Abstract
The objective of this study was to investigate risk factors for the development of systemic inflammatory response syndrome following ureteroscopic laser lithotripsy. We retrospectively collected data of 469 patients who underwent ureteroscopic laser lithotripsy at our single institution from February 2008 to June 2016. Details for the patient, the stone, and the surgical factors that potentially contributed to postoperative infection were extracted. Using a logistic regression model, we analyzed how the clinical factors affected the incidence of systemic inflammatory response syndrome. Twenty-seven patients (5.7%) were postoperatively diagnosed with systemic inflammatory response syndrome; of these, 25 patients were diagnosed within 24 h after ureteroscopy. One patient required intensive care unit admission, but no death was reported. A preoperative stent was significantly associated with postoperative systemic inflammatory response syndrome only on univariate analysis, and the reasons for stenting were varied. Multivariate analysis revealed that obstructive pyelonephritis, a positive preoperative bladder urine culture result, and female gender were significantly associated with postoperative systemic inflammatory response syndrome. Patients who experienced obstructive pyelonephritis preceding ureteroscopic laser lithotripsy or had a positive preoperative bladder urine culture result were at an increased risk of systemic inflammatory response syndrome despite receiving appropriate preoperative antibiotic therapy. Regarding the impact of a preoperative stent on postoperative infection, further investigation focusing on reasons for stenting is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infectious complications are one of the most troubling problems for urologists when treating urolithiasis. Ureteroscopy (URS) is generally less invasive than percutaneous nephrolithotomy (PNL), which targets large renal stones and is characterized by a renal puncture; however, URS has recently been applied in the treatment of larger stones, using a staged procedure. There has been increasing awareness of post-URS infection associated with the expansion of its application [1]. The incidence of lethal post-URS infection is no longer negligible [2], but only a limited number of studies have focused on risk factors associated with post-URS infection. Therefore, appropriate assessment of the incidence and risk factors for post-URS infection is urgently needed.
To determine the accurate incidence of critical post-URS infection, we adopted the systemic inflammatory response syndrome (SIRS) criteria as the representative index of infectious complications in this study, because SIRS has definite diagnostic criteria based on data that can be obtained by routine checking of vital signs and laboratory tests even outside the intensive care unit (ICU). The reported rate of post-PNL SIRS ranges from 9.8 to 16.7% [3,4,5], whereas the rate of post-URS SIRS is reported to be 4.4–8.1% [6,7,8]. However, our comprehensive understanding of the risk factors, regardless of the equipment used, the stone location, and the stone size, is limited. The purpose of this study was to analyze preoperative and intraoperative risk factors for post-URS SIRS.
Patients and methods
Patients and study design
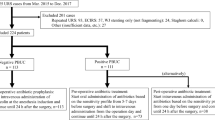
After obtaining institutional review board approval, we retrospectively reviewed all URS performed at Kidney Stone Center, Tokyo Metropolitan Ohtsuka Hospital, from February 2008 through June 2016. Patients who underwent PNL or endoscopic combined intrarenal surgery (ECIRS) were excluded. Patients who underwent URS for their staghorn calculi were also excluded. When a staged procedure was performed in the case of a high stone burden, clinical data of the initial URS were extracted. In the cases in which we could not approach a stone due to a narrow ureter at the initial URS, clinical data of the second URS, which was performed after passive dilation, were extracted. All patients were evaluated by medical history, physical examination, complete blood count (CBC) and chemistry studies, mid-stream bladder urine culture, and non-contrast computed tomography (NCCT) of the abdomen and pelvis.
Cumulative stone diameter was defined as the sum of the maximum diameter of each stone. Cumulative stone volume was defined as the sum of each stone volume that we measured three-dimensionally using the ellipsoid formula. We measured the mean and the highest CT-attenuation value of the region of interest. Stone composition was analyzed by infrared spectroscopy. Stone composition occupying 60% or more of the analyzed fragment was defined as representative. Stone location was represented by that of the largest stone in cases of multiple stones. A positive preoperative bladder urine culture (PBUC) result was defined as 10,000 colony forming unit/ml or greater; sensitivity to antibiotics was analyzed when a PBUC result was positive. A bedridden state was equal to Eastern Cooperative Oncology Group performance status (ECOG-PS) 4.
