Abstract
We analyzed our stone-free rates of PNL with regard to stone burden and its ratio to the renal collecting system volume. Data of 164 patients who underwent PNL were analyzed retrospectively. Volume segmentation of renal collecting system and stones were done using 3D segmentation software with the images obtained from CT data. Analyzed stone volume (ASV) and renal collecting system volume (RCSV) were measured and the ASV-to-RCSV ratio was calculated after the creation of a 3D surface volume rendering of renal stones and the collecting system. Univariate and multivariate statistical analyses were performed to determine factors affecting stone-free rates; also we assessed the predictive accuracy of the ASV-to-RCSV ratio using the receiving operating curve (ROC) and AUC. The stone-free rate of PNL monotherapy was 53% (164 procedures).The ASV-to-RCSV ratio and calyx number with stones were the most influential predictors of stone-free status (OR 4.15, 95% CI 2.24–7.24, <0.001, OR 2.62, 95% CI 1.38–4.97, p < 0.001, respectively). Other factors associated with the stone-free rate were maximum stone size (p < 0.029), stone surface area (p < 0.010), and stone burden volume (p < 0.001). Predictive accuracy of the ASV-to-RCSV ratio was AUC 0.76. Stone burden volume distribution in the renal collecting system, which is calculated using the 3D volume segmentation method, is a significant determinant of the stone-free rate before PCNL surgery. It could be used as a single guide variable by the clinician before renal stone surgery to predict extra requirements for stone clearance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous nephrolithotomy (PNL) is a standard treatment for patients with larger renal stones in the last decade. Compared with other methods, such as open surgery, it has lower morbidity and better postoperative patient comfort [1]. Although PNL has a high success rate, a recent publication from the Clinical Research Office of the Endourological Society (CROES) reported a SFR of 75.5% [2]. But, clinical results vary widely due to the stones’ characteristics, where bigger and more complex stones show lesser stone-free rates (SFR) [3, 4].
Several prediction PNL result models have been proposed according to the preoperatory characteristics of the patients, renal collecting system anatomy, and the stones but their success is not sufficient for the prediction of SFR [5, 6]. In the modern management of renal stones, three factors are important in the efficiency of PNL: the total stone burden, the location of the stone, and the anatomy of the collecting system. A standardized measure of renal collecting system volume and stone burden would have advantages over subjective assessment. Furthermore, a validated instrument for assessing stone complexity could facilitate accurate predictions regarding surgical outcomes and would represent a valuable asset in planning surgery and counselling patients.
Volume segmentation is an important part of computer-based medical applications for diagnosis. We have applied this novel method for the analysis of stone burden distribution in renal collecting system using automatic or semiautomatic extraction of the renal collecting system and stone. Three-dimensional (3D) segmentation of renal collecting system and renal stones provides a more detailed analysis of the collecting system anatomy, and it is an additional tool to better define the volumetric stone burden distribution in the collecting system.
In this study, we evaluated the 3D segmentation volume of the renal collecting system and its ratio to the stone volume burden as a predictor of the stone-free rate (SFR) after PNL. To the best of our knowledge, this study is the first series of its type and it is also a new armamentarium for planning PNL surgery.
Patients and methods
Patients
After approval of this study by the Institutional Ethics Committee of Okmeydanı Research and Training Hospital, we performed a retrospective analysis of 320 evaluable patients who underwent single- or multiple-tract PNL for renal stones between November 2009 and March 2016. We excluded 156 of 320 patients from the study who had a history of ipsilateral PNL for secondary or tertiary PNL, renal anatomical malformations, such as a horseshoe or ectopic kidney or sponge kidneys, or unclear computed tomography (CT) images. Preoperative data were collected prospectively, and retrospective data were collected by reviewing the hospital’s and physician’s records and by contact with the patients. All PNL procedures were performed by urologists during one session. The remaining 164 patients who met the inclusion criteria for the current study and who were treated with PNL were included.
