Abstract
Recently, we reported that atorvastatin prevents renal tubular cell injury by oxalate and inhibits renal crystal retention. In this study, we investigated the mechanism by which atorvastatin inhibits renal crystal retention. Male Sprague-Dawley rats were separated into four experimental groups, and the ethylene glycol model of hyperoxaluria and the atorvastatin treatment model were analyzed. To clarify the mechanism by which atorvastatin inhibits renal crystal retention, the removed kidneys were used for the quantitative analysis of superoxide dismutase (SOD) and catalase. The subunits of the NADPH oxidase system were evaluated using real-time polymerase chain reaction analysis. Furthermore, the level of transforming growth factor-β (TGF-β) in kidney tissue was compared in each group. Atorvastatin treatment increased the SOD and catalase level compared with the stone-forming control group. Atorvastatin treatment decreased the expression of NOX-1 mRNA. Furthermore, the level of TGF-β was suppressed by atorvastatin treatment. We found that atorvastatin have inhibited calcium oxalate (CaOX) urolithiasis formation. We hypothesize that the mechanism of action of atorvastatin involves inhibiting TGF-β and NADPH oxidase, and increasing the SOD and catalase level. We believe that atorvastatin will be helpful in the treatment of CaOX urolithiasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of urolithiasis in Japan has increased steadily over the last 40 years; the prevalence of urolithiasis in Japan in 2005 was 192.0 per 100,000 people. Most importantly, the calcium oxalate (CaOX) urolithiasis recurrence rates are as high as 35 and 50% at 5 and 10 years, respectively. The development and spread of the use of low-invasive surgery using shock wave lithotripsy (SWL) and endoscopy in recent years have led to significant progress in the treatment of urolithiasis. However, the rate of recurrence of urolithiasis remains high, and increases after SWL treatment [1]. Therefore, there is a pressing need to prevent this disease and its recurrence. To reduce the occurrence of urolithiasis, it is essential to elucidate the mechanism of lithogenesis and to develop effective treatments for the prevention of CaOX urolithiasis.
Although urolithiasis has been known since ancient times, researchers are still trying to elucidate the mechanism of CaOX renal stone formation. A recent study indicated that the exposure of renal tubular cells to oxalate leads to the production of reactive oxygen species (ROS) and the development of oxidative stress, followed by injury and inflammation [2]. Renal tubular cell injury and inflammation appear to play significant roles in the development of CaOX urolithiasis.
3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors are potent inhibitors of cholesterol biosynthesis. These agents slow the progression and foster the regression of atherosclerosis, thereby improving cardiovascular outcomes in humans with elevated and normal serum cholesterol levels [3]. Atorvastatin, which is a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, reduces high cholesterol levels and that has anti-inflammatory and antioxidant activities [4–6], and it has been reported to control the expression of transforming growth factor-β (TGF-β1) [7]. TGF-β, a prototype cytokine, stimulates extracellular matrix synthesis. When an injury has occurred to glomerular or tubular cells in the kidneys, TGF-β secretion initially causes secondary infiltrating macrophages to invade the interstitial space. The tubulointerstitial inflammation is one of key role for calcium oxalate during stone formation, in particular adhesion step on the renal tubular cell.
Recently, we reported that atorvastatin treatment inhibited the renal tubular cell injury and on oxidative stress caused by ROS [8]. In addition, atorvastatin treatment decreased the apoptosis of renal tubular cells and the formation of renal crystal deposits in kidney tissues. NADPH oxidase is surmised to be the most important source of ROS production in kidneys as reported in blood vessels and other organs. TGF-β is reported to participate in ROS production through the activation of NADPH oxidase.
In the present study, we investigated the mechanism by which atorvastatin inhibits the renal tubular cell injury and oxidative stress caused by ROS, in particular in relevance with TGF-β and NADPH oxidase.
Materials and methods
Stone-forming rat model and experimental design
All experimental procedures were performed with the approval of the Animal Care Committee of the Faculty of Medicine, Osaka University Graduate School of Medical Sciences. The stone-forming rat model [8] involved 200-g, 10-week-old, specific-pathogen-free, male Wistar rats that were force-fed daily with 0.12 ml of 1% ethylene glycol (Wako, Tokyo, Japan) dissolved in 1 ml of water and administered in two doses, and with 0.5 μg of vitamin D3 dissolved in 1 ml of salad oil administered orally for 2 weeks. Twenty-four rats were divided into four groups: group A (n = 6) received 1 ml of water and 0.5% carboxymethyl cellulose every day; group B (n = 6) received 1 ml of water and atorvastatin (2 mg/kg) in 0.5% carboxymethyl cellulose every day; group C (n = 6) received 0.12 ml of 1% ethylene glycol in 1 ml of water and 0.5 μg vitamin D3 dissolved in 1 ml of salad oil (stone-forming rat model group); and group D (n = 6) received 0.12 ml of 1% ethylene glycol dissolved in 1 ml of water and 0.5 μg vitamin D3 dissolved in 1 ml of salad oil and atorvastatin (2 mg/kg) in 0.5% carboxymethyl cellulose every day. On Day 12, the rats were killed and all the kidneys were resected for further examination. The kidneys were fixed with 10% formalin and embedded in paraffin.