A single dose of cefazorin was intravenously administered at anesthesia induction and at postoperative day 1 (POD1) except when a patient had certain infectious risks, such as a positive PBUC result or a history of obstructive pyelonephritis preceding URS. For patients with these infectious risks, an antibiotic specific to the patient’s pathogens was orally administered before admission and an adequate dose of broad-spectrum antibiotic was administered from admission to POD1. The administration period of antibiotic prophylaxis before admission was left to the discretion of the treating urologists. Vital signs were closely monitored postoperatively. CBC and chemistry were routinely checked within 24 h after URS. Stone-free state was defined as the absence of residual stones or fragments on postoperative kidney–ureter–bladder (KUB) radiography or CT at 4 weeks after URS.
SIRS criteria
According to the criteria established in 1992 by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee, patients with two or more of the following clinical findings were diagnosed with SIRS [9]: (1) body temperature higher than 38 °C or lower than 36 °C; (2) heart rate higher than 90 beats per minute or PaCO2 lower than 32 mmHg; (3) respiratory rate higher than 20 breaths per minute; and (4) white blood cell count higher than 12,000/mm3 or lower than 4000/mm3; or the presence of greater than 10% immature neutrophils.
URS technique
URS was performed in the lithotomy position under general or spinal anesthesia. A semi-rigid ureteroscope (Fibre Uretero-Renoscope; Richard Wolf, Knittlingen, Germany) was used primarily for lower ureteral stones, and flexible fiberoptic or video ureteroscopes (URF-P6 or URF-V2; Olympus, Tokyo, Japan) were used for upper ureteral stones and renal stones after the insertion of a ureteral access sheath (12/14 or 10/12 Fr ReTrace; Coloplast, Humlebaek, Denmark). Stones were fragmented by holmium:YAG laser (VersaPulse; Lumenis, Tel Aviv, Israel) and picked out by a nitinol stone retrieval basket (Escape; Boston Scientific, Natick, MA, USA). A 4.7–6 Fr ureteral stent and an 18 Fr urethral catheter were indwelled at the end of operations in all cases.
Statistical analysis
To find significant factors associated with SIRS, Pearson test and Wilcoxon test were performed to compare proportions between the SIRS group and the non-SIRS group. Multivariate logistic regression analysis was performed to identify predictive risk factors of post-URS SIRS; thus, the variables were limited to those that could be obtained preoperatively and intraoperatively. The variables which were significantly associated with post-URS SIRS in univariate analysis were selected for multivariate analysis. When performing multivariate analysis, a continuous variable was converted into a binary variable based on the median. The strength of association between various factors and SIRS was reported as the odds ratio (OR) and 95% confidence interval (CI). All statistical tests were two-sided, with p < 0.05 considered significant. Statistical analysis was performed using JMP, version 10.0 (SAS, Cary, NC, USA).
Results
We identified 469 consecutive patients who underwent URS successfully during the study period. Table 1 reports patient and stone demographics. Stone composition and PBUC were analyzed in 460 patients. Overall, 100 patients (21.3%) experienced obstructive pyelonephritis preceding URS. In these patients, drainage by a ureteral stent or percutaneous nephrostomy was established and an antibiotic specific for their pathogens was administered. URS was performed after treatment for pyelonephritis had been completed in terms of laboratory data and the patient’s general condition.
Forty-two patients (8.9%) developed a fever over 38 °C after URS. Twenty-seven patients (5.7%) were diagnosed with SIRS; among whom, 25 were diagnosed with SIRS within 24 h after URS. One patient required admission to the ICU for vasopressor-refractory shock, but no death was reported.
Table 2 shows the comparison between patients with post-URS SIRS versus those without SIRS. On univariate analysis, female gender, a lower body mass index (BMI), a bedridden status, obstructive pyelonephritis preceding URS, a positive PBUC result, a magnesium ammonium phosphate (MAP) stone, and a preoperative stent were significantly correlated with SIRS. The SIRS group had a higher rate of preoperative stents than the non-SIRS group (62.9 vs 23.5%, p < 0.0001), but there was no difference in preoperative nephrostomy (0 vs 2.2%, p = 0.42). The duration of antibiotic prophylaxis before admission was left to the discretion of the treating urologists, and the medians of the duration in the SIRS group and the non-SIRS group turned out to be both 6 days. The median of stent indwelling duration of the SIRS group was 19 days and that of the non-SIRS group was 22 days. There was no significant difference between the two groups (p = 0.779).