Clinical and imaging assessments
Preoperatively, patients were evaluated using plain film X-rays, intravenous urography, ultrasonography, urinalysis, urine culture, complete blood count, serum biochemistry, and coagulation tests before the procedure. Non-contrast CT was performed routinely. Preoperative factors that were analyzed included skin-to-stone length, number of calyces with stones, maximum diameter of the stone (mm), average stone density (HU), stone surface area (mm2), stone side (right or left), age, sex, body mass index (BMI), stone burden volume (mm3), and renal collecting system volume (mm3). The perioperative factors recorded were tract number, surgery time, fluoroscopy time, stone-free status, and hospitalization time.
Two types of stone surface areas were measured: the calculated surface area which was calculated using the formula π × r 2 (r being the radius, or half the largest diameter of the stone) [7] and analyzed stone surface area, which was determined by segmentation of the renal stones (Dornheim Segmenter, Mainz, Germany). The calculated stone burden volume was estimated using Ackermann’s formula (Volume = 0.6 × stone surface1.27), as proposed in the recommendations of the European Association of Urology in 2009 [8]. Analyzed stone volume (ASV) and renal collecting system volume (RCSV) were measured and the ASV-to-RCSV ratio was calculated after the creation of a 3D surface volume rendering of renal stones and the collecting system. For skin-to-stone measurement, we took a measurement from the point of the largest stone diameter at a 45° angle from the horizontal. We used the axial image from a CT scan during the measurement procedure.
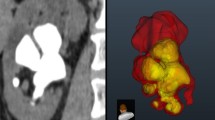
For volume segmentation of the collecting system, images were obtained from transverse CT images (Toshiba Alexion™ multislice CT) and performed using the seed-based region growing method. With the seed-based region growing tool, well-visualized images of the intrarenal collecting system from the unenhanced CT data were selected and using a starting point images were chosen manually. Stone volume segmentation was performed using the thresholding method, and a range of values from the CT data of the renal stones were selected and data that fell outside the range of the stone threshold values were not included in the analysis. Segmentation of the RCSV and stone burden of all patients depicting the pelvicalyceal anatomy and the volumetric stone burden distribution in the collecting system were analyzed by a urologist (HAA) and a radiologist (RB) (Fig. 1).
Three-dimensional volume segmentation and coronal CT images of different renal collecting system and stones. Three needed multiple-stage percutaneous nephrolithotomy (PNL) with two tracts, while 1, 2, and 4 needed one-stage PNL with single tract. The volumetric data are as follows: (1a) calculated stone volume (CSV)—4320 mm3, (1b) analyzed stone volume (ASV)—4732 mm3, renal collecting system volume (RCSV)—67260 mm3, ASV/RCSV (%)—4.8%. (2a) CSV—6693 mm3, (2b) ASV—7825 mm3, RCSV—55150 mm3, ASV/RCSV (%)—10.4%. (3a) CSV—24125 mm3, (3b) ASV—29927 mm3, RCSV—68350 mm3, ASV/RCSV (%)—43.7%. (4a) CSV—3252 mm3, (4B) ASV—3573 mm3, RCSV—45670 mm3, ASV/RCSV (%)—7.8%.
PNL surgery was performed by expert surgeons with the patient under general anesthesia. After a 5 or 6F open-ended ureteral catheter was inserted, patients were placed in the prone position and percutaneous access was achieved under fluoroscopic guidance. Tract dilatation was accomplished using Amplatz or balloon dilators of up to 22 or 30F. Fragmentation and stone removal were accomplished using pneumatic or ultrasound energy and retrieval graspers through rigid 22F–26F nephroscopes. After completion, a 16F re-entry catheter or alternatively an 18F Foley catheter as nephrostomy tube was inserted. After the nephrostomy tube was removed, if the patient was comfortable, afebrile, and there was no longer drainage from the nephrostomy site, patient was discharged the next day.
On postoperative day 1, stone-free status was determined based on plain X-ray of the kidneys, ureters, and bladder or on a non-contrast CT if the treated stone was radiolucent. The size of residual stones was noted. The need for retreatment and the stone-free status 3 months postoperatively was also recorded, with the latter determined using low-dose non-contrast CT.
