Abstract
The objective of the study is to evaluate the effectiveness and safety of miniaturized percutaneous nephrolithotomy (mini-PNL) method in infantile patients <3 years of age diagnosed with renal stones. We studied 48 renal units in 40 patients of infantile patients <3 years of age who underwent mini-PCNL at our institute. The mean age of the patients was 24.02 (5–36) months. The mean diameter of the stones was 22.3 mm (11–45 mm). Intrarenal access was achieved under fluoroscopic (n = 43) or ultrasonographic (n = 5) guidance under general anesthesia. A 20 Fr peel-away sheath, a 17 Fr rigid nephroscope and a pneumatic intracorporeal lithotripsy were used. Mean operative time for PNL was 85 (25–135) min. Mean fluoroscopy time was estimated as 3.7 min. The mean hospital stay was 4.3 days (2–10). Mean hemoglobin loss was 0.89 g/L (11.56–10.67) and three of the patients, including one case during the perioperative period, required blood transfusions. Colonic perforation developed in one case. In two patients, urinary drainage persisted for more than 24 h after withdrawal of the nephrostomy tube. Seven patients developed urinary tract infections (UTI). At the end of the postoperative first week, the stone-free rate was estimated to be 81.2 %. In conclusion, for percutaneous management of renal stones in the infantile age group, mini-PNL is an applicable treatment modality that can be applied through small incisions. It has higher stone-free rates, shorter hospital stays, and excellent esthetic outcomes. In this age group especially, surgical exposure to hypothermia and radiation should be avoided.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urolithiasis is a serious problem worldwide. In recent studies, an increase in the incidence of stone disease has been observed. This condition also demonstrates similar trends in the pediatric population. In an epidemiological study in Turkey, the incidence of stone disease was estimated as 14.8 % [1]. In our country, stone disease is most frequently seen in the Southeastern Anatolia Region, where our hospital is situated.
Stone disease in children is often associated with anatomic and metabolic abnormalities or infectious diseases, and the risk of recurrence is high [2–5]. These factors make minimally invasive procedures more important in this age group.
According to EAU guidelines, shock wave lithotripsy (SWL) is preferred in the management of renal stones smaller than 2 cm in diameter. However, SWL requires repetitive sessions and is performed under anesthesia [6, 7]. However, in the management of renal stones larger than 2 cm in diameter, percutaneous nephrolithotomy (PNL) has been considered to be the gold standard. In addition, in cases with cystine stones, SWL refractory stones, or residual stones following open surgery/SWL, PNL have been recommended [7].
In line with advances in technology, as a result of the use of relatively more miniature endoscopic instruments, serious changes have been made recently in the management of pediatric renal stones [6, 8]. Various studies have been published about the safe use of PNL in the pediatric age group. In these studies, stone-free rates up to 90 % have been reported for PNL procedures [6]. However, a limited number of studies have been performed about the management of renal stones by PNL in the infantile age group (0–3 years). Because of the relatively small and fragile kidneys, and smaller ureteral and urethral calibers of the infants, this intervention poses difficulties and creates concerns for urologists. Urologists have been reluctant to perform PNL in children because of concerns regarding the use of large instruments in pediatric kidneys, parenchymal damage and the associated effects on renal function, radiation exposure with fluoroscopy, and the risk of major complications including sepsis and bleeding [9].
In this study, we aimed to evaluate the effectiveness and safety of miniaturized PNL (mini-PNL) method in infantile patients <3 years of age diagnosed with renal stones, and we present our single-center experiences on this issue.
Patients and methods
Our hospital is a tertiary care diagnostic and therapeutic research center that provides health care to nearly seven million people in the Southeastern Anatolia Region. During the period between January 2011 and January 2014, mini-PNL was applied to 48 renal units of 40 infants (17 girls and 23 boys), including eight patients with bilateral kidney stones. Symptomatic patients with kidney stones up to 2 cm were first referred for SWL treatment. Mini-PNL was the first treatment option for the patients with stones >2 cm and shock wave-resistant stones <2 cm. Also mini-PNL was mainly performed in the case of retrograde intrarenal surgery (RIRS) failure or patient preference. Patients’ sex, age, stone size, location, and laterality were the preoperative factors. The demographic values, perioperative and preoperative measures including age, stone location and size, presence of hydronephrosis, diagnostic imaging methods, laterality, operation type, number and type of access, and fluoroscopy time were prospectively recorded into a patient entry system. The postoperative parameters evaluated were the duration of the hospitalization, stone clearance rates, hemoglobin decline, complication rates, auxiliary methods and insertion of double-J (DJ) stent if applicable. Pretreatment evaluation included physical examination, a detailed history, creatinine, urine culture, urine analysis, plain radiography, intravenous urography (IVU) and ultrasonography. Computed tomography (CT) was not regularly performed unless the surgeon opted. Whenever detected, positive urine cultures were treated with appropriate antibiotics preoperatively.