Table 3 lists the pathogens identified from PBUC. Escherichia coli and Enterococcus were the most common in the SIRS group. Table 4 shows the reasons for preoperative stenting; the most common reason in the SIRS group was drainage for obstructive pyelonephritis. The reasons for preoperative nephrostomy were all drainage for obstructive pyelonephritis. The SIRS group required a significantly longer time to discharge after URS than the non-SIRS group (median 8 days vs 2 days, p < 0.0001).
Table 5 shows multivariate analysis of factors associated with post-URS SIRS. On a reduced model of multivariate analysis, obstructive pyelonephritis preceding URS (OR 4.58, 95% CI 1.90–11.6, p = 0.0009), a positive PBUC result (OR 3.49, 95% CI 1.42–8.35, p = 0.005), and female gender (OR 3.00, 95% CI 1.21–8.17, p = 0.021) remained significant.
Discussion
European Association of Urology (EAU) guidelines concerning urolithiasis recommend that PNL should be the first-line therapy for renal stones of 2 cm or greater [10]; however, several single-institutional case series of URS for large renal stones have been reported [11]. Our initial experience of URS with large renal stones showed successful outcomes with a high stone-free rate, but the infectious complication rate was not negligible [12]. Skolarikos et al. reported that 4.6% of 1,210 patients who underwent URS for a solitary kidney stone had a stone greater than 20 mm, and they showed a higher probability of post-URS fever in patients with large stones [13]. From the viewpoint of achieving a high stone-free rate and a low complication rate, the appropriate renal stone size for URS remains debatable, while there have been some proposals [14]. The advantage of URS is its minimally invasive design, but we should be aware of the potential for more complex complications with the extended application of URS.
Among 469 patients included in this study, 27 patients (5.7%) were diagnosed with post-URS SIRS. This rate was consistent with the reported rate of previous studies [6,7,8]. Although one patient required ICU admission, no one died from post-URS infection during the study period. This suggested that SIRS did not necessarily shift to fatal status, but the fact that the SIRS group required a significantly longer time to discharge after URS than the non-SIRS group indicated that post-URS SIRS imposed a physical and economic burden on the patients. Comparison between patients with post-URS SIRS and those without suggested multiple factors associated with post-URS SIRS: female gender, a lower BMI, a bedridden state, obstructive pyelonephritis, a positive PBUC result, a MAP stone, and a preoperative stent. Multivariate analysis revealed that obstructive pyelonephritis, a positive PBUC result, and female gender were significantly associated with post-URS SIRS. Hereafter, among these results, we focus on the following three risk factors: a preoperative stent, a bedridden state, and obstructive pyelonephritis.
A ureteral stent and percutaneous nephrostomy, which are both administered in patients with obstructive pyelonephritis, were analyzed as separate potential risk factors in our study. The optimal drainage for obstructive pyelonephritis has yet to be established. In previous studies, comparisons between a ureteral stent and percutaneous nephrostomy focused mainly on the incidence of complications, the control of infection, and the impact on quality of life [15]. To our knowledge, however, no studies evaluate how the two types of drainage prior to URS affect the rate of post-PNL or post-URS infection. In our univariate analysis, a preoperative stent was significantly associated with post-URS SIRS (p < 0.0001), but preoperative nephrostomy was not (p = 0.42). Table 4 shows that 20.6% (12/58) of patients stented for drainage in obstructive pyelonephritis and 7.9% (5/63) of those stented for other reasons presented post-URS SIRS. A ureteral stent, which is reportedly associated with biofilm colonization [16] and causes reflux of bladder urine, may have a negative impact on post-URS infection even after the cure of obstructive pyelonephritis. On the other hand, percutaneous nephrostomy can play an important role in maintaining good intraoperative irrigation and preventing high renal pelvic pressure. These hypotheses are in contrast to the results of subgroup analysis of Blackmur et al.’s study indicating that a preoperative stent might reduce the risk for post-URS SIRS in patients with a positive PBUC result [6]. However, a preoperative stent and nephrostomy were not treated as separate variables in their tables, and the accurate number of preoperative stents and their reasons was not mentioned. Although the effect of a preoperative stent on the rate of post-URS infection remains to be determined, we propose that we should be aware of the background details of preoperative stenting.