Statistical analysis
Continuous variables with normal distribution were expressed as the mean and range. The distribution of variables was assessed using the Kolmogorov–Smirnov test. The Mann–Whitney U test was used to compare quantitative data and the Chi-square test was used in the analysis of qualitative independent data.
We performed a univariate and multivariate logistic regression analysis to assess all the preoperative and perioperative characteristics that determined the presence of SFR. In both the univariate and the multivariate analyses, we used an odds ratio (OR) as the risk measure and its 95% confidence interval (95% CI).
Finally, to determine the predictive ability of the ASV-to-RCSV ratio for SFR, data were analyzed via the diagnostic efficiency derived from the receiver operating characteristic (ROC) curve and area under the ROC curve. A p < 0.05 or an OR with 95% CI was considered statistically significant and the software used was SPSS 22.0™ (IBM Corporation, California).
Results
This study included 164 patients (109 male and 55 female) with a mean age of 46.3 ± 13.1 years. All PNL procedures were performed by urologists during one session with single or multiple tracts. Of 32 procedures (19.5%), multi-tract PNL was used in two tracts in 27 PNL patients and in three tracts in 5 patients. The BMI range of patients was 21–35 kg/m2. The mean operating time was 97 ± 24 min and the mean length of stay in hospital was 5 ± 2 days. Overall, the stone-free rate for PNL monotherapy was 53% (87 kidneys).
The patient and stone characteristics were compared between the stone-free patients and those with residual stones (Table 1). Patients with residual stones had a larger stone burden than stone-free individuals (p < 0.001). In residual group, the number of calyces with stones was significantly higher than the stone-free group (p < 0.001). No differences were observed between the RCSV (p = 0.834), but the ASV-to-RCSV ratio was significantly higher in residual stone patients compared with those without residual stones (p < 0.001). Additionally, in patients with residual stones, multiple tracts were necessary in 27.3% of patients compared with 12.6% of those without residual stones (p = 0.018). Hospitalization time and mean surgical time were longer in patients with residual stones compared with those without residual stones (p < 0.001). The other parameters tested were not significantly different (p > 0.05).
The univariate and multivariate analyses are illustrated in Table 2. Univariate analysis revealed that SFR did not correlate with patient sex (p = 0.471), BMI (p = 0.670), age (p = 0.692), side of surgery (p = 0.890), preoperative creatinine level (p = 0.218), skin-to-stone tract length (p = 0.071), and stone density (p = 0.385). Additionally, no association was observed between the SFR and RCSV before PNL (p = 0.691) and the access number (p = 0.0522). Perioperative factors that did not affect the stone-free rate were time to access to a collecting system and fluoroscopy time (p = 0.119 and p = 0.891). There was significant association between calyx number with stones and ASV-to-RCSV ratio with the stone-free rate (p < 0.001). In addition, variables that define stone burden were significantly correlated with SFR (p < 0.05). A stepwise multivariate regression analysis showed that the stone burden variables were influential predictors of SFR (stone surface area, stone burden volume, and maximum stone size, p < 0.05). Additionally, the ASV-to-RCSV ratio and calyx number with stones were the most influential predictors of stone-free status (OR 4.15, 95% CI 2.24–7.24, <0.001, OR 2.62, 95% CI 1.38–4.97, p < 0.001, respectively).
As shown in Table 3, the area under the ROC curve (95% CI) for the prediction of stone-free status was 0.762 (0.684–0.840) for the ASV-to-RCSV ratio. The optimal cut-off value in the prediction of residual stones was 38% for the ASV-to-RCSV ratio, with 81.8% sensitivity and 65.5% specificity.
Discussion
This study showed that the major determinant of stone-free status in PNL is the volumetric stone burden distribution in the collecting system. The stone burden and the number of calyces with stones are the other parameters affecting SFR. We have also observed that RCSV has no impact on the stone-free rate. If the ASV-to-RCSV ratio is a quantitative measure, it would be a guide for clinicians before renal stone surgery to predict extra requirements for stone clearance.