Stone size was determined by measuring the longest axis on preoperative radiologic investigation; in cases of multiple calculi, stone size was defined as the sum of the longest axis of each stone. The decision to perform stenting was made according to the duration of the procedure, stone load, and hematuria or the degree of visible trauma. The patients were monitored for postoperative complications.
The nephrostomy tube was removed 1–2 days after the procedure, when the drainage was clear, in the absence of pain, fever, or urine leakage around the tube, and the Double-J stent was extracted 4 weeks later. On postoperative day 1, urethral and ureteral catheters were withdrawn. The patient was discharged from the hospital when the nephrostomy tube was removed, as long as there was no leakage from the PNL site and the urine was clear.
Kidney ureter bladder graphy (KUB) was done to assess the stone-free rate in all patients after 1 day and 1 week of follow-up. Clearance was defined as no residual stone on KUB and ultrasound. All fragments <4 mm were considered Clinically Insignificant Residual Fragments (CIRF). Data were recorded prospectively in a database.
Mini-PNL technique
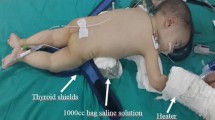
All procedures were performed under general anesthesia. The urologist inserted a 4F/5F retrograde ureteral catheter into the patient in the lithotomy position. The tip of the catheter was sited at the renal pelvis or within the upper pole calyx, and its position was confirmed by instilling a small amount of radiographic contrast medium into the collecting system. A 10–12 F Foley catheter, depending on patient’s age, was inserted into the urethra and taped to the ureteric catheter. Then, the patient was turned to the prone position with appropriate padding placed under the chest region to avoid pressure sores and to provide adequate ventilation during the procedure. In addition, the gonads of the patient were protected from X-rays using gonad shields (Fig. 1).
During the operation, patients were kept warm because of an increased risk of hypothermia in the pediatric population [10]. To that end, an external heater was used routinely (Nellcor WarmTouch, 220–240 V, 50/60 Hz, USA).
The renal collecting system was opacified by retrograde injection of contrast agent via the ureteral catheter, and a mobile fluoroscopy C-arm was used to determine the calyx to be punctured. The selected calyx was punctured under fluoroscopic imaging using a 16-ga intravenous cannula (angiocath) [11]. Once the calyx was accessed, an straight tip, 0.035 in. diameter, 180 cm length, hydrophilic guidewire was negotiated past the stone into the distal ureter and preferably into the bladder. Later, the track was dilated sequentially initially using plastic fascial dilators 6, 8, and 10 F. The guide wire was kept in place if possible during the operation.
A Fr peel-away sheath was used in renal units. The majority of cases were accessed via the lower pole calyx. In some cases, the access tract was established via a middle posterior calyx. A 17 Fr rigid nephroscope (Richard-Wolf, Knittlingen, Germany) and a pneumatic lithoclast were used in some cases. Small fragmented stones were extracted using grasping forceps. When necessary, X-ray or ultrasound was used to search for fragments. At the end of the procedure, fragmentation and clearance were assessed by fluoroscopy. Extracted fragmented stones were sent to analysis for stone typing.
At the end of the operation, a 14-Fr foley catheter was inserted as a nephrostomy tube. Anterograde pyelography was performed to evaluate the collecting system and assess the amount of extravasations. A nephrostomy tube was not inserted (tubuless) at the end of the same operation. For example, operating time was between 30 and 60 min, single access, and no residual stone fragments were present in such cases.
Results
Between January 2011 and January 2014, 40 infant patients underwent 48 mini-PNL procedures in our institute (eight patients had treatment for bilateral renal stones). There were 17 girls and 23 boys. Presenting complaints of the patients are shown in Table 1 in order of decreasing frequency.