Evaluation of superoxide dismutase level
The kidney samples were thawed and homogenized in 1 ml of 25 mM Tris–HCL, pH 7.6, 150 mM NaCl, 1% sodium deoxycholate, and 1% SDS and centrifuged at 10,000g for 10 min at 4°C. The supernatant was used for determination of superoxide dismutase (SOD). SOD was measured using Superoxide Dismutase assay kit provided by Oxis research, USA SOD-525 method [9].
Evaluation of superoxide catalase level
The kidney samples were thawed and homogenized in 1 ml of 25 mM Tris–HCL, pH 7.6, 150 mM NaCl, 1% sodium deoxycholate, and 1% SDS and centrifuged at 10,000g for 10 min at 4°C. The supernatant was used for determination of catalase. Catalase assay kit provided by Cayman Chemical Company, USA [10].
Detection of NADPH oxidase subunits (NOX-1, Rac-1) using real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (PCR) was performed with the Thermal Cycler Real Time System (Takara Bio, Japan). The cDNA was obtained from the rat kidneys and an aliquot (2 μl) was amplified in a 25-μl reaction mix that contained 1× SYBR Premix Taq (Takara Bio) and the following primer pairs: for NOX-1, sense 5′-GGCATCCCTTTACTCTGACCT-3′ and antisense 5′-TGCTGCTCGAATATGAATGG-3′; and for Rac-1, sense 5′-GTACATCCCCACCGTCTTTG-3′ and antisense 5′-CCCAGATTCACTGGTTTTC-3′. After a 15 min Taq activation step at 95°C, PCR was conducted for 40 cycles of 30 s at 94°C, 30 s at 60°C, and 30 s at 72°C. The relative expression of the real-time reverse-transcriptase PCR products was determined using the ΔΔC t method. One of the control samples was chosen as the calibrator sample and used in each PCR. Each sample was run in triplicate, and the mean C t was used in the ΔΔC t equation.
Evaluation of expression TGF-β level in kidney tissue
The kidney samples were homogenized in RIPA Lysis Buffer (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and protein concentrations were measured. After a 1:100 dilution, the activated TGF-β concentrations were measured with an ELISA kit (R&D Systems, Minneapolis, MN, USA) [11].
Statistical analysis
All data are expressed as mean ± the standard error. The statistical analysis was performed with the Mann–Whitney U test with p < 0.05 being considered as significant.
Results
Evaluation of SOD and catalase levels
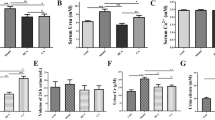
The quantitative analysis of the SOD and catalase levels is shown in Figs. 1, 2. The SOD and catalase levels were significantly higher in the atorvastatin-positive stone-forming rats than in the atorvastatin-negative stone-forming rats.
Detection of NADPH oxidase subunits (NOX-1, Rac-1)
The real-time PCR quantitative analyses of the NADPH oxidase subunits (Rac-1 and NOX-1) are shown in Fig. 3a, b. Atorvastatin treatment significantly decreased the expression of Nox-1 mRNA, but not that of Rac-1 mRNA.
a The Real-time PCR quantitative analyses of the NADPH oxidase subunits Rac-1 is shown. Atorvastatin treatment did not significantly decrease the expression of Rac-1 mRNA. b The Real-time PCR quantitative analyses of the NADPH oxidase subunits NOX-1 is shown. Atorvastatin treatment significantly decreased the expression of NOX-1 mRNA
TGF-β level in kidney tissue
The TGF-β level in kidney tissue detected by ELISA analysis is shown in Fig. 4. Atorvastatin treatment significantly decreased the expression of TGF-β in kidney tissue.
Discussion
Many researchers are attempting to determine the mechanism of CaOX renal stone formation. Recently, Khan has reported that renal cellular exposure to oxalate or CaOX crystals leads to the production of ROS and the development of oxidative stress, followed by injury and inflammation. Renal injury and inflammation appear to play significant roles in stone formation [2]. Therefore, reducing the production of ROS in the kidney can be expected to reduce renal stone formation. SOD and NADPH oxidase are thought to play key roles in the increased release and production of ROS. A predominant source of ROS production is the NADPH oxidase system.
NADPH oxidase consists of various subunits, including Rac-1, p22phox, gp91phox, NOX-1, p40phox, p47phox, and p67phox [12, 13]. In the kidney, NOX-1 is a membrane-bound NADPH oxidase subunit, and its levels are increased in rat kidneys that are subjected to stress by the infusion of angiotensin II [14]. Consequently, inhibition of the NADPH oxidase system should reduce ROS production. Atorvastatin is a specific inhibitor of NADPH oxidase. Recently, we reported that atorvastatin treatment inhibited the renal tubular cell injury and oxidative stress caused by ROS [8]. In the present study, to clarify the mechanism by which atorvastatin inhibits the renal tubular cell injury and oxidative stress caused by ROS, we investigated the expression of NADPH oxidase subunits in a rat stone-forming kidney model. We found that while there was no significant difference in the level of Rac-1 NADPH oxidase subunits between the atorvastatin-positive and atorvastatin-negative stone-forming groups, atorvastatin treatment significantly decreased the levels of NOX-1, which is a membrane-bound NADPH oxidase subunit. Wassmann et al. reported that the expression of NADPH oxidase subunits (nox-1, p22phox and Rac-1) was reduced in the aortas of spontaneously hypertensive rats with the atorvastatin [7]. In this study, the reason why atorvastatin treatment significantly decreased the levels of NOX-1 and no significant difference in the level of Rac-1, it may be that NOX-1 is a membrane-bound of kidney NADPH oxidase subunit. Therefore, one of mechanisms by which atorvastatin inhibits the renal tubular cell injury and oxidative stress caused by ROS is the inhibition of NOX-1.
Superoxide dismutase coverts reactive oxygen into H2O2, which is then converted into H2O and O2 by catalase. SOD, glutathione peroxidase, and catalase are enzymes found in the vasculature that ultimately lead to the elimination of free radicals with the generation of water and oxygen [15]. Wassmann et al. reported that atorvastatin treatment did not exert a significant effect on the vascular expression of mRNAs for the antioxidative enzymes SOD and glutathione peroxidase in vivo [5]. In the present study, the SOD and catalase levels in the atorvastatin-positive stone-forming rats were significantly higher than those in the atorvastatin-negative stone-forming rats. Therefore, one of the mechanisms by which atorvastatin inhibits the renal tubular cell injury and oxidative stress caused by ROS is that atorvastatin stimulates the production of SOD which converts reactive oxygen into H2O2, and catalase which converts H2O2 into H2O and O2.
TGF-β leads to renal tubular damage and interstitial fibrosis. When an injury has occurred to glomerular or tubular cells in the kidneys, TGF-β secretion initially causes secondary infiltrating macrophages to invade the interstitial space. The next phase connected to epithelial cell injury occurs when the CaOX crystals attached to the cells and confined to the interstitial space lead to macrophages infiltration and TGF-β secretion into the interstitial space, causing extensive inflammation. The tubulointerstitial inflammation is another key role for CaOX during stone formation [7]. Mizuguchi et al. [16] reported that in unilateral obstruction (UUO) the obstructed kidney shown increased TGF-β production and atorvastatin decreased renal TGF-β levels in UUO. In addition, TGF-β increases the activity of NADPH oxidase. And then, TGF-β leads to the production of ROS and the development of oxidative stress. In the present study, atorvastatin inhibited the expression of TGF-β. The suppression of TGF-β in kidney tissue was thought to be additional mechanism to inhibit renal crystal retention by atorvastatin. Our group reported that TGF-β immunostaining and a running slice comparison revealed the co-localization of TGF-β with crystal deposits [17]. Whether the localization was in the interstitial space or the cytosol was unclear because the structure of the tubular cell lumen was disrupted due to crystal deposits. This activated TGF-β may have enhanced interstitial inflammation, which would promote CaOX stone formation. The suppression of TGF-β in kidney tissue is thought to be an additional mechanism inhibiting crystal deposits.
Many substances can inhibit renal tubular cell injury and consequently, renal stone formation. Ebisuno et al. concluded that urinary glycosaminoglycans play a critical role in preventing crystal adhesion by inhibiting renal tubular cell injury [18]. The reduction of angiotensin production via the inhibition of angiotensin-converting enzyme (ACE) or blocking angiotensin receptors has been shown to reduce significantly renal CaOX crystal deposition, as well as the development of interstitial inflammation [19, 20]. Agiotensin II induces oxidative stress by activating membrane-associated NADPH oxidase, which leads to the production of superoxide [21, 22]. Umekawa et al. reported that CaOX-induced monocyte chemoattractant protein-1 and osteopontin induces ROS by activating NADPH oxidase [23].