Other studies support our findings, but in these studies, the results that affect the stone-free status results were provided as a qualitative data. El Nahas and co-workers investigated the factors affecting the stone-free rate of PNL surgery for treatment of renal stones in 241 patients, and they found that complete renal stones and the presence of secondary calyx stones were the independent risk factors affecting the incidence of stone-free status after PNL [9]. In our study, the stone burden volume and the RCSV are calculated separately using the 3D volume segmentation method, and the ratio between these variables is given as a quantitative value. This is the most important difference that distinguishes our study from other studies.
To achieve stone-free status in patients with high volumetric stone distribution, multiple tracks or a surgery conducted in stages may be required. Soucy and co-workers had evaluated the PNL efficiency in staghorn calculi by commonly selecting the lower calix as an access tract in 509 patients, and they concluded that with attention paid to an accurate tract selection, complete stone clearance could be achieved using a single access [10]. They also showed that stone-free status was achieved in 127 patients using single tract, but in patients with more complex stone cases and/or a larger stone burden, stone clearance was not obtained even with multiple accesses. The variables responsible for residual stones in patients with complex stone cases or a large stone burden were not objectively discussed in their study. In our study, we retrospectively evaluated the patients who had a single- or multiple-tract access and found that additional surgeries were required to clear the stones if the ASV-to-RCSV ratio was greater than 38%.
Preoperative planning to select the optimal calyx for access can be facilitated using intraoperative fluoroscopy or preoperative CT urography. However, because of the impaired architecture of the renal collecting system, conventional imaging modalities may be inadequate to describe the architectural structure of calyx anatomy in patients with complex renal stones. 3D segmentation can be an alternative imaging method for analyzing the renal collecting system and stone distribution. Additionally, conversion of DICOM file format (acquisition from CT images) to stereolithography (STL) format allows the collecting system and renal stones to be printed using a 3D printer.
Generating 3D models that are anatomically identical to the patient’s renal collecting system and the renal stones allow surgeons, trainees, and patients to interact with the renal unit in a tangible way rather than using conventional imagery. There are few reports of bio-modeling for planning endourological procedures. Gadzhiev and co-workers investigated the usefulness of plasticine bio-modeling in surgical percutaneous treatment of patients with complex renal stones. In this study, they had created a plasticine replicate of the pelvicalyceal system, which was based on the gathered 3D CT image data, and they used 3D models as a reference during surgery [11]. They concluded that the plasticine 3D models seem to provide better appreciation of the preoperative renal collecting system and serve as a reference tool during surgery, which might increase SFR and lower complication rates after PNL in patients with complex renal stones.
Numerous nomograms have been established to predict and aid in decision-making during PNL surgery. They all are aimed to predict stone-free rates and complications while serving as disease stratification tools that provide both the surgeon and the patient with information on the individual procedure complexity. Cumulatively, the Guy’s Score, S.T.O.N.E. nephrolithometry, and nephrolithometric nomogram incorporate a total of 11 variables [12,13,14]. Of these, four variables are shared in three nomograms: stone location, staghorn status, stone size and number, and CT are suggested for the measurement of variables. However, stone location, stone size, and number could be underestimated or overestimated using two-dimensional CT images. Additionally, the presence of a hydronephrosis variable has been included separately in S.T.O.N.E nephrolithometry nomogram and has been defined as a quantitative data. Variables in nomograms could be better calculated and analyzed using “3D volume segmentation” and also the presence of hydronephrosis could be defined as a qualitative data rather than a quantitative one. With 3D volume segmentation, the prediction efficiency of nomograms could be enhanced before PNL surgery. Additionally, the ASBV-to-RCSV ratio could be an alternative tool for predicting PNL success as a single parameter in renal stones.