The mean age of the patients was 24.02 (5–36) months. Stones were localized inside the right (n = 27) or left (n = 21) kidneys. They were detected in the renal pelvis (n = 22), lower pole (n = 4), lower pole + pelvis (n = 12), middle pole (n = 1), upper pole (n = 1), staghorn (n = 6) and proximal ureter (n = 2). The mean diameter of the stones was 22.3 mm (11–45 mm). Renal units were reached through one (n = 38 renal units) or two (n = 10) access tracts. Intrarenal access was achieved under fluoroscopic (n = 43 renal units) or ultrasonographic (n = 5) guidance. Radiological evaluation of the stones revealed opaque and non-opaque stones in 37 and 11 patients, respectively. Demographic characteristics of the patients, mean size of the stones, grade of hydronephrosis, and access type are summarized in Table 2.
The type of approach was decided upon stone location, and middle (n = 10) or lower (n = 38) poles were used for access to stones. Upper pole access was not required in any patients. On perioperative evaluation, tubeless PNL was applied without any massive bleeding with shorter operative times in a total of nine patients. Mean operative time for PNL was 85 (25–135 min) minutes. Mean fluoroscopy time was estimated as 3.7 min. On postoperative day 1, complete stone-free state was achieved in 70.8 % of renal units (34/48). Still, clinically significant (n = 9 renal units) or insignificant (n = 5) residual stones were detected. At the end of the postoperative first week, 81.2 % of renal units (39/48) were spontaneously relieved of their clinically insignificant residual stone fragments. Patients with clinically significant stones underwent SWL (n = 4), re-PNL (n = 3) or URS (n = 2).
Stone analysis showed 2 uric acid stones, 4 cystine stones, 6 calcium oxalate (CaOx) stones, 7 calcium phosphate (CaP) stones and 5 calcium oxalate–calcium phosphate stones. Also the stone composition was not known in 16 patients. Distribution of stone compositions was shown in Table 3.
Mean hemoglobin loss was 0.89 g/L (11.56–10.67) and three of the patients, including one case during the perioperative period, required blood transfusions. Mean postoperative serum creatinine levels were not statistically different when it compared to its mean preoperative levels (p > 0.05).
Upon detection of a foul-smelling discharge around the nephrostomy tube, radiological examinations were performed which revealed colonic perforation. Oral intake by the patient was discontinued. DJ stent was implanted on an emergency basis, and the patient was given antibiotic therapy for 7 days. The nephrostomy tube was withdrawn under control, and the patient was managed conservatively without any requirement for additional surgical intervention.
The mean hospital stay was 4.3 days (2–10 days). In two patients, urinary drainage persisted for more than 24 h after withdrawal of the nephrostomy tube. In one patient, a conservative approach was used because of minimal urinary leakage that ceased on postoperative day 5. In another patient, a DJ stent was implanted on the postoperative day 2 because of profuse drainage. The stent of this patient was removed 30 days postoperatively. In four patients, a subfebrile state developed that could be brought under control using antipyretics during the early postoperative period. Seven patients developed urinary tract infections (UTI). Peri- and postoperative complications that developed secondary to the PNL procedure are shown in Table 4. Also, when complications were investigated in terms of stone composition, there was no statistical correlation between them (p > 0.05).
Discussion
Presenting symptoms of pediatric stone disease vary with the age of the patients. Especially in older children, complaints of classical flank pain and hematuria have been observed. However, in relatively younger children, the incidence of gastrointestinal symptoms such as sudden abdominal cramps, nausea and vomiting, and nonspecific symptoms as irritability increases [4, 5]. Generally, painless or painful macroscopic hematuria is less frequently seen, and microscopic hematuria is more frequently observed, which might be the only finding in the diagnosis of stone disease. In our series, infantile patients presented most frequently with complaints of abdominal pain (n = 22; 55 %), nausea/vomiting (n = 16; 40 %), UTI (n = 13; 32 %), hematuria (n = 11; 27 %), restlessness (n = 9; 22 %), incidental stone disease (n = 4; 10 %), septic manifestations (n = 3; 8 %), and anuria/oliguria (n = 3; 8 %). Medical history of 13 patients revealed that these patients had previously suffered from UTI. Based on these results, we recommend attentive evaluation of patients presenting with recurrent UTI for the presence of urolithiasis.