Although urolithiasis has been known since ancient times, there are few effective ways of preventing CaOX urolithiasis. The development of drugs that prevent renal tubular cell injury represents a novel strategy for preventing urolithiasis. We believe that atorvastatin will become recognized as an effective drug for preventing CaOX urolithiasis.
References
Siener R, Glatz S, Nicolay C et al (2003) Prospective study on the efficacy of a selective treatment and risk factors for relapse in recurrent calcium stone patients. Eur Urol 44:467–474
Khan SR (2005) Hyperoxaluria-induced oxaidative stress and antioxidants for renal protection. Urol Res 33:349–357
Takemoto M, Liao JK (2001) Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 21:1712–1719
Glorioso N, Troffa C, Filigheddu F et al (1999) Effect of the HMG-CoA reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia. Hypertension 34:1281–1286
Wassmann S, Laufts U, Muller K et al (2002) Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol 22:300–305
Kim SI, Han DC, Lee HB (2000) Lovastatin inhibits transforming growth factor-beta1 expression in diabetic rat glomeruli and cultured rat mesangial cells. J Am Soc Nephrol 11:80–87
Toblli JE, Ferder L, Stella I et al (2002) Effects of angiotensin II subtype 1 receptor blockade by losartan on tubulointerstitial lesions caused by hyperoxaluria. J Urol 168:1550–1555
Tsujihata M, Momohara C, Yoshioka I et al (2008) Atorvastatin inhibits renal crystal retention in a rat stone forming model. J Urol 180:2212–2217
Nebat C, Moutet M, Huet P et al (1993) Spectrophotometric assay of superoxide dismutase activity based on the activated autoxidation of a tetracyclic catechol. Anal Biochem 214:442–451
Shevalye H, Stavniichuk R, Xu W et al (2010) Poly (ADP-ribose) polymerase (PARP) inhibition counteracts multiple manifestations of kidney disease in long-term streptozotocin-diabetic rat model. Biochem Pharmacol 79:1007–1014
Wheeler CR, Salzman JA, Elsayed NM (1990) Automated assays for superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase activity. Anal Biochem 184:193–199
Babior BM (1999) NADPH oxidase: an update. Blood 93:1464–1476
Griendling KK, Sorescu D, Ushio-Fukai M (2000) NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86:494–501
Suh YA, Arnold RS, Lassegue B et al (1999) Cell transformation by the superoxidegenerating oxidase mox1. Nature 401:79–82
Chabrashvili T, Kitiyakara C, Blau J et al (2003) Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Renal Integr Comp Physiol 285:R117
Mizuguchi Y, Miyajima A, Kosaka T et al (2004) Atorvastatin ameliorates renal tissue damage in unilateral ureteral obstruction. J Urol 172:2456–2459
Yoshioka I, Tsujihata M, Akane W et al (2011) Angiotensin type-1 receptor blocker candesartan inhibits calcium oxalate crystal deposition in ethylene glycol-treated rat kidneys. Urology. doi:10.1016/j.urology.2010.11.019
Andreoli TE (2000) Free radicals and oxidative stress. Am J Med 108:650–651
Ebisuno S, Kohjimoto Y, Tamura M et al (1995) Adhesion of calcium oxalate crystal to Madin-Darby canine kidney cells and some effects of glycosaminoglycans on cell injuries. Eur Urol 28:68–73
Toblli JE, Ferder L, Stella I et al (2001) Protective role of enalapril for chronic tubulointerstitial lesions of hyperoxaluria. J Urol 166:275–280
Antus B, Exton MS, Rosivall L (2001) AngiotensinII: a regulator of inflammation during renal disease? Int J Immunopathol Pharmacol 14:25–30
Umekawa T, Hatanaka Y, Kurita T et al (2004) Effect of angiotensin II receptor blockage on osteopontin expression and calcium oxalate crystal deposition in the rat kidneys. J Am Soc Nephrol 15:635–644
Umekawa T, Tsuji H, Uemura H et al (2009) Superoxide from NADPH oxidase as second messenger for the expression of osteopontin and monocyte chemoattractant protein-1 in renal epithelial cells exposed to calcium oxalate crystals. Br J Urol 104:115–120
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsujihata, M., Yoshioka, I., Tsujimura, A. et al. Why does atorvastatin inhibit renal crystal retention?. Urol Res 39, 379–383 (2011). https://doi.org/10.1007/s00240-011-0370-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-011-0370-1