A bedridden state was also significantly associated with post-URS SIRS on univariate analysis. Although it did not remain significant on multivariate analysis, we consider that URS for a bedridden patient should be attempted with extreme caution. Bedridden patients are likely to have a MAP stone and a positive PBUC result, which often have acquired antibiotic resistance, and their stones are likely to grow larger asymptomatically. In this study, a positive PBUC result was an independent risk factor for post-URS SIRS on multivariate analysis, and the presence of a MAP stone was significantly related to post-URS SIRS on univariate analysis. These complex factors make their perioperative management more difficult. The negative impact of a poor performance status on postoperative infection has been reported in previous studies [4, 17]. Martov et al. from the CROES URS Global Study showed that female gender, a high American Society of Anesthesiologists (ASA) score, a high stone burden, and Crohn’s and cardiovascular disease were significant risk factors of postoperative UTI or fever in patients with a negative baseline urine culture. We did not adopt a high ASA score or paraplegia as potential risk factors in the present study, because we considered the longstanding bedridden state itself to largely contribute to stone formation and increased operation difficulty. The ASA-PS classification cannot precisely extract bedridden patients, and bedridden patients are not necessarily paraplegic.
We regarded obstructive pyelonephritis preceding URS as the most important risk factor among those we identified, since its OR was the highest on multivariate analysis. Kanno et al.’s study reported that 8% of patients with obstructive pyelonephritis and 6% of patients with no obstructive pyelonephritis presented post-URS fever or urosepsis and their rates were not significantly different [18]. However, the tendency for patients with obstructive pyelonephritis to present with post-URS fever could be observed. In the aforementioned study by Blackmur et al., their matched pair analysis showed that urinary tract infection requiring hospital treatment in the 90 days preceding URS was not associated with post-URS SIRS [6]; this was not consistent with our findings. Their results seemed to be more persuasive due to a matched pair analysis and the large number of patients included. In contrast, Youssef et al. showed that patients with sepsis preceding URS had a significantly higher complication rate, a longer hospital length of stay, and longer courses of postoperative antibiotics [19]. Clearly, there is a lack of consensus concerning risk factors for post-URS infection, and this can be attributed to the limitations of retrospective studies. A prospective multi-institutional study of post-URS infection is now required.
The number of patients included in our study was large compared with previous single-institutional studies, but we experienced a relatively small number of SIRS events. In general, the incidence of post-URS infection is reported to be lower than that of post-PNL infection due to the less invasive nature of the procedure, and consequently, a study on post-URS infection requires a larger number of patients to obtain significant statistical results. Therefore, further investigation by a multi-institutional study will be helpful. We analyzed cumulative stone volume three-dimensionally using the ellipsoid formula and expected that this measuring method would lead to more accurate stone burden estimation [20]. Finally, we focused on the potential effect of a preoperative stent on post-URS SIRS and examined the reasons for stenting. A preoperative stent was significantly associated with post-URS SIRS only on univariate analysis, but there is still room for argument about the negative impact of preoperative stenting on post-URS infection.
Conclusions
Obstructive pyelonephritis preceding URS, a positive PBUC result, and female gender were significantly associated with post-URS SIRS. Patients who experienced obstructive pyelonephritis preceding URS or had a positive PBUC result were at an increased risk for post-URS SIRS, even though they received appropriate preoperative antibiotic therapy.
