Abstract
As the origin(s) of life on Earth remains an open question, detailed characteristics about the “last universal ancestor” (LUA) continue to be obscured. Here we provide arguments that strengthen the bacterial-like nature of the LUA. Our view attempts to recreate the evolution of archaeal lipids, the major components of the distinctive membrane that encapsulates these ancient prokaryotes. We show that (S)- 3-O-geranylgeranylglyceryl phosphate synthase (GGGPS), a TIM-barrel protein that performs the committed step in archaeal lipid synthesis, likely evolved from the duplication and fusion of a (βα)4 half-barrel ancestor. By comparison to the well-characterized HisA and HisF TIM-barrel proteins, we propose a time line for the invention of this diagnostic archaeal biosynthetic pathway. After excluding the possibility of horizontal gene transfer, we conclude that the evolutionary history of GGGPS mirrors the emergence of Archaea from the LUA. We illustrate aspects of this “lipid capture” model that support its likelihood in recreating key evolutionary events and, as our hypothesis is built on a single initiating event, we suggest that the appearance of GGGPS represents an example of enzyme-driven speciation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Irrespective of the growing phylogenetic evidence (Ciccarelli et al. 2006), many fundamental characteristics about the last universal ancestor (LUA) will likely remain enigmatic and continue to be disputed (Forterre and Philippe 1999; Glansdorff 2000; Kurland et al. 2006; Penny and Poole 1999). One debate regarding early life concerns the nature of the primitive lipid bilayer (Koga et al. 1998; Martin and Russell 2003; Peretó et al. 2004; Wächtershäuser 1988, 2003). This controversy is particularly interesting because a defining characteristic of Archaea is their unique membrane lipids (Woese et al. 1990). In general, three features distinguish archaeal membrane lipids (AMLs) from their bacterial and eukaryotic counterparts. The phospholipid backbone is built upon sn-glycerol-1-phosphate (G1P), not sn-glycerol-3-phosphate (G3P) as found in Bacteria and Eukarya; the archaeal hydrophobic lipid chains are isoprenoid derivatives instead of fatty acids; and the isoprenoids are bound to G1P through ether, not ester, linkages (De Rosa et al. 1986).
Notably, ether-linked lipids also exist in eukaryotes and bacteria (Paltauf 1994), and phospholipid fatty acids have recently been described in archaea (Gattinger et al. 2002). In fact, “hybrid” lipids from as yet unidentified organisms have also been reported (Schouten et al. 2000). To date, however, there is no known exception to the G1P backbone stereochemistry of AMLs or the G3P backbone found in bacteria and eukaryotes. This leaves open the question of the nature of the membrane lipids in the LUA. Hence, the relevant biosynthetic pathways are implicated in the emergence of Archaea, and life in general (Glansdorff 2000; Koga et al. 1998; Martin and Russell 2003; Peretó et al. 2004; Segré et al. 2001; Wächtershäuser 1988, 2003).
In the first portion of this report, we examine the evidence that both substrates of (S)-3-O-geranylgeranylglycerylphosphate synthase (GGGPS), the enzyme which performs the committed step in archaeal lipid synthesis, were present within the LUA. As the downstream enzyme, (S)-2,3-di-O-geranylgeranylglycerylphosphate synthase (DGGGPS), is known not to be stereospecific, we conclude that the chirality of AMLs is determined by GGGPS. Based on sequence and structural similarities, we then present evidence which suggests that GGGPS evolved by gene duplication and subsequent fusion of a (βα)4 half-barrel ancestor protein (or duplication of an ancient hisF-like gene). Upon the advent of GGGPS, we speculate that a small fraction of the LUA community would have acquired the ability to synthesize archaeal-like membrane lipids instead of bacterial-like membrane lipids. Assuming that this created a physical isolation, albeit a temporary one, we propose that these early “Archaea” would have been free to develop other distinctly archaeal features.
Materials and Methods
Sequence and Structural Alignments
A search of the Protein Data Bank (http://www.rcsb.org) was performed with DALI (Holm and Sander 1996) using the GGGPS-G1P-complex crystal structure (PDB-ID 2F6X). The program LSQMAN (Kleywegt 1996) was used for all structural alignments and to aid in structure-based sequence alignments. Molecular figures were prepared with the programs MOLSCRIPT (Kraulis 1991) and RASTER3D (Merritt and Murphy 1994).
GGGPS Assay
GGGPS from Archaeoglobus fulgidus and the Asp13-to-Ala13 (D13A) mutant (created by the Stratagene Quikchange protocol) were purified as previously described (Payandeh et al. 2006). To avoid the use of radioactivity, we implemented an enzyme-coupled assay to detect the free pyrophosphate liberated by the GGGPS reaction. The PiPer Pyrophosphate Assay (Molecular Probes) was performed as described by the manufacturer in the “background management” setting. GGGPS (20 μM) was prepared in 100 mM Tris, pH 7.5, and 1 mM MgCl2, and the assays were run with fixed substrate concentrations: 25 μM rac-glycerophosphate (Sigma- Aldrich) and 25 μM GGPP (Sigma-Aldrich). Absorbence was monitored at 565 nm and reactions were incubated at 37°C for 2 h.
Results and Discussion
GGGPS Is Responsible for the Chirality of AMLs
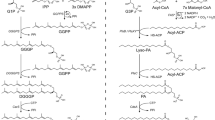
The biosynthesis of AMLs is illustrated in Fig. 1. Glycerol-1-phosphate dehydrogenase (G1PDH) has received most of the attention regarding the advent of AMLs by virtue of supplying the required precursor, G1P (Daiyasu et al. 2002; Han and Ishikawa 2005; Koga et al. 1998, 2003; Peretó et al. 2004). However, G1PDH does not represent the committed step in AML biosynthesis and, therefore, could not fix the nature of AMLs. In contrast, GGGPS catalyzes the committed step and, in a single reaction, provides all three distinguishing characteristics of AMLs: the backbone stereochemistry, an isoprenoid chain, and an ether linkage (Fig. 1) (Chen et al. 1993; Nemoto et al. 2003; Payandeh et al. 2006; Soderberg et al. 2001). Accordingly, GGGPS is in a position to have fixed the nature of AMLs. In fact, recent analysis has demonstrated that the downstream enzyme, DGGGPS, can efficiently transfer a prenyl group onto both (R)-geranylgeranylglyceryl phosphate and (S)-geranylgeranylglyceryl phosphate (Zhang et al. 2006). Moreover, enzymes downstream of DGGGPS are also known not to be stereospecific (Morii and Koga 2003; Morii et al. 2000). These facts strongly support the notion that the chirality of AMLs is determined solely by GGGPS.
Schematic of archaeal membrane lipid biosynthesis. Enzymatic steps of outstanding interest are indicated in red. In brief, dimethylallyl diphosphate (DMAPP) and its isomer isopentenyl diphosphate (IPP) are synthesized by a mevalonate-like pathway in Archaea (Boucher et al. 2004; De Rosa et al. 1986). Long isoprenoid chains, e.g., geranylgeranyl diphosphate (GGPP), are produced from these five-carbon precursors by consecutive condensations through the action of a prenyl diphosphate synthase. G1P is produced by G1P dehydrogenase (G1PDH), which is structurally distinct from its bacterial and eukaryotic counterpart G3P dehydrogenase (G3PDH). The committed step in AML biosynthesis occurs with the formation of an ether linkage between G1P and an isoprenoid diphosphate. The enzyme catalyzing this reaction, (S)-3-O-geranylgeranylglyceryl phosphate synthase (GGGPS), represents the only known prenyltransferase structure with a TIM-barrel fold. The enzyme catalyzing the second prenyltransfer reaction, (S)-2,3-di-O-geranylgeranylglyceryl phosphate synthase (DGGGPS), has been characterized as a member of the UbiA family of prenyltransferases and is known not to be stereospecific (Zhang et al. 2006). In ensuing reactions, various headgroups can be formed and isoprenoid double bonds may be reduced. Synthesis of the so-called archaeal tetraether lipids remains largely unexplored, and is not illustrated here. Note that the isoprenoids DMAPP, IPP, and GGPP are essentially ubiquitous across all three domains of life. DHAP, dihydroxyacetone phosphate; GGGP, geranylgeranylglyceryl phosphate; DGGGP, di-geranylgeranylglyceryl phosphate; CAS, CDP-archaeol synthase (Morii et al. 2000); AS, archaetidylserine synthase (Morii and Koga 2003); DGGGPR, 2,3-di-O-geranylgeranylglycerylphospholipid reductase.
G1PDH and DGGGPS Were Recruited from the LUA
G1PDH, GGGPS, and DGGGPS (Fig. 1) are entirely restricted to Archaea (Boucher et al. 2004; Hemmi et al. 2004; Koga et al. 1998; Peretó et al. 2004). Although G1PDH is structurally distinct from its bacterial and eukaryotic counterpart G3P dehydrogenase (G3PDH) (Fig. 1), it has been established that G1PDH is a close homologue of glycerol dehydrogenase (GDH) (Daiyasu et al. 2002; Han and Ishikawa 2005; Koga et al. 1998, 2003; Peretó et al. 2004). In this context, it seems particularly relevant that the GDH proteins are somewhat promiscuous enzymes (Ruzheinikov et al. 2001). Thus, if we assume that the pathways for the uptake and utilisation of glycerol-like molecules are ancient, as they appear to be (Lin 1976; Zardoya 2005), then perhaps G1P may have been produced within the LUA by a promiscuous GDH-like protein (Daiyasu et al. 2002; Han and Ishikawa 2005; Jensen 1976; Koga et al. 2003; Peretó et al. 2004). This would suggest that G1P was available within the LUA, ready for the appearance of GGGPS. While G1P is also a component of the phosphoglycolipids and lipoteichoic acid polymers found in Gram-positive bacteria (Fischer and Arneth-Seifert 1998), the membrane-derived oligosaccharide of E. coli (Kennedy et al. 1976), and the unacylated moiety of phosphatidylglycerol from bacteria and eukaryotes (Itabashi and Kuksis 1997), note that the G1P structures present within these compounds are produced by an alternate biosynthetic mechanism (Fischer and Arneth-Seifert 1998; Kennedy et al. 1976).
We address the existence of isoprenoid diphosphates (Fig. 1) within the LUA by focusing on the UbiA prenyltransferase family (Hemmi et al. 2004). Members of the UbiA family transfer prenyl groups onto various acceptors; and these enzymes perform essential biosynthetic roles in anoxygenic and oxygenic respiration and photosynthesis (Garcia-Gil et al. 2003; Mulkidjanian et al. 2006; Shineberg and Young 1976; Suvarna et al. 1998; Xiong et al. 2000). UbiA-like enzymes are presumably deeply rooted evolutionarily (Mulkidjanian et al. 2006; Xiong et al. 2000), particularly because the fossil record indicates the existence of complex photosynthetic life >3.4 billion years ago (Nisbet and Sleep 2001; Tice and Lowe 2004). We infer the presence of a ubiA-like gene or gene family within the LUA, probably menA- or bchG-like (Garcia-Gil et al. 2003; Mulkidjanian et al. 2006; Shineberg and Young 1976; Suvarna et al. 1998; Xiong et al. 2000). Based on the similarity of their substrates, an early UbiA-like enzyme could have performed a DGGGPS function upon the advent of GGGPS (Hemmi et al. 2004; Jensen 1976; Zhang et al. 2006). Hence, as it relates to present-day AML biosynthesis (Fig. 1), we suspect that an ancestral GDH-like enzyme supplied G1P, and an ancestral UbiA-like protein catalyzed the second prenyltransfer reaction.
Archaeal Membrane Lipids in Just Three Steps?
Before we proceed, perhaps the significance of a minimal biosynthetic pathway should be rationalized. Most notably, the structural core of AMLs, 2,3-di-O-geranylgeranylglyceryl phosphate (unsaturated archaetidic acid), is created through a three-step pathway: G1PDH, GGGPS, and DGGGPS (Fig. 1). The remainder of the biosynthetic pathway seems to underscore the evolutionary relevance of this unsaturated “precursor.” For instance, at least two headgroup modifications (and possibly more) are known to occur on the unsaturated lipid species (Daiyasu et al. 2005; Morii and Koga 2003; Morii et al. 2000). Also, the enzyme which catalyzes the reduction of isoprenoid double bonds, 2,3-di-O-geranylgeranylglycerylphospholipid reductase (DGGGPR), recognizes unsaturated archaetidic acid and its derivatives as substrates, i.e., those containing phosphoglycerol and phosphoethanolamine headgroups (Nishimura and Eguchi 2006). The incomplete saturation or “cold adaptation” of AMLs (Nichols et al. 2004), as well as the remarkable physicochemical properties of AMLs (e.g., the presumed temperature range in which they maintain a liquid crystalline phase) (Albers et al. 2000; Koga and Morii 2005), seems to further indicate the limited evolutionary (enzymatic) requirements of this pathway.
Although we have not specifically included all biosynthetic proteins in our hypothesis for reasons of simplicity, it seems that the enzymes downstream of DGGGPS (Fig. 1) could also be accommodated (Daiyasu et al. 2005; Morii and Koga 2003; Morii et al. 2000; Nishimura and Eguchi 2006). For instance, the isoprenoid reductase protein (DGGGPR) is related to bacterial and eukaryotic homologues involved in the biosynthesis of bacteriochlorophyll and chlorophyll, bchP and chlP, respectively (Nishimura and Eguchi 2006). Hence, the evolution of DGGGPR would presumably follow suit with the proposals that we have stated, both above and below, for DGGGPS.
GGGPS Is a Member of the HisA/HisF TIM-Barrel Family
We now return our focus to the committed step of AML biosynthesis, GGGPS (Fig. 1). We believe that GGGPS’s sequence and structural relatedness to HisA and HisF will establish its (GGGPS’s) evolutionary history. The TIM-barrel proteins HisA, HisF, TrpF, and TrpC catalyze sequential reactions in the biosynthesis of histidine and tryptophan, respectively. These four (βα)8 TIM-barrel proteins have been studied extensively; in part, because they are closely related in structure and function. For example, HisF can catalyze the HisA reaction (Lang et al. 2000); HisA and HisF can be converted into a TrpF activity through a single amino acid substitution (Leopoldseder et al. 2004); and in some bacteria, only one enzyme performs both HisA and TrpF functions (Kuper et al. 2005).
It is intriguing that the structures of HisA and HisF have a remarkable degree of internal (two-fold) symmetry (Figs. 2B and C). This finding suggests that these enzymes have evolved by duplication and fusion of a (βα)4 half-barrel ancestor protein (Lang et al. 2000). While already evident within their primary sequence (e.g., Fig. 3), there is also other evidence to support this notion. These data include the production of stable (βα)4 half-barrel proteins (Höcker et al. 2001) and the creation of novel (βα)8 full-barrel proteins (Höcker et al. 2004), the ability to circularly permutate TIM-barrels (Sterner and Höcker 2005), and the occurrence of naturally split variants (Nagano et al. 2002; Sterner and Höcker 2005). In fact, recent structural work has led to the identification of a possible “living” half-barrel ancestor (Gaspar et al. 2005). Moreover, large-scale structural analysis of TIM-barrel proteins has indicated the existence of internal four-fold symmetry, as identified by specific sequence hallmarks (Nagano et al. 2002). It is notable that all four sequence hallmarks are present within HisF (Fig. 3) (Nagano et al. 2002).
Superposition of GGGPS with HisA and HisF. A The dimeric structure of GGGPS from A. fulgidus (AfGGGPS). The red subunit is not used in the alignments; the N- and C-terminal “half-barrels” of the other protomer are colored light and dark purple, respectively. B N- and C-terminal half-barrels of HisA from Thermotoga maritima (TmHisA) are colored light and dark blue, respectively. C N- and C-terminal half-barrels of HisF from T. maritima (TmHisF) are colored light and dark green, respectively. D A table of the calculated RMSD/Cα values for the alignments shown in E–H. E, F Alignments of the full-length proteins. G, H N- and C-terminal half-barrels of TmHisA and TmHisF have been aligned onto the canonical (i.e., C-terminal) half-barrel of AfGGGPS. These views are rotated 90° with respect to E and F.
Structure-based sequence alignment of GGGPS and HisF. The GGGPS sequence from A. fulgidus (AