation and winter in the main river at lower elevation, whereas a few (15%) reside all year on the lower river. They also found that selenium and other contaminant concentrations in eggs reflected the concentrations in the prey of their nesting environment, rather than of their wintering areas (Morrissey et al. 2004b).
Sandpipers nest on gravel bars and stream and lake edges throughout the study area and winter along the coast. After hatching, the young remain in the nest for a few days before leaving it to forage with the parent before they can fly. In general, at least 2 weeks are needed for recently arrived migratory birds to accumulate tissue concentrations of selenium that reflect local conditions (Heinz et al. 1990). Waterfowl collected in spring before the egg-laying season were expected to have reached equilibrium with respect to local environmental contaminant levels (Braune et al. 1999). Because sandpipers establish territories and court for about a month before laying eggs, any contaminants in their eggs would reflect the local nesting environment, rather than their wintering areas.
Study Locations
Based on previous data on selenium concentrations in water, sediments, and benthic macroinvertebrates, we selected the portions of the Fording River and 2 tributaries (Line and lower Michel Creeks) that receive coalmine runoff as exposed locations (Figure 1). There were five reference areas: Boivin Creek, upper Elk River, Lynx Creek, upper Michel Creek, and Gold Creek (Figure 1). These reference areas were located upstream or out of the drainage basin of any mine runoff, except for upper Michel Creek, which was far enough downstream and sufficiently diluted with clean tributaries that its selenium concentrations were also background. Gold and Lynx creeks are on the Alberta side of the Continental divide. Since Gold, Lynx, and Boivin creeks had not been sampled previously, at the beginning of the study in May 2002, we took samples of water and riffle benthic invertebrates, as well as a water sample from the upper Elk River. The water samples from these two reference area creeks confirmed that they represented background selenium concentrations (all concentrations ≤0.1 μg/L Se) (Table 1). These are consistent with other reported background water concentrations that ranged from ≤0.1 to ≤ 3.0 μg/L (Adams et al. 1998; Casey and Siwik 2000; Ohlendorf et al. 1990; Seiler 2003; U.S. Department of the Interior 1998).
The benthos samples from Gold and Lynx creeks (mainly Ephemeroptera [mayflies], Diptera [flies], Perlodid stoneflies, and Hydropsychid caddis flies) averaged 0.92 μg/g wet wt (7.1 μg/g dry wt), and were in the range of other, previously sampled reference area selenium concentrations (0.34 to 0.95 μg/g wet wt) (Table 1). However, a composite sample from Boivin Creek, on the west side of the Elk Valley, where there are no coal mines, had 1.66 μG/g Se wet wt (12.8 μg/g dry wt). Selenium concentrations in riffle benthos in the Elk River Valley ranged from 1.5 to 10.7 μg/g dry wt overall; from 1.5 to 6.84 μg/g dry wt in upriver reference sites, and 3.1 to 10.7 μg/g dry wt at sites below mine runoff (EVS Environment Consultants 2002; McDonald and Strosher 1998). This range is a little higher than other reported background concentrations in aquatic invertebrates that ranged from 0.4 to 4.5 μg/g dry wt (Hoffman et al. 2002; Ohlendorf et al. 1986; Saiki and Lowe 1987).
Nesting and Productivity Observations
During March and April 2002, we monitored courtship and nesting behaviour to locate nests. Dippers were present throughout the later winter period, but the first spotted sandpiper was seen on May 13. When courtship behaviour indicated that nesting was imminent, we monitored each pair’s behaviour to determine when egg-laying and incubation were initiated. This allowed us to predict a hatching date for each nest. When egg-laying was complete for each nest and the female began incubating, we counted the eggs. We began collecting eggs on May 15 for dippers and on June 19 for sandpipers. About 2 to 4 days before the expected hatching time, when embryos would be nearly fully formed (except for the first dipper nest, which we sampled sooner), we counted the eggs again (to determine whether more had been laid) and collected one from each nest for chemical analysis, up to the number allowed by our federal permit (seven eggs each in exposed and reference locations for each species). However, at one dipper nest each from an exposed and a reference area, we collected all eggs to test for within-clutch variability of selenium concentrations. Tissue collection and storage procedures followed Canadian Wildlife Service (1992, 1997) protocols.
When parental behaviour indicated that nestlings were present, from 1 to 10 days after hatching, we counted and checked them for mortality and teratogenic effects, using a mirror on a pole when necessary to see inside the nests. Live nestlings were not examined in detail, however, to avoid excessive disturbance that could have led to nest abandonment. When the nestlings began to appear outside the nests, we used binoculars or a 20× spotting scope to identify any obvious teratogenic effects or mortality. We continued these observations until the young were fledged and left the nest area. After the incubation period, any remaining eggs were salvaged for examination and chemical analysis.
We continued monitoring the nests until fledging was complete, about 3 weeks for dippers. The precocious sandpipers, however, left the nest 1–2 weeks after hatching and before they were fledged enough to fly, so that nestling survival was based on the number of nestlings last observed in the nests, usually about 1 week post-hatch.
Egg Examinations
We weighed and measured each egg and opened it to examine the contents under a hand lens for general condition and viability. If no embryo was present (indicated by no visible structures in the egg, other than yolk and albumin), we considered it infertile. Because we timed our collections for the later stage of incubation, nearly all eggs from active nests had visible embryos. However, for unhatched eggs that we salvaged after the incubation period or after a nest had been abandoned, it is possible that we identified eggs as infertile that had been fertilized but had died at an early stage of incubation. Because of this uncertainty, we analysed the data on the basis that the eggs either hatched or not.
If an embryo was present, we examined the eyes, bill, wings, legs, and feet to identify any visible abnormalities. If an embryo was present but dead, we recorded it as inviable. After examination, we placed the egg contents (without the shells) into acid-washed sample jars and shipped them to the laboratory. There they were frozen at −40°C until the field collections were complete so that all of the eggs could be analyzed together in one batch, eliminating the possibility of interbatch variation.