References
Giusti G, Proietti S, Peschechera R, Taverna G, Sortino G, Cindolo L, Graziotti P (2015) Sky is no limit for ureteroscopy: extending the indications and special circumstances. World J Urol 33:257–273
Cindolo L, Castellan P, Scoffone CM, Cracco CM, Celia A, Paccaduscio A, Schips L, Proietti S, Breda A, Guisti G (2016) Mortality and flexible ureteroscopy: analysis of six cases. World J Urol 34:305–310
Korets R, Graversen JA, Kates M, Mues AC, Gupta M (2011) Post-percutaneous nephrolithotomy systemic inflammatory response: a prospective analysis of preoperative urine, renal pelvic urine and stone cultures. J Urol 186:1899–1903
Draga RO, Kok ET, Sorel MR, Bosch RJ, Lock TM (2009) Percutaneous nephrolithotomy: factors associated with fever after the first postoperative day and systemic inflammatory response syndrome. J Endourol 23:921–927
Erdil T, Bostanci Y, Ozden E, Atac F, Yakupoglu YK, Yilmaz AF, Sarikaya S (2013) Risk factors for systemic inflammatory response syndrome following percutaneous nephrolithotomy. Urolithiasis 41:395–401
Blackmur JP, Maitra NU, Marri RR, Housami F, Malki M, Mcllhenny C (2016) Analysis of factors’ association with risk of postoperative urosepsis in patients undergoing ureteroscopy for treatment of stone disease. J Endourol 30:963–969
Fan S, Gong B, Hao Z, Zhang L, Zhou J, Zhang Y, Liang C (2015) Risk factors of infectious complications following flexible ureteroscope with a holmium laser: a retrospective study. Int J Clin Exp Med 8:11252–11259
Zhong W, Leto G, Wang L, Zeng G (2015) Systemic inflammatory response syndrome after flexible ureteroscopic lithotripsy: a study of risk factors. J Endourol 29:25–28
Bone RC, Balk RA, Cerra FB, Dellinger RP, Ferin AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655
Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, Knoll T (2016) EAU guidelines on interventional treatment for urolithiasis. Eur Urol 69:475–482
Aboumarzouk OM, Monga M, Kata SG, Traxer O, Somani BK (2012) Flexible ureteroscopy and laser lithotripsy for stones > 2 cm: a systematic review and meta-analysis. J Endourol 26:1257–1263
Takazawa R, Kitayama S, Tsujii T (2012) Successful outcome of flexible ureteroscopy with holmium laser lithotripsy for renal stones 2 cm or greater. Int J Urol 19:264–267
Skolarikos A, Gross AJ, Krebs A, Unal D, Bercowsky E, Eltahawy E, Somani B, de la Rosette J (2015) Outcomes of flexible ureterorenoscopy for solitary renal stones in the CROES URS global study. J Urol 194:137–143
Takazawa R, Kitayama S, Tsujii T (2015) Appropriate kidney stone size for ureteroscopic lithotripsy: when to switch to a percutaneous approach. World J Nephrol 4:111–117
Mokhmalji H, Braun PM, Martinez Portillo FJ, Siegsmund M, Alken P, Köhrmann KU (2001) Percutaneous nephrostomy versus ureteral stents for diversion of hydronephrosis caused by stones: a prospective, randomized clinical trial. J Urol 165:1088–1092
Kehinde EO, Rotimi VO, Al-Hunayan A, Abdul-Halim H, Boland F, Al-Awadi KA (2004) Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J Endourol 18:891–896
Martov A, Gravas S, Etemadian M, Unsal A, Barusso G, D’Addessi A, Krambeck A, de la Rosette J, Clinical Research Office of the Endourological Society Ureteroscopy Study Group (2015) Postoperative infection rates in patients with a negative baseline urine culture undergoing ureteroscopic stone removal: a matched case-control analysis on antibiotic prophylaxis from the CROES URS global study. J Endourol 29:171–180
Kanno T, Matsuda A, Sakamoto H, Higashi Y, Yamada H (2013) Safety and efficacy of ureteroscopy after obstructive pyelonephritis treatment. Int J Urol 20:917–922
Youssef RF, Neisius A, Goldsmith ZG, Ghaffar M, Tsivian M, Shin RM, Cabrera F, Ferrandino MN, Scales CD, Preminger GM, Lipkin ME (2014) Clinical outcomes after ureteroscopic lithotripsy in patients who initially presented with urosepsis: matched pair comparison with elective ureteroscopy. J Endourol 28:1439–1443
Merigot de Treigny O, Bou Nasr E, Almont T, Tack I, Rischmann P, Soulié M, Huyghe E (2015) The cumulated stone diameter: a limited tool for stone burden estimation. Urology 86:477–481
Author information
Authors and Affiliations
Contributions
Uchida: project development, data collection, data analysis, and manuscript writing. Takazawa: project development, data collection, and manuscript editing. Kitayama: data collection. Tsujii: supervision
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional research committee and with 1964 Helsinki declaration and its later amendments or comparable ethical standards. The institutional review board approved protocol number is 2016-42. For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Uchida, Y., Takazawa, R., Kitayama, S. et al. Predictive risk factors for systemic inflammatory response syndrome following ureteroscopic laser lithotripsy. Urolithiasis 46, 375–381 (2018). https://doi.org/10.1007/s00240-017-1000-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-017-1000-3