Surgical management of urolithiasis in children has evolved dramatically in the last two decades. In the 1980s, the advent of SWL revolutionized pediatric stone management, and it is currently the procedure of choice for treating most upper tract calculi in industrialized nations. Today, SWL is one of the main modalities for treating pediatric renal stones, and selected cases can be managed effectively and safely using SWL. However, the long-term effects of shock waves on developing kidneys are not clear and many studies have shown that the success rate of SWL decreases significantly with increasing stone size and number [12–14]. The requirement for multiple sessions and the need for general anesthesia in children are other drawbacks of this procedure [15].
Percutaneous nephrolithotomy (PNL) has significantly higher stone-free rates and lower requirements for ancillary procedures compared with SWL [9, 16, 17]. This trend is further promoted by the introduction of mini-PNL, which is postulated to be less invasive compared with standard PNL because of the miniaturized instruments [18]. However, PNL may present problems in children, despite modifications such as the “mini-perc,” because of the small size and mobility of the pediatric kidney, friable renal parenchyma, and the small size of the collecting system. Today, PNL is typically reserved for larger stone burdens and failed SWL treatments because of its more invasive nature [15, 17, 18].
Due to the location of our hospital in an endemic stone region, we very often encounter cases of pediatric stone disease. Recently, we have not performed open stone surgery due to increased experience at the pediatric PNL. In compliance with urological guidelines, we perform SWL as first-line management in children with stones smaller than 2 cm in size. For stones refractory to SWL or those with a density of more than 1,000 HU, we prefer PNL. Generally, in the pediatric age group, higher stone-free rates (73–96 %) have been reported in PNL series [19–21]. Unsal et al. [9] reported stone-free rates of 83.3 % in a pediatric population <5 years of age using mini-PNL monotherapy. In our study, the mean stone size was 22.3 mm, and the stone-free rate was estimated to be 81.2 %. In our study, the stone-free rate was similar to those reported in the literature.
Few centers have reported their experiences with PNL in infants and very young children [9, 10, 14]. In a mini-PNL series performed in 19 infants, Guohua Zeng et al. [6] indicated stone-free rates of 85 and 95 % for stones with a mean diameter of 2.2 cm at the end of the first and second PNL sessions, respectively. In the recently published original study, Guven et al. reported a stone-free rate (SFR) of 94.7 % in 17 infants and small children with large renal calculi who underwent PNL. Unlike in adults as well as older children, management of large stones in infants requires a high level of performance and experience. We believe that an SFR of 94.7 % for PNL monotherapy in infants could be successful [16]. As far as we know, ours is the largest series investigating the efficacy of mini-PNL in this age group. We achieved a mean stone-free rate of 81.2 % in 48 renal units of 40 infants at the end of the first post-PNL week. We believe that an SFR of 81.2 % for PNL monotherapy in infants is acceptable.
However, it should be kept in mind that the higher incidence of metabolic and anatomic abnormalities in infants (when compared to the adult population) is a major concern in stone formation and may influence the choice of management option and the ultimate effectiveness of treatment [8].
Percutaneous nephrolithotomy with adult-size instruments may present problems in infants and preschool-aged children because of the small size and mobility of the pediatric kidney, friable renal parenchyma, and the small size of the collecting system [7]. One of the most frequent and serious complications encountered during percutaneous nephrolithotomy is massive bleeding which appears to approach 7–15 % in many studies reported in the literature. Bleeding is an important factor affecting both patient mortality and stone-free rates. Calibers of the instruments used, stone burden, and operating time have been reported as influential factors on the requirement for blood transfusion in the pediatric age group [22, 23]. In our study, bleeding requiring blood transfusion occurred in a total of three patients (7.5 %) during the peri- and postoperative periods (n = 1 and 2 patients, respectively).
Pediatric PNL conveys a higher risk of hypothermia compared to adult patients [24]. Especially in cases with challenging calyceal access or those with excess stone burden, pediatric patients can easily enter into hypothermia during prolonged operations because of cold irrigation, and the partially uncovered body of the patient might delay recovery from anesthesia [25]. To refrain from these problematic conditions, we believe that ambient room temperature should be adjusted, and irrigation fluids should be brought to body temperature to avoid exposure of the patient to a cold environment during preoperative and postoperative periods. In our study group, hypothermic complications were not observed in any patients. We recommend routine warming of the irrigation fluid and use of external heaters during PNL, especially in the infantile age group.
One of the unavoidable downsides of PNL surgery is exposure to radiation. Exposure to radiation is a much more important issue for pediatric patients. One of the measures to be taken to avoid this problem is to use ultrasonographic guidance during creation of an access tract. Penbegül et al. [26] reported significantly less exposure to radiation in their series of 19 cases in which they created access tracts under ultrasonographic guidance. In our study, we planned to make US-guided intrarenal accesses in 10 patients; however, we could only achieve US-guided intrarenal entry in seven of these cases without the aid of fluoroscopic support. One of the important issues concerning alleviation of radiation exposure during PNL relates to protection of the patients’ gonads. Meticulous care should be exerted in this age group due to incomplete gonadal maturation. In our routine practice, we achieve gonadal protection by placing a thyroid shield under the gonadal region of the patient [27].
One of the most serious complications encountered during percutaneous nephrolithotomy is colonic perforation, which has been reportedly encountered more often during entry into the upper and lower poles of the horseshoe kidneys. In PNL series cited in the literature, colonic perforations have been reported at a rate of 0.2–0.8 % [28]. Intestinal trauma can be revealed on antegrade pyelograms obtained at the end of the operation that demonstrates leakage of gas or colonic contents from the inside or periphery of the nephrostomy tract, or development of colocutaneous fistula after removal of the nephrostomy tube. Various therapeutical approaches have been described concerning the management of colonic perforation developed during percutaneous nephrolithotomy. In cases with retrorenal colonic perforations, placement of a stent inside the pelviocalyceal system to divert the urinary system from the colon and then pulling nephrostomy tube back into the colon so as to allow formation of a controlled colocutaneous fistula are recommended approaches. Also in our series, colonic perforation developed in one case. Foul-smelling drainage around the nephrostomy tube was observed on the postoperative day 1. We detected retrorenal colonic perforation. As has been recommended in the literature, we inserted a DJ stent and created a controlled nephrocutaneous tract. The patient was discharged uneventfully following a conservative therapeutic approach.
As reported in the study by Bilen et al. [15], after surgery in tubeless mini-PNL operations, the lack of bleeding or minimal bleeding may indicate that there is no need for a nephrostomy tube, or that placing a urethral stent might be sufficient and even less invasive. In recent years, many studies have been performed related to tubeless PNL. In these studies, relatively shorter hospital stays and lesser requirements for analgesia have been reported in cases with tubeless PNL [29, 30]. We also applied tubeless PNL in which we achieved complete stone-free rates in relatively shorter operative times in nine (18.7 %) patients without using a lithotriptor for stone extraction.
One of the minor complications encountered after percutaneous nephrolithotomy is prolonged postoperative urinary leakage around nephrostomy tract, which is seen in 8 % of cases [23, 31]. In the literature, in cases of prolonged drainage exceeding 24 h, implantation of DJ–stent has been recommended. In our series, in two cases prolonged drainage developed. In one patient, a conservative approach was applied. On postoperative day 2, a DJ stent was inserted in the other patient.
Conclusions
For percutaneous management of renal stones in the infantile age group, mini-PNL is an applicable treatment modality that can be applied through small incisions. It has higher stone-free rates, shorter hospital stays, and excellent esthetic outcomes. In this age group especially, surgical exposure to hypothermia and radiation should be avoided.