Chemical Analysis
Egg contents were submitted to a private laboratory (ALS Environmental of Vancouver, British Columbia, certified by the Canadian Association for Environmental Analytical Laboratories Inc. and accredited by the Standards Council of Canada). Eggs were analyzed for arsenic, mercury, and vanadium, as well as selenium, because of the possible antagonistic or synergistic effects these metals may have with selenium. Analysis followed U.S. Environmental Protection Agency and Environment Canada protocols for the Puget Sound–Georgia Strait region (Puget Sound Water Quality Action Team 1995). The eggs were homogenized and digested with nitric acid followed by repeated additions of hydrogen peroxide. Instrument analysis for As, Se, and Vn was by ICP/MS (Inductively coupled plasma–mass spectrometry; EPA Method 6020) and Hg by cold-vapour AAS (Atomic Absorption Spectrophotometry; EPA Method 7000 series). The laboratory’s proprietary micro-digestion technique was used to produce acceptable detection limits for the small (about 4 g for dippers and 7 g for sandpipers) aliquot sizes of individual egg contents. This is the same as routine digestion (HNO3−H2O2) except that everything was scaled down and instead of using a hotplate and a flask, analysts used a testube and hotblock digester. To maximize the tissue available for selenium and metals determination, percent moisture was not routinely measured and results were reported in wet weight only. However, two dipper eggs, collected separately, were analysed for percent moisture, the mean of which (86.9%) was used to calculate dry weight for all dipper eggs.
The laboratory’s Data Quality Objectives (accuracy 70%–120% recovery, precision 45% relative difference) were met for all samples. For the ICPMS analysis, a series of internal standards covered the mass ranges of Li6, Sc45, Y89, In115, Re187, and Th232. Five laboratory method blanks were all less than the detection limit (<1.0 mg/kg). Recoveries of selenium in seven standard reference materials (2 NIST Bovine Liver, 2 1577b, NRC Dogfish Liver, DOLT-2 and 3 NRC Lobster Hepatopancreas, TORT-2) were all 100% except for one of the three lobster samples that had 117% recovery (mean of 106%). Hence, the selenium concentrations might have been overstated by about 6%. We analyzed two pairs of duplicate samples of sandpiper eggs, with a difference of 5% for selenium in one sample and 11% for selenium in the other. For arsenic, the difference was not calculable in one duplicate because one of the pair was below the detection limit, and was 0.00% in the other. For vanadium, both QA duplicates were below detection limit. Analytical results were reported in wet weight, which were used for all statistical comparisons.
Statistical Analysis
We compared the number of eggs laid per nesting pair, the proportion of eggs incubated to full term that hatched (“hatchability”), and proportion of those that survived long enough to leave the nest (“nestling survival”). Counts of eggs incubated to full term omitted those collected from active nests for selenium analysis, or lost for other causes, such as flooding or predation. Eggs that disappeared before completion of incubation were assumed to have been taken by predators. Counts of fledglings omitted nests with nestlings that were destroyed by snow or flooding. Descriptive statistics were performed with SPSS®. Hatchability and survival of hatchlings were compared in reference and exposed areas by correspondence analysis of K × C “Non-central” Chi Squared (X2) contingency tables (BMDP Statistical Software, Inc.). The hatchability test compared the number of eggs that hatched versus those that failed to hatch in exposed and reference areas, whereas the nestling survival test compared the number of nestlings that survived to fledging versus those that did not. Hatchability was based on the subset of nests with counts of both eggs incubated and eggs hatched (i.e., nestlings). Nestling survival was based on the subset of nests with counts of both nestlings and fledglings. Selenium concentrations were compared by one-way analysis of variance (ANOVA) for unequal sample sizes using log-transformed, wet weight concentrations.
Prior to testing for relationship of egg health to selenium concentrations, to determine whether results from the two species could be combined, we used a two-way ANOVA on selenium concentration data, with Species x Treatment (exposed or reference) as the groups (BMDP software: BMDP2V—analysis of variance and covariance with repeated measures). Because the interaction term was significant (p = 0.0013), indicating different sequestering of selenium in the eggs, we analysed the health status of the two species separately.
In sandpipers, to determine whether some of the differences in selenium concentration could be attributed to egg size or number, we analysed within-nest variation by ANOVA (BMDP software: BMDP3V—general mixed model analysis of variance). We encountered some infertile and inviable eggs and tested their frequencies for significance between exposed and reference areas by chi-square.
Results
Weather
Approximately double the usual amount of snow fell in February (73 cm compared to a 20-year normal of 34 cm) and persisted late into the spring because of low temperatures. The monthly average minimum temperature for March was colder than normal, −10.8°C compared to a 20-year normal of −5.0°C, whereas precipitation was higher than normal throughout the spring and sharply higher in May (100 mm compared to a 20-year normal of 60 mm), most of it falling as snow. Snow or flooding destroyed at least nine American dipper nests, whereas sandpipers delayed nesting until declining water levels began to expose gravel bars in late June and July. As a result, courtship and nesting for both species were much later than usual, and more spread out.
Selenium in American Dipper Eggs and Effects on Productivity
In 45 American dipper pair territories monitored, we found 36 nests. However, snow and floods destroyed 9 nests, leaving 27 active first nests. One pair re-nested after fledging a full clutch, bringing the total number of active nests from which we collected eggs and made productivity observations to 28. The first eggs appeared about May 12. The first brood appeared on June 2. By July 11, dippers had laid eggs at all of the study area creeks and young had fledged on 7 of 10 of the reference and exposed creeks. Delayed nesting or re-nesting after a failed first attempt occurred during July. By the end of July, dippers had laid eggs at all creeks in the study area. We collected eggs and/or made productivity observations at 14 nests in both exposed and reference areas.
