Abstract
The aim of this research was to examine whether a daily instillation of tetra sodium ethylenediaminetetraacetic acid (EDTA) solution could reduce the rate at which encrustation by crystalline Proteus mirabilis biofilms blocks urinary catheters. Sets of three bladder models were fitted with size 14 all-silicone catheters. Tetra sodium EDTA solution was instilled into the catheter following biofilm development. Catheters were examined by digital photography and scanning electron microscopy for evidence of encrustation. The results showed that the mean time to blockage of the control catheters was 45 h for saline, 57 h for water and 67 h for those exposed to daily instillations of the EDTA solution. Statistical analysis confirmed that the mean encrustation rate on the EDTA-treated catheters was significantly lower than on the control-treated devices (P = 0.047). This in vitro study indicates that EDTA may have beneficial effects in reducing the complication of catheter encrustation and blockage by crystalline biofilms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients who undergo long-term bladder catheterisation are prone to encrustation of their catheters [1, 2] caused by urease-producing bacteria, particularly Proteus mirabilis and other urease-producing bacteria such as Providencia spp. and Morganella spp. [3–5]. Within an attached state, Pr. mirabilis has been shown to produce the enzyme urease which hydrolyses urea to ammonia and carbon dioxide [6]. Elevated levels of both ammonia and carbon dioxide lead to an alkaline pH in urine and the catheter biofilm. Under alkaline conditions, magnesium and calcium phosphate crystals (struvite and apatite) are formed resulting in the formation of a crystalline biofilm. This crystalline structure material builds up in the catheter and eventually occludes the catheter eyehole or drainage lumen preventing the flow of urine from the bladder. This blockage is known to lead to serious consequences for patients’ health and welfare [1, 7]. Presently, there are no procedures that are proving very effective in controlling this problem. Consequently, as urease-producing bacteria are prevalent in urine all catheters are considered susceptible to biofilms and encrustation problem [8].
Conceptually, the simplest way to prevent the biofilm formation by urease-producing bacteria within a urinary catheter is to impregnate catheters with a broad spectrum antimicrobial agent. Therefore, planktonic bacteria could be attacked before they colonise the catheter surface and develop into a biofilm [9]. However, difficulties do arise in delivering effective concentrations of antimicrobial agents from catheters for prolonged periods. Therefore, the usefulness of antimicrobial catheters in patients undergoing long-term bladder management is limited. In view of the importance of Pr. mirabilis in the process of encrustation and blockage of urinary catheters, we decided to develop a strategy that specifically targeted Pr. mirabilis utilising the ‘anti-biofilm’, permeation and chelating properties of tetra sodium ethylenediaminetetraacetic acid (EDTA). Tetra sodium EDTA has a very important role to play as an ‘anti-biofilm’ agent and therefore may have important implications for use in controlling biofilms in catheters [10–13].
The objective of the investigation was to examine whether the daily instillation of tetra sodium EDTA solution reduced the rate at which encrustation by crystalline Pr. mirabilis biofilms blocks urinary catheters.
Methods
The in vitro catheter model system
The laboratory model of the catheterised model used in this paper and the details of the artificial urine supplied to the model have been described in the literature [14]. In essence, the model consisted of a glass chamber (the bladder) maintained at 37°C by a water jacket. Each model was sterilised by autoclaving and then a size 14 all-silicone catheter (BARD Ltd., Crawley, United Kingdom) was inserted into the bladder chamber through a section of silicone tubing (the urethra) at the base of the model. Catheters were then secured in place at the outlet of the bladder by inflation of their balloons with 10 ml of sterile deionised water. Catheters were subsequently connected to drainage bags in the normal way. Sterile urine was pumped into the chambers so that residual volumes collect below the catheter eyeholes before flowing through the drainage tube to the collection bags.
Preparation of EDTA
Eighty milligrams/millilitre of tetra sodium EDTA, batch number 600083, was manufactured at Randy’s compounding pharmacy, Port Orchard, WA and stored at room temperature until required.
Experimental protocol
Sets of three catheter models were fitted with size 14 all-silicone catheters (BARD Ltd.). Urine at a 1 in 2 dilution of the normal concentration was pumped into the bladder chambers to the level of the catheter eyeholes. 10 ml volumes of urine were then removed and replaced by 10 ml of a 4-h urine culture (approximate inoculum size was 108 cfu/ml) of a test strain of Pr. mirabilis (NSM 6). This organism had been isolated from a patient’s encrusted catheter and was known to induce rapid crystalline biofilm formation. The models were supplied with artificial urine at 1.0 ml/min after a 1-h period to allow the bacteria to establish themselves in the bladder. In each experiment, model 1 (control) was allowed to run until the catheter blocked with crystalline biofilm. Model 2 ran for 18 h and then the catheter was clamped near to the drainage tube junction to prevent the flow of urine from the bladder. The tetra sodium EDTA solution (approximately 6 ml) was instilled into the catheter up to the level of the eyehole. After 30 min, the clamp was removed, the EDTA solution drained from the catheter and normal drainage of urine from the bladder resumed. These instillations were repeated at 24 h intervals and the models were allowed to run for up to 4 days or until the catheters blocked. Model 3 was operated as per model 2 except that the periodic catheter instillation was performed with distilled water as a further control. In each case, the time to catheter blockage was noted. The catheter was removed from the model and the extent of encrustation was measured by chemical analysis to determine the amounts of calcium and magnesium in the material deposited on the catheters. In two of the experiments, the catheters were also examined by digital photography for evidence of encrustation. Each experiment was performed five times (n = 5).
Determination of encrustation rates
On removal from the models, each catheter was cut into 1 cm sections and placed in 100 ml of nitric acid (7%). These were subjected to gentle sonication for 5 min at 35 kHz to break up the crystalline material and facilitate dissolution. The resulting solutions were then assayed for calcium and magnesium by atomic adsorption spectroscopy. The total amount of calcium and magnesium recovered from each catheter was calculated (µm/catheter) and the rate of encrustation per hour was determined by dividing these values by the time each catheter took to block.
Visualisation of encrustation
At blockage, the extent of encrustation on the catheters was recorded by taking digital photographs of catheter sections. Catheters were removed from the models and sections (1 cm in length) were cut from each catheter. Section A was the eyehole area. Sections B and C were lumenal sections taken from directly beneath the eyehole and from beneath the catheter balloon, respectively.
Scanning electron microscopy (SEM)
Catheters were removed from the models at the end of the experimental periods. Two sections (1 cm in length) were cut from each catheter, one included the eyehole, the second from immediately below the eyehole. These sections were viewed directly in a JEOL 5200 SEM (Jeol Ltd., Tokyo, Japan) using the low vacuum setting.
Statistical analysis
The Kruskal–Wallis test was used to compare the mean encrustation rates. The statistical software programme Minitab® release 14 (Minitab Inc., PA, USA) was employed to perform these tests. When reporting the mean of the data, the standard deviation (±SD) of the mean was also indicated.
Results
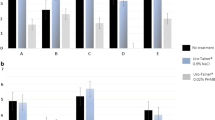
Table 1 shows that the mean time to blockage of the control catheters was 45 h, compared to 57 h for the catheters receiving daily instillations of water and 67 h for those exposed to daily instillations of the EDTA solution. It is important to note that the mean values for the catheters exposed to the EDTA instillations were an underestimate of the research findings. This was because in two of the experiments all catheters drained for the full 4-day experimental period. Consequently, the main conclusion drawn from these data is that the effect of the EDTA instillations was variable and the factors known to induce this are presently unknown but could relate to the low levels of EDTA used, i.e. 80 mg/ml. It is possible that higher concentrations of EDTA may have the ability to enhance the overall reduction in encrustation in catheters.
From Table 2, it is shown that the mean rate of encrustation on the control catheters was 943 μg Ca and Mg/catheter/h. The equivalent values for the catheters exposed to instillations of water or EDTA solutions were 750 and 357 μg Ca + Mg/catheter/h, respectively. These data suggest that the EDTA in particular caused dissolution of the calcium and magnesium salts that were deposited on the catheters. Using the non-parametric Kruskal–Wallis test, there was no significant difference between the mean encrustation rates on the control- and water-treated catheters (P = 0.917). The mean rate of encrustation on the EDTA-treated catheters was significantly lower than that on both the control- and water-treated catheters (P = 0.047).
The images presented in Figs. 1 and 2 show the blockage of the eyeholes and luminal sections of the catheters by crystalline material. In Fig. 1, it seems that encrustation due to Pr. mirabilis blocked both the eyehole and central lumen of the control catheter. The lumen of the catheter exposed to the EDTA, however, appeared to be only partially blocked and it was the eyelet that seemed to be occluded. The catheter exposed to tetra sodium EDTA is shown in Fig. 2. This catheter was shown to continue to drain for the full 4-day experimental period. This clearly demonstrates the effectiveness of EDTA on reducing encrustation of the catheter.
A representative example of digital images of cross-sections of catheters that were removed from Proteus mirabilis infected bladder models at the time of catheter blockage (shown at the base of each column) or at 93 h (experiment end, catheter still draining). a is the eyehole section, b the first lumenal section below the eyehole and c the luminal section directly below the catheter balloon
Discussion
A technique to effectively control the blockage of catheters by crystalline Pr. mirabilis biofilm is considered to be significant for the care of patients undergoing long-term bladder catheterisation [15–17]. Presently, no technique or chemical has been shown to prevent the blockage of a urinary catheter despite a number of studies that have been undertaken in this area [18–23].
Within this study, a chemically defined artificial urine was supplied to the models to ensure standardization of the experimental tests. It has previously been shown that phosphates start to crystallize from this urine when the pH rises to 6.9 and that when it is supplied at 1.0 ml/min to the models, all-silicone catheters block at mean times of around 49 h [24]. These experimental conditions are thus intended to produce a high rate of crystalline biofilm formation that will block catheters more rapidly than would normally happen in most patients. The results presented in this paper demonstrated that daily instillations of tetra sodium EDTA extended the life-span of catheters. It is important to note that the mean values for the catheters exposed to the EDTA instillations were an underestimate of the overall research findings. This was because in a number of experiments all catheters drained for the full 4-day experimental period.
SEMs illustrated the extensive encrustation of their eyeholes and central channels when control or water instillations were used. In contrast, the test catheter using tetra sodium EDTA was often clear of crystalline biofilm after 48 h. This has very important implications in the control of encrustation.
In conclusion, the encouraging results from this in vitro investigation should support the need for an examination of the efficacy of this strategy in patients undergoing long-term catheterisation who are prone to recurrent complications of catheter encrustation and blockage.
References
Cools HJ, Van der Meer JW (1998) Restriction of long-term indwelling urethral catheterisation in the elderly. Br J Urol 58:683–688
Kohler-Ockmore J, Feneley RC (1996) Long-term catheterization of the bladder: prevalence and morbidity. Br J Urol 77:347–351
Mobley HL, Warren JW (1987) Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol 25:2216–2217
Kunin CM (1989) Blockage of urinary catheters: role of microorganisms and constituents of the urine on formation of encrustations. J Clin Epidemiol 42:835–842
Stickler DJ, Morris NS, Winters C (1993) Simple physical model to study formation and physiology of biofilms on urethral catheters. Methods Enzymol 310:494–501
Stickler DJ, Lear JC, Morris NS et al (2006) Observations on the adherence of Proteus mirabilis onto polymer surfaces. J Appl Microbiol 100:1028–1033
Kunin CM (1997) Urinary tract infections: detection, prevention and management, 5th edn. Williams and Wilkins, Baltimore, pp 226–278
Morris NS, Stickler DJ, Winters C (1997) Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br J Urol 80:58–63
Danese PN (2002) Antibiofilm approaches: prevention of catheter colonization. Chem Biol 9:873–880
Raad I, Hachem R, Tcholakian RK et al (2002) Efficacy of minocycline and EDTA lock solution in preventing catheter-related bacteremia, septic phlebitis, and endocarditis in rabbits. Antimicrob Agents Chemother 46:327–332
Kite P, Eastwood K, Sugden S, Percival SL (2004) Use of in vivo-generated biofilms from hemodialysis catheters to test the efficacy of a novel antimicrobial catheter lock for biofilm eradication in vitro. J Clin Microbiol 42:3073–3076
Percival SL, Kite P, Eastwood K et al (2005) Tetrasodium EDTA as a novel central venous catheter lock solution against biofilm. Infect Control Hosp Epidemiol 26:515–519
Devine DA, Percival RS, Wood DJ et al (2007) Inhibition of biofilms associated with dentures and toothbrushes by tetrasodium EDTA. J Appl Microbiol 103:2516–2524
Stickler DJ, Morris NS, Winters C (1999) Simple physical model to study formation and physiology of biofilms on urethral catheters. Methods Enzymol 310:494–501
Stickler D, Ganderton L, King J et al (1993) Proteus mirabilis biofilms and the encrustation of urethral catheters. Urol Res 21:407–411
Stickler DJ (2002) Susceptibility of antibiotic-resistant Gram-negative bacteria to biocides: a perspective from the study of catheter biofilms. J Appl Microbiol 92:163–170
Stickler DJ, Jones GL, Russell AD (2003) Control of encrustation and blockage of Foley catheters. Lancet 361:1435–1437
Getliffe KA, Hughes SC, Le Claire M (2000) The dissolution of urinary catheter encrustation. BJU Int 85:60–64
Getliffe K (2004) The effect of acidic maintenance solutions on catheter longevity. Nurs Times 100:32–34
Getliffe KA (1994) The characteristics and management of patients with recurrent blockage of longterm urinary catheters. J Adv Nurs 20:140–149
Getliffe KA (1992) Encrustation of urinary catheters in community patients. PhD Thesis. University of Surrey
Hesse A, Shreyger F, Tuschewisztki GJ et al (1989) Experimental investigations on dissolution of incrustations on the surfaces of catheters. Urol Int 44:364–369
Roe BH (1989) Use of bladder washouts: a study of nurses’ recommendations. J Adv Nurs 14:494–500
Stickler DJ, Morgan SD (2006) Modulation of crystalline Proteus mirabilis biofilm development on urinary catheters. J Med Microbiol 55:489–494
Acknowledgment
The study was supported by a grant provided by Aseptica Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Percival, S.L., Sabbuba, N.A., Kite, P. et al. The effect of EDTA instillations on the rate of development of encrustation and biofilms in Foley catheters. Urol Res 37, 205–209 (2009). https://doi.org/10.1007/s00240-009-0196-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-009-0196-2