References
Akinci M, Esen T, Tellaloglu S (1991) Urinary stone disease in Turkey: an updated epidemiological study. Eur Urol 20:200–203
Baştuğ F, Gündüz Z, Tülpar S et al (2013) Urolithiasis in infants: evaluation of risk factors. World J Urol 31:1117–1122
Demirkesen O, Önal B, Tansu N et al (2006) Efficacy of extracorporeal shock wave lithotripsy for isolated lower caliceal stones in children compared with stones in other renal locations. Urology 67:170–174
Dursun I, Poyrazoglu HM, Dusunsel R et al (2008) Pediatric urolithiasis: an 8-year experience of single centre. Int Urol Nephrol 40:3–9
Faerber GJ (2001) Pediatric urolithiasis. Curr Opin Urol 11:385–389
Zeng G, Zhao Z, Zhao Z et al (2012) Percutaneous nephrolithotomy in infants: evaluation of a single-center experience. Urology 80:408–411
Etemadian M, Maghsoudi R, Shadpour P et al (2012) Pediatric percutaneous nephrolithotomy using adult sized instruments: our experience. Urol J 9:465–471
Dogan HS, Tekgul S (2007) Management of pediatric stone disease. Curr Urol Rep 8:163–173
Unsal A, Resorlu B, Kara C et al (2010) Safety and efficacy of percutaneous nephrolithotomy in infants, preschool age, and older children with different sizes of instruments. Urology 76:247–252
Jackman SV, Hedican SP, Peters CA et al (1998) Percutaneous nephrolithotomy in infants and preschool age children: experience with a new technique. Urology 52:697–701
Penbegul N, Soylemez H, Bozkurt Y (2012) An alternative and inexpensive percutaneous access needle in pediatric patients. Urology 80:938–940
Lottmann HB, Traxer O, Archambaud F et al (2001) Monotherapy extracorporeal shock wave lithotripsy for the treatment of staghorn calculi in children. J Urol 165:2324–2327
Nazli O, Cal C, Ozyurt C et al (1998) Results of extracorporeal shock wave lithotripsy in the pediatric age group. Eur Urol 33:333–336
Kumar R, Anand A, Saxena V et al (2011) Safety and efficacy of PCNL for management of staghorn calculi in pediatric patients. J Pediatr Urol 7:248–251
Bilen CY, Gunay M, Ozden E et al (2010) Tubeless mini percutaneous nephrolithotomy in infants and preschool children: a preliminary report. J Urol 184:2498–2503
Guven S, Istanbulluoglu O, Gul U et al (2011) Successful percutaneous nephrolithotomy in children: multicenter study on current status of its use, efficacy and complications using clavien classification. J Urol 185:1419–1424
Straub M, Gschwend J, Zorn C (2010) Pediatric urolithiasis: the current surgical management. Pediatr Nephrol 25:1239–1244
Helal M, Black T, Lockhart J et al (1997) The Hickman peel-away sheath: alternative for pediatric percutaneous nephrolithotomy. J Endourol 11:171–172
Salah MA, Tóth C, Khan AM et al (2004) Percutaneous nephrolithotomy in children: experience with 138 cases in a developing country. World J Urol 22:277–280
Bilen CY, Kocak B, Kitirci G et al (2007) Percutaneous nephrolithotomy in children: lessons learned in 5 years at a single institution. J Urol 177:1867–1871
Desai MR, Kukreja RA, Patel SH (2004) Percutaneous nephrolithotomy for complex pediatric renal calculus disease. J Endourol 18:23–27
Erdenetsesteg G, Manohar T, Singh H (2006) Endourological management of pediatric urolithiasis: proposed clinical guidelines. J Endourol 20:737–748
Kapoor R, Solanki F, Singhania P (2008) Safety and efficacy of percutaneous nephrolithotomy in the pediatric population. J Endourol 22:637–640
Roberts S, Bolton DM, Stoller ML (1994) Hypothermia associated with percutaneous nephrolithotomy. Urology 44:832–835
Vorrakitpokatorn P, Permtongchuchai K, Raksamani EO et al (2006) Perioperative complications and risk factors of percutaneous nephrolithotomy. J Med Assoc Thai 89:826–833
Penbegül N, Tepeler A, Sancaktutar AA et al (2012) Safety and efficacy of ultrasound-guided percutaneous nephrolithotomy for treatment of urinary stone disease in children. Urology 79:1015–1019
Söylemez H, Sancaktutar AA, Altunoluk B et al (2012) Re: radiation protection in pediatric radiology. Urol Res 40:621–622
Vallancien G, Capdeville R, Veillon B (1985) Colonic perforation during percutaneous nephrolithotomy. J Urol 134:1185–1187
Aghamir SM, Hosseini SR, Gooran S (2004) Totally tubeless percutaneous nephrolithotomy. J Endourol 18:647–648
Gupta NP, Kesarwani P, Goel R et al (2005) Tubeless percutaneous nephrolithotomy. A comparative study with standart percutaneous nephrolithotomy. Urol Int 74:58–61
Zeren S, Satar N, Bayazit Y (2002) Percutaneous nephrolithotomy in the management of pediatric renal calculi. J Endourol 16:75–78
Conflict of interest
None to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bodakci, M.N., Daggülli, M., Sancaktutar, A.A. et al. Minipercutaneous nephrolithotomy in infants: a single-center experience in an endemic region in Turkey. Urolithiasis 42, 427–433 (2014). https://doi.org/10.1007/s00240-014-0677-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-014-0677-9