In addition to the 14 eggs allowed by our federal permit, we salvaged 7 eggs that failed to hatch, well after the incubation period. In all, we collected 11 dipper eggs from reference streams and 10 from exposed streams.
Summary results of chemical analysis for mean egg selenium (MES) and productivity observations are illustrated in Figure 2 and summarized in Table 2. Arsenic and vanadium were mostly less than detection limits of 0.01 to 0.02 μg/g wet weight and 0.2 μg/g wet weight, respectively. Mercury was detected in all eggs with a mean of 6.8 pg/g wet weight and was not correlated with selenium.
The eggs that we collected from active nests were no different in selenium concentration, size, or weight (p > 0.05) from the eggs that were salvaged after they failed to hatch. Therefore, we combined them for further analysis.
Because of the number of nests destroyed by snow and floods, sample sizes were unexpectedly low (Table 2). The MES concentration from the exposed areas was 1.10 μg/g ± SE 0.058 wet weight and in the reference areas, 0.96 μg/g ± SE.059 wet weight (Table 2). These were not significantly different (p = 0.34), but because of the small sample size, the statistical power was low (0.11). On a dry weight basis, exposed and reference area means were 8.4 ± SE 0.44 μg/g dry weight and 7.4 ± SE 0.45 μg/g dry weight, respectively.
Because of high water, we were unable to count eggs, nestlings, or fledglings in some of the nests. All nests incubated to full term produced at least 1 hatchling. Three nests (all in exposed areas) were destroyed by floods or predators after eggs were laid, but before the expected hatch date. In the exposed areas, dippers laid 4.4 ± SE 0.18 eggs per nest (Table 2). Of 24 eggs incubated to full term, 5 failed to hatch. Overall hatchability was 79% (19 of 24 incubated to full term) (Figure 2). Of 30 nestlings (including some in nests for which we had been unable to count the eggs), 23, or 77%, survived to fledge.
In the reference areas, dippers laid 4.5 ± SE 0.29 eggs per nest (Table 2). Although not significantly different from the exposed area (p > 0.10), the power of this test was very low (0.20) because of the small sample size. Of 49 eggs incubated to full term, 3 failed to hatch, 46 nestlings hatched, and 36 of them fledged. Overall hatchability was 94% (Figure 2). Nestling survival was 78% overall. The difference in hatchability between exposed and reference areas was not significant at 5% probability (X2 = 3.6, p = 0.056). The difference in nestling survival was also not significant (X2 = 1.7, p = 0.27), but the sample size was low and the statistical power was only 0.26. There was no correlation between selenium concentrations and eggs laid, brood size, or clutch size (linear regression, p > 0.05). Nest success (the number of nests monitored that produced at least 1 fledgling) was nearly identical: 92.9% (13 of 14 nests) in the exposed areas and 92.3% (12 of 13) in the reference areas.
We saw no abnormalities in the 14 dipper embryos that were sufficiently developed that terata might have been visible, or in the 76 juveniles that we observed. Of the eggs collected for examination, three were infertile or inviable in the reference areas and four in the exposed areas. Egg measurements in relation to selenium concentrations are shown in Figure 3. Because of the small numbers of eggs in this dataset, selenium concentration with egg weight was examined as a simple correlation for each group (reference and exposed); the r values were very low, 0.21 and 0.02, respectively, neither of which was significant at p = 0.10.
The range of selenium concentrations in the seven inviable eggs spanned the range for dipper eggs from lowest (0.6 μg/g wet wt) to highest (1.4 μg/g wet wt). The frequencies of viable vs. inviable eggs were identical (not counting two broken eggs). Therefore, there was no relationship of selenium concentration with egg size or health, nor would it be expected, since variability was as high within nests as between nests.
Selenium in Spotted Sandpiper Eggs and Effects on Productivity
In the 82 territories that we monitored, we found 60 nests representing all of the study creeks except Line Creek. Although three pairs of sandpipers occupied territories on Line Creek and raised one fledgling each, we were unable to locate any nests there.
In addition to the 14 eggs collected (7 each in reference and exposed areas) from active nests, we salvaged 26 eggs that failed to hatch and remained in the nests until well after the incubation period. Most (19) of these were on lower Michel Creek (one of the three exposure locations). The total sandpiper egg sample size was 40 sandpiper eggs: 14 from reference areas and 26 from exposed areas.
With sandpipers, there was enough “replication” (eggs from the same nest) to examine variation within nests compared to between nests. We used a general mixed model ANOVA, maximum likelihood method (BMDP software: BMDP3V) to test whether the selenium concentration was significantly different in reference versus experimental sites, while accounting for variation within nests (a random variable) and any possible effect of egg weight as a covariate. The result was that the random factor of eggs coming from the same nest was not significant (p = 0.372), and therefore all the eggs were treated in the same way in the analysis without regard to their having nestmates in the analysis or not. The weight of eggs also was not a factor affecting selenium concentration in the subsample of replicates, because we found no significant correlation between egg weight and selenium concentration. This result suggested that the amount of selenium in an egg was primarily determined by other factors, such as exposure to selenium or random variation. After excluding any problems with unequal replication and covariance on weight, the main treatment effect, we then examined the reference sites vs. exposed sites, and found the difference highly significant at p = 0.001: exposed sites had higher selenium concentrations (Table 3).
There was no difference (p < 0.05) in selenium concentrations between the eggs that were collected randomly from active nests and those that were salvaged after they failed to hatch, both in the whole data set and when separated by whether they were from exposed areas or not. Therefore, randomly and nonrandomly collected sandpiper eggs were combined for further analysis.
The MES concentration in sandpipers from the exposed areas (2.2 ± SE 0.13 μg/g wet wt) was about twice that of dippers. The mean selenium concentration in the reference areas was 1.2 ± SE 0.057 μg/g wet weight. On a dry weight basis, based on an assumed moisture content of 70% (Ohlendorf and Hothem 1995; Ohlendorf et al. 1986), sandpipers in exposed and reference areas had MES concentrations of 7.3 ± SE 0.43 and 3.8 ± SE 0.19 μg/g dry weight, respectively.
Arsenic and vanadium were mostly less than the detection limits of 0.01 and 0.1 μg/g wet weight, respectively. We detected mercury in all spotted sandpiper eggs with a mean of 25.6 pg/g wet weight. Mercury was not correlated with selenium.
Sandpipers had a relatively high incidence of nests in which the whole clutch failed to hatch (5 of 29 nests in an exposed area, lower Michel Creek. and 2 of 28 nests in a reference area, upper Michel Creek). In these cases, we could not determine whether the parents abandoned the nests after the eggs failed to hatch, or if they failed to hatch because the parents abandoned the nests. In two of those nests—both from the exposed area—the eggs held dead embryos. In the exposed areas, the mean clutch size was 3.8 ± SE 0.09, whereas in the reference areas, all clutches consisted of 4 eggs (4.0 ± SE 0.0 per nest), the difference being insignificant (X2 = 4.0, p = 0.135). Of 99 sandpipers’ eggs incubated to full term in the exposed areas, 77 hatched, or 78% hatchability (Table 3; Figure 4). In the reference areas, 102 eggs incubated to full term produced 95 hatchlings (92% overall hatchability). The difference was highly significant (X2 = 9.6, p < 0.01). In both exposed and reference areas, nestling survival (the proportion that survived long enough to leave the nest partly fledged) was 100%. Nest success was 84.4% (29 of 32 nests) in the exposed areas and 96.4% (28 of 29 nests) in the reference areas.
There was no correlation between selenium concentration and either clutch or brood size, nor with eggs size or weight (linear regression, p < 0.05); nor was it expected, in view of the variation of selenium within and between nests in each of the treatment areas.
Of the 13 embryos large enough to examine, and in the 57 nestlings that we observed, we saw no visible, physical abnormalities. Among the 14 eggs we collected from active nests there were no dead embryos, but we found 11 (1 from a reference area and 10 from an exposed area) in eggs that we salvaged after they failed to hatch. There was no relationship between selenium and egg measurements (linear regression, p > 0.05) (Figure 5).
Discussion
Selenium concentrations
The percent moisture we used to convert from wet to dry weight in dipper eggs (\( \overline{\rm X} \) = 86.9%, N = 2) seems high compared to the waterfowl and shorebird moisture contents that have been more frequently reported. If so, it may have resulted in our overstating the dry weights. However, they were similar to the egg percent moisture results from 2003 (\( \overline {\rm X} \) = 83%, N = 15) that we found in red-winged blackbirds (Agelaius phoeniceus), another songbird (L. Harding, unpublished data).
The MES concentrations in dippers were higher in this study (7.4 ± SE 0.45 μg/g dry weight in reference areas and 8.4 ± SE 0.44 μg/g dry weight in exposed areas) than those that Morrissey et al. (2004b) found in American dippers from coastal streams, which were less than 3.0 μg/g dry weight. MES concentrations in American dipper eggs at coal mine–affected sites in Alberta were similar to those in this study, and higher in exposed than in reference areas (6.75 and 5.30 μg/g dry wt, respectively) (Wayland and Lindeman 2003). These results suggest that dippers in the Elk Valley may be accumulating selenium slightly in both exposed and reference areas (recalling the naturally elevated Se concentrations in benthos from latter), but the small sample size and consequent low statistical power preclude distinguishing between exposed and reference areas.
The higher sandpiper MES concentrations in exposed areas (7.3 ± SE 0.43 μg/g dry wt) compared to reference areas (3.8 ± SE 0.19 μg/g dry wt) suggest uptake from coal mine runoff. We found no egg selenium concentrations for sandpipers feeding in freshwater environments in the literature, but data for killdeers (Charadrius vociferus), American avocets (Recurvirostra americana), and black-necked stilts (Himantopus mexicanus), all of which are in the same order of shorebirds, Charadriiformes, are available. At Kesterson Reservoir in California, a highly selenium-contaminated and well-studied site, black-necked stilts showed lower embryotoxicity to selenium than ducks, American avocets lower than the stilts, and killdeers lowest of all (Ohlendorf et al. 1986, 1989). The MES concentrations associated with embryotoxicosis at Kesterson were 24.8 ppm dry weight to 28.2 ppm dry weight for black-necked stilts and 6.0 to 16.4 ppm dry weight for American avocets; by contrast, reference area black-necked stilts had MES of 2.4 ppm dry weight and American avocets at MES 1.9 ppm dry weight, and both species had 100% hatchability. In comparison, our Elk River sandpiper selenium concentrations appear slightly elevated, relative to avocets and stilts in those reference areas.
The differences in uptake between dippers and sandpipers may relate to differences in their prey (the sandpipers take smaller invertebrates, and more Dipterans, from the water’s edge, rather than feeding on riffle benthos) or metabolism.
Toxicity Thresholds
Skorupa and Ohlendorf (1991), in a synthesis of toxicity data, suggested a toxicity threshold in birds’ eggs of about 8.0 μg/g dry weight. Other studies also showed effects at the 3 to 8 μg/g dry weight level (Fairbrother et al. 1994; Heinz et al. 1990; Lemly 1996, 1997; Skorupa 1998b; Stanley et al. 1994; U.S. Department of the Interior 1998; U.S. Environmental Protection Agency 1998). More recently, higher effects thresholds of 12 to 15 μg/g dry weight MES for embryotoxicity and nestling mortality have been recommended (Adams et al. 2003; Fairbrother et al. 1999, 2000; Ohlendorf 2003). These were based mainly on laboratory toxicity studies of mallard (Anas platyrhynchos) and black-necked stilts.
Some species, however, such as stilts and avocets, are less sensitive than mallards, whereas others such as coots Fulica americana, more so (DuBowy 1989; Fairbrother et al. 1999; Ohlendorf et al. 1989; Williams et al. 1989). The embryotoxicity threshold for American avocets, for example, was about 10 times higher (60 mg/kg Se dry wt) than for black-necked stilts (Skorupa 1998a). Based on these values, Elk River sandpipers’ and dippers’ egg selenium concentrations would be below the reported effects threshold at all sites. Our findings of reduced hatchability in sandpipers at MES concentrations of around 7.3 ± SE 0.43 μg/g dry weight suggest a lower threshold for this species.
Teratogenesis is a less sensitive endpoint than either hatchability or nestling mortality (Fairbrother et al. 1994; Heinz et al. 1989; Seiler 2003; Skorupa 1998a), except for some field effects datasets where high variability has resulted in approximately equivalent teratogenesis and hatchability thresholds (Adams et al. 2003). The most recent review favours an EC10 threshold range for teratogenesis of 16 to 24 μg/g dry weight selenium (Adams et al. 2003). Since all of our bird eggs’ selenium concentrations were below that threshold, visible deformations in embryos were not expected, and none was found in the eggs that we examined and the chicks that we observed.
Despite our finding of reduced hatchability for sandpipers, overall productivity for both species was above normal ranges for the two species: Nest success for dippers (92% and 93% in exposed and reference areas, respectively) was above the range (50% to 84%) found in unpolluted streams over 3 years in Colorado (Price 1983). Elsewhere in British Columbia, 36 dipper broods produced a mean of 2.1 young (Campbell et al. 1997), whereas in our study, mean brood size in the exposed area was 3.0. Had we not collected eggs for analysis, the brood sizes would have been even higher. Throughout British Columbia, spotted sandpiper broods (N = 252) had a mean of 2.4 young and 75% had 2–4 young (Campbell et al. 1990), whereas in exposed areas of this study, brood sizes (averaged 2.7 (again, after we collected eggs) and 85% had 2–4 young. In this study, the modest influence of selenium on sandpiper hatchability was insufficient to affect number of young recruited to the local population.
The high overall productivity of sandpipers, despite reduced hatchability of fully incubated eggs, may result from (1) the dominance of other, nontoxicity factors, such as predation and floods, in limiting productivity; and (2) the lack of effects on nestling survival (once they hatched, dippers and sandpipers in exposed areas were just as likely to survive to fledging as those in reference areas). Adams et al. (2003) emphasised the variability of field effects that result from such confounding factors as weather and starvation. A possible alternative explanation for the lower hatchability in exposed areas compared to reference areas is a differential disruption of nesting by flooding, which reduced the area available for nesting and may have altered the parent’s behaviour, food availability, or susceptibility of the parents, eggs, and young to predators. It is also possible that the highway and industrial activity along lower Michel Creek, where most of the nest failures occurred, could have contributed to a relatively high incidence of nest abandonment in that area.
Prediction of Toxicity from SeleniumConcentrations in Water
These results beg the question: why was only modest uptake of selenium demonstrated in one species and none in the other, when water selenium concentrations in the exposed areas would have predicted much higher MES concentrations, and consequently more severe effects?
At some sites, uptake and effects of selenium are less than expected from water concentrations alone. An example is the Kennecott Utah Copper mine in Utah, U.S.A. There, selenium in the diets of American avocets, black-necked stilts, coots, and several other waterfowl and shorebird species exceeded levels reported in other studies as associated with reproductive impairment (Ecological Planning and Toxicology Inc. and Parametrix & Inc. 1997). However, as in the Elk River valley, there was no difference in reproductive success at that site compared to the same species using nearby reference sites.
One reason might be the mechanism of bioaccumulation. Selenium occurs in water as dissolved selenite and selenate and as particulate matter (in plankton, suspended organic detritus, elemental selenium, and selenite adsorbed on clay particles). Organic selenides occur in a variety of poorly known compounds including selenomethionine, which is highly biologically reactive. It is generally agreed that ingestion is by far the most important uptake route in consumer organisms, that organic detritus is a key pathway, and that bacterial conversion of selenium to organic forms, which occurs mostly in lentic (standing or stagnant water, such as ponds and swamps) systems, is a critical transformation process (U.S. Environmental Protection Agency 1998).
Other reasons for the lack of severe effects at these exposures might have been the presence of other elements such as boron, arsenic, and mercury, which are known to act antagonistically with selenium in waterfowl, ameliorating adverse effects; or differences in diet or condition (Adams et al. 2003; Hoffman and Heinz 1998; Hoffman et al. 1992; Stanley et al. 1994, 1996). However, arsenic and mercury were only present at trace concentrations in American dipper and spotted sandpiper eggs in this study (arsenic was below detection limits in most eggs), and were not correlated with selenium concentrations. Thus, antagonistic effects from other elements do not appear to be responsible for the observed lack of adverse effects.
Most of the field studies of selenium toxicity in waterfowl and waterbirds have been in lentic systems. Lotic (rapidly flowing) systems, such as those of the study area, have not been well studied. Mechanisms that facilitate transformation of selenium into bioavailable forms and uptake in aquatic systems—uptake of selenium by rooted plants and oxidization by photosynthesis and uptake by bottom-dwelling invertebrates and detritus-feeding fish and wildlife—are prominent in lentic systems, but scarce in lotic systems (Lemly 1999; Lemly and Smith 1987). Lemly (1999) found that 90% of the total selenium in an aquatic system may be in the upper few centimetres of sediment and overlying detritus. The streams sampled for this study, however, arise from glaciers and permanent snowfields and run at high velocity over clean boulders and gravel. Gradients ranged from −0.6% (Fording River) to −4.1% (Boivin Creek). These are lotic streams that have little sediment, scant organic detritus reservoirs, and short residence times, in contrast to the lentic systems that comprise the bulk of selenium studies.
Adams et al. (1998) found that the risk to birds from selenium varies by site, as well as by species. They analyzed data from 15 sites with a wide variety of physiographic and physicochemical features and developed models based on log-linear correlations that relate selenium in water to selenium in the food chain and selenium in the food chain to selenium in birds’ eggs. Based on their model, the selenium in the water in this study area predicts about twice the concentration in aquatic macroinvertebrates that actually occurs (EVS Environment Consultants 2003b; McDonald and Strosher 1998). However, their model predicts approximately the same concentrations in birds’ eggs from the concentrations of selenium in their prey species that we found. We hypothesize that the relatively low uptake of selenium into the eggs of the two waterbirds in this study, despite the high concentrations in the streams, is likely due to their lotic environment’s low biological transformation and uptake rates.
References
Acchuleta S (1996) American dippers as indicators of water quality. Department of Interior, U.S. Fish and Wildlife Service Environmental Contaminants Program Project ID 6F27, Lakewood, Colorado
WJ Adams KV Brix KA Cothern LM Tear RD Cardwell A Fairbrother J Toll (1998) Assessment of selenium food chain transfer and critical exposure factors for avian wildlife species: need for site-specific data EE Little AJ DeLonay BM Greenberg (Eds) Environmental toxicology and risk assessment: seventh volume. ASTM STP 1333 American Society for Testing and Materials Philadelphia, Penusylvania
WJ Adams KV Brix M Edwards LM Tear DK DeForest A Fairbrother J Toll (2003) ArticleTitleAnalysis of field and laboratory data to derive selenium toxicity thresholds for birds Environ Toxicol Chem 22 2020–2029 Occurrence Handle10.1897/1551-5028(2003)022<2020:AOFALD>2.0.CO;2 Occurrence Handle1:CAS:528:DC%2BD3sXotF2gsLc%3D Occurrence Handle12959526
Braune BM, Malone BJ, Burgess NM, Elliott JE, Garrity N, Hawkings J, Marshall JHH, Marshall WK, Rodrigue J, Wakeford B, Wayland M, Weseloh DV, Whitehead PE (1999) Chemical residues in waterfowl and gamebirds harvested in Canada, 1987–95. Canadian Wildlife Service technical report series no. 326
RW Campbell NK Dawe I McTaggart-Cowan JM Cooper GW Kaiser MCE McNall GEJ Smith (1990) The birds of British Columbia volume 2: nonpasserines-diurnal birds of prey through woodpeckers UBC Press Vancouver, B.C.
RW Campbell NK Dawe I McTaggart-Cowan JM Cooper GW Kaiser MCE McNall GEJ Smith (1997) The birds of British Columbia volume 3: passerines—flycatchers through vireos UBC Press Vancouver, B.C.
Canadian Wildlife Service (1992) Protocols for field collection and storage of wild bird specimens for biomarkers studies. Environment Canada, Hull, P.Q.
Canadian Wildlife Service (1997) Waterfowl dissection protocol, updated February 28, 1997 to reflect current practices. Canadian Wildlife Service Tissue Preparation and Specimen Bank, National Wildlife Research Centre, Hull, P.Q.
R Casey P Siwik (2000) Overview of selenium in surface waters, sediment and biota in river basins of west-central Alberta In Planning for end land uses in mine reclamation: proceedings of the Twenty-fourth Annual British Columbia Mine Reclamation Symposium Williams Lake BC 184–194
Clements WH (1992) Bioaccumulation of heavy metals by brown trout (Salmo trutta) in the Arkansas River: importance of food chain transfer. Colorado Water Resources Research Institute Completion Report Project 10, Fort Collins, Colorado
R Dallinger H Kautzky (1985) ArticleTitleThe importance of contaminated food uptake for heavy metals by rainbow trout (Salmo gairdneri): a field study Oecologica 67 82–89 Occurrence Handle10.1007/BF00378455
PJ DuBowy (1989) ArticleTitleEffects of diet on selenium bioaccumulation in marsh birds Journal of Wildlife Management 53 776–781
Ecological Planning and Toxicology Inc, Parametrix Inc (1997) Ecological risk assessment of the Kennecott Wetlands along the Great Salt Lake. Report for Kennecott Utah Copper, Magna, Utah, pp 125
Environment Canada/Health Canada (1995) Canadian water guidelines. Summary of guidelines for water quality in Canada, 1995. Department of Supply and Services, Ottawa, Ontario
EVS Environment Consultants (2002) Elk Valley selenium monitoring study. Draft annual report for 2001. Prepared for the Elk Valley Mines Environmental Management Committee, North Vancouver, B.C., 73 pp
EVS Environment Consultants (2003a) Elk Valley mines selenium trend analysis. Prepared for the Elk Valley Mines Environmental Management Committee, North Vancouver, B.C., 56 pp
EVS Environment Consultants (2003b) Elk Valley selenium monitoring study. Draft annual report for 2002. Prepared for the Elk Valley Mines Environmental Management Committee, North Vancouver, B.C., 41 pp
A Fairbrother M Fix T O’Hara CA Ribic (1994) ArticleTitleImpairment of growth and immune function of avocet chicks from sites with elevated selenium, arsenic and boron J Wildl Dis 30 222–233 Occurrence Handle1:CAS:528:DyaK2cXltVClsbs%3D Occurrence Handle8028107
A Fairbrother KV Brix JE Toll S McKay WJ Adams (1999) ArticleTitleEgg selenium concentrations as predictors of avian toxicity Hum Ecol Risk Assess 5 1229–1253 Occurrence Handle1:CAS:528:DC%2BD3cXnvF2gsA%3D%3D
A Fairbrother KV Brix DK DeForest WJ Adams (2000) ArticleTitleEgg selenium thresholds for birds: a response to J. Skorupa’s critique of Fairbrother et al., 1999 Hum Ecol Risk Assess 6 203–212 Occurrence Handle1:CAS:528:DC%2BD3cXitVOhsbc%3D
P Hayes BM Matsuda KR Summers (1993) Critical Waterfowl Habitats in British Columbia. Technical report series no. 183, Canadian Wildlife Service Pacific and Yukon Region, Vancouver, B.C. 112
GH Heinz DJ Hoffman LG Gold (1989) ArticleTitleImpaired reproduction of mallards fed an organic form of selenium Wild Manage 53 418–428
GH Heinz GW Pendleton AJ Krynitsky LG Gold (1990) ArticleTitleSelenium accumulation and elimination in mallards Arch Environ Contam Toxico1 19 374–379 Occurrence Handle10.1007/BF01054981 Occurrence Handle1:CAS:528:DyaK3cXktlKjtrc%3D
DJ Hoffman CJ Sanderson LJ LeCaptain E Cromartie GS Pendleton (1992) ArticleTitleInteractive effects of selenium, methionein and dietary protein on survival, growth, and physiology in mallard ducklings Arch Environ Contam Toxicol 23 163–168 Occurrence Handle10.1007/BF00212270 Occurrence Handle1:CAS:528:DyaK38XltlSgsrY%3D Occurrence Handle1514839
DJ Hoffman GH Heinz (1998) ArticleTitleEffects of mercury and selenium on glutathione metabolism and oxidative stress in mallard ducks Environ Toxicol Chem 17 116–166
DJ Hoffman CM Marn KC Marois E Sproul M Dunne JP Skorupa (2002) ArticleTitleSublethal effects in avocet and stilt hatchlings from selenium-contaminated sites Environ Toxicol Chem 21 561–566 Occurrence Handle10.1897/1551-5028(2002)021<0561:SEIAAS>2.0.CO;2 Occurrence Handle1:CAS:528:DC%2BD38XhvVehs7w%3D Occurrence Handle11878470
CJ Kennedy LE McDonald R Loveridge MM Strosher (2000) ArticleTitleThe effect of bioaccumulated selenium on mortalities and deformities in the eggs, larvae and fry of a wild population of cutthroat trout (Oncorhynchus clarki lewisi) Arch Environ Contam Toxicol 39 46–52 Occurrence Handle10.1007/s002440010078 Occurrence Handle1:CAS:528:DC%2BD3cXjvFCkurs%3D Occurrence Handle10790501
AD Lemly (1993) ArticleTitleGuidelines for evaluating selenium data from aquatic monitoring and assessment studies Environ Monit Assess 26 181–204 Occurrence Handle1:CAS:528:DyaK3sXmslyitbs%3D
AD Lemly (1996) ArticleTitleAssessing the toxic threat of selenium to fish and aquatic birds Environ Monit Assess 43 19–35 Occurrence Handle10.1007/BF00399568 Occurrence Handle1:CAS:528:DyaK28XmsFOms7k%3D
AD Lemly (1997) ArticleTitleEnvironmental implications of excessive selenium: a review Biomed Environ Sci 10 415–435 Occurrence Handle1:STN:280:DyaK1c7htVCjsA%3D%3D Occurrence Handle9448924
AD Lemly (1999) ArticleTitleSelenium transport and bioaccumulation in aquatic systems: a proposal for water quality criteria based on hydrologic units Ecotoxicol Environ Safety 42 150–156 Occurrence Handle10.1006/eesa.1998.1737 Occurrence Handle1:CAS:528:DyaK1MXhsVels7w%3D Occurrence Handle10051364
Lemly AD, Smith GJ (1987) Aquatic cycling of selenium: implications for fish and wildlife. U.S. Department of Interior, Fish and Wildlife Service, Washington, D.C.
JW Logie DM Bryant DL Howell JA Vickery (1996) ArticleTitleBiological significance of UK critical load exceedance estimates for flowing waters: assessments of dipper Cinclus cinclus populations in Scotland J Appl Ecol 33 1065–1076
LE McDonald MM Strosher (1998) Selenium mobilization from surface coal mining in the Elk River basin, British Columbia: A survey of water, sediment and biota Ministry of Environment, Lands and Parks Cranbrook, H.C 55
McDonald LE, Strosher MM (2000) Selenium in the Elk River Basin, British Columbia: a review of findings and discussion of implications for assessment and management. In: 24th annual British Columbia Mine Reclamation Symposium, Williams Lake, B.C.
CA Morrissey LI Bendell-Young JE Elliott (2004a) ArticleTitleSeasonal trends in population density, distribution and movement of American dippers within a watershed of southwestern British Columbia, Canada Condor 106 815–825
CA Morrissey JE Elliott LI Bendell-Young (2004b) ArticleTitleLinking contaminant profiles to the diet and breeding location of American dippers using stable isotopes J Appl Ecol 11 502–512 Occurrence Handle10.1111/j.0021-8901.2004.00907.x
NK Nagpal K Howell (2001) Water quality guidelines for selenium Ministry of Water, Lands and Air Protection Victoria, B.C.
HM Ohlendorf (2003) Ecotoxicology of selenium JGA Hoffman DJ Rattner BA Burton JJ Cairns (Eds) Handbook of ectoxicology EditionNumbersecond Lewis Publishers Boca Raton, Florida 465–500
HM Ohlendorf RL Hothem (1995) Agricultural drainwater effects on wildlife in central California DJ Hoffman BA Rattner GA Burton J Cairns (Eds) Handbook of ecotoxicology Lewis Publishers Boca Raton, Florida 577–595
HM Ohlendorf RL Hothem CM Bunck TW Aldrich JF Moore (1986) ArticleTitleRelationships between selenium concentrations and avian reproduction Trans North Am Wildl Nat Resources Conf 51 330–342
HM Ohlendorf RL Hothem D Welsh (1989) ArticleTitleNest success, cause-specific nest failure, and hatchability of aquatic birds at selenium-contaminated Kesterson Reservoir and a reference site Condor 91 787–796
HM Ohlendorf RL Hothem CM Bunck KC Marois (1990) ArticleTitleBioaccumulation of selenium in birds at Kesterson Reservoir, California Arch Environ Contam Toxicol 19 495–507 Occurrence Handle10.1007/BF01059067 Occurrence Handle1:CAS:528:DyaK3cXkvFejs7Y%3D Occurrence Handle2386406
HM Ohlendorf JP Skorupa MK Saiki DA Barnum (1993) Food chain transfer of trace elements to wildlife RG Allen CMU Neale (Eds) Management of irrigation and drainage systems—integrated perspectives: National Conference on Irrigation and Drainage Engineering ASCE New York
Price FEB (1983) Population ecology of the Dipper (Cinclus mexicanus) in the Front Range of Colorado. Copper Ornithological Society
InstitutionalAuthorNamePuget Sound Water Quality Action Team (1995) Recommended guidelines for measuring metals in Puget Sound marine water, sediment and tissue samples U.S. Environmental Protection Agency Olympia, Washington 71
MK Saiki TP Lowe (1987) ArticleTitleSelenium in aquatic organisms from subsurface agricultural drainage water, San Joaquin Valley, California Arch Environ Contam Toxicol 16 657–670 Occurrence Handle10.1007/BF01055416 Occurrence Handle1:CAS:528:DyaL2sXlsVyrt74%3D Occurrence Handle3674972
Seiler RL (2003) Irrigation-induced contamination of water, sediment, and biota in the western United States—synthesis of data from the National Irrigation Water Quality Program. U.S. Dept. of the Interior, U.S. Geological Survey, Denver, Colorado
Skorupa JP (1998a) Selenium. In: Martin PL, Larsen DE (eds) Guidelines for interpretation of the biological effects of selected constituents in biota, water, and sediment. National Irrigation Water Quality Program Information report no. 3, U.S. Department of Interior, Denver, Colorado
JP Skorupa (1998b) Selenium poisoning of fish and wildlife in nature: lessons from twelve real-world examples WT Frankenberger SuffixJr RA Engberg (Eds) Environmental chemistry of selenium Marcel Dekker Inc. New York, New York 315–354
JP Skorupa HM Ohlendorf (1991) Contaminants in drainage water and avian risk thresholds A Dinar D Silberman (Eds) The economics and management of drainage in agriculture Kluwer Academic Press San Diego 345–368
A Sorace P Formichetti A Boano P Andreani C Gramegna L Mancini (2002) ArticleTitleThe presence of a river bird, the dipper, in relation to water quality and biotic indices in central Italy Environ Pollut 118 89–96 Occurrence Handle10.1016/S0269-7491(01)00237-8 Occurrence Handle1:CAS:528:DC%2BD38Xms1egtg%3D%3D Occurrence Handle11996386
TR Stanley JW Spann GJ Smith R Roscoe (1994) ArticleTitleMain and interactive effects of arsenic and selenium on mallard reproduction and duckling growth and survival Arch Environ Contam Toxicol 26 444–451 Occurrence Handle10.1007/BF00214145 Occurrence Handle1:CAS:528:DyaK2cXis1emtbo%3D
TRJ Stanley GJ Smith H DJ GH Heinz R Roscoe (1996) ArticleTitleEffects of boron and selenium on mallard reproduction and duckling growth and survival Environ Toxicol Chem 15 1121–1132
SM Strom HS Ramsdell AS Archuleta (2002) ArticleTitleAminolevulinic acid dehydratase activity in American dippers (Cinclus mexicanus) from a metal-impacted stream Environ Toxicol Chem 21 115–120 Occurrence Handle1:CAS:528:DC%2BD38Xht1yisw%3D%3D Occurrence Handle11804044
SJ Tyler SJ Ormerod (1992) ArticleTitleA review of the likely causal pathways relating the reduced density of breeding dippers Cinclus cinclus to the acidification of upland streams Environ Pollut 78 49–55 Occurrence Handle1:STN:280:DC%2BD2c7psFGktA%3D%3D Occurrence Handle15091927
U.S. Department of the Interior (1998) Guidelines for interpretation of the biological effects of selected constituents in biota, water, and sediment: selenium. National Irrigation Water Quality Program information report no. 3
U.S. Environmental Protection Agency (1998) Results of the peer consultation workshop on selenium aquatic toxicity and bioaccumulation. EPA 822-R-98-07
Wayland M, Lindeman D (2003) Assessing the potential risk of coal mining on selenium exposure and toxicity to birds in the Alberta foothills. A report to the Environmental Assessment Research and Development Fund, Environment Canada, Prairie & Northern Region, Edmonton. Environment Canada, Prairie & Northern Wildlife Research Centre, Saskatoon, Saskatchewan, 26 pp
ML Williams RL Hothem HM Ohlendorf (1989) ArticleTitleRecruitment failure in American avocets and black-necked stilts nesting at Kesterson Reservoir, California, 1984–1985 Condor 91 797–802
Acknowledgments
This study was supported by the Elkview Coal Corporation, Fording Coal Ltd., and Line Creek Mine Ltd. under the direction of a government–industry task force. Dave Ryder of Elkview Coal Corporation, Roger Berdusco of Fording Coal Ltd., Bob Logan and Bill Kovach and their colleagues of Line Creek Mine Ltd., and Mark Strosher and Les McDonald of the British Columbia Ministry of Water, Lands and Air Protection provided advice and guidance throughout the study. Emily Robertson of Biometrics performed the statistical analysis and Hannah Diamond of SciWrite Environmental Sciences Ltd. proofread the manuscript. We thank Dr. Peter M. Chapman, Dr. Harry M. Ohlendorf, and two anonymous referees for their reviews of earlier versions of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harding, L.E., Graham, M. & Paton, D. Accumulation of Selenium and Lack of Severe Effects on Productivity of American Dippers (Cinclus mexicanus) and Spotted Sandpipers (Actitis macularia). Arch Environ Contam Toxicol 48, 414–423 (2005). https://doi.org/10.1007/s00244-004-0004-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-004-0004-5