There are several limitations to the present study. First, we evaluated the variables retrospectively; a prospectively designed study may be more appropriate. Second, we did not evaluate the complications and their relationship with stone burden distribution. Third, 3D volume segmentation of the renal collecting system is a rather time-consuming method, but in the future, segmentation will be performed automatically.
Conclusion
In conclusion, stone burden volume distribution in the renal collecting system, which is calculated using the 3D volume segmentation method, is a significant determinant of the stone-free rate before PNL surgery. It could be used as a single guide variable by the clinician before renal stone surgery to predict extra requirements for stone clearance.
Abbreviations
- PNL:
-
Percutaneous nephrolithotomy
- SFR:
-
Stone-free rate
- 3D:
-
Three dimensional
- CT:
-
Computed tomography
- BMI:
-
Body mass index
- RCSV:
-
Renal collecting system volume
- ABV:
-
Analyzed stone burden volume
- AUC:
-
Area under the curve
- DICOM:
-
Digital imaging and communications in medicine
- OR:
-
Odds ratio
- ROC:
-
Receiver operating characteristic
References
Preminger GM, Clayman RV, Hardeman SW et al (1985) Percutaneous nephrostolithotomy vs open surgery for renal calculi: a comparative study. JAMA 254:1054–1058
De La Rosette J, Assimos D, Mahesh D et al (2011) The Clinical Research Office of the Endourological Society Percutaneous Nephrolithotomy Global Study. J Endourol 25(1):11–17
Desai M, De Lisa A, Turna B et al (2011) The Clinical Research Office of the Endourological Society Percutaneous Nephrolithotomy Global Study: Staghorn versus nonstaghorn stones. J Endourol 25:1263–1268
Ghani KR, Patel U, Anson K (2009) Computed tomography for percutaneous renal access. J Endourol 23:1633–1639
Zhu Z, Wang S, Xi Q et al (2011) Logistic regression model for predicting stone-free rate after minimally invasive percutaneous nephrolithotomy. Urology 78:32–36
Shahrour K, Tomaszewski J, Ortiz T et al (2012) Predictors of immediate postoperative outcome of single-tract percutaneous nephrolithotomy. Urology 80:19–25
Tiselius HG (2008) How efficient is extracorporeal shockwave lithotripsy with modern lithotripters for removal of ureteral stones? J Endourol 22:249–255
Ackermann D, Dunthorn M, Newman RC et al (1989) Calculation of stone volume and urinary stone staging with computer assistance. J Endourol 3:355–359
El-Nahas AR, Eraky I, Shokeir AA et al (2012) Factors affecting stone-free rate and complications of percutaneous nephrolithotomy for treatment of staghorn stone. Urology 79(6):1236–1241
Soucy F, Ko R, Duvdechai M et al (2009) Percutaneous nephrolithotomy for staghorn calculi: a single center experience of 15 years. J Endourol 23(10):1669–1673
Gadzhiev N, Brovkin S, Grigoryev V et al (2015) Sculpturing in urology, or how to make percutaneous nephrolithotomy easier. J Endourol 29(5):512–517
Thomas K, Smith NC, Hegarty N et al (2011) The Guy’s stone score–grading the complexity of percutaneous nephrolithotomy procedures. Urology 78:277–281
Okhunov Z, Friedlander JI, George AK et al (2013) S.T.O.N.E. nephrolithometry: novel surgical classification system for kidney calculi. Urology 81:1154–1159
Smith A, Averch TD, Shahrour K et al (2013) A nephrolithometric nomogram to predict treatment success of percutaneous nephrolithotomy. J Urol 190:149–156
Acknowledgements
We thank Ertan Koç for assistance with statistics that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing financial interests exist.
Rights and permissions
About this article
Cite this article
Atalay, H.A., Canat, L., Bayraktarlı, R. et al. Evaluation of stone volume distribution in renal collecting system as a predictor of stone-free rate after percutaneous nephrolithotomy: a retrospective single-center study. Urolithiasis 46, 303–309 (2018). https://doi.org/10.1007/s00240-017-0995-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-017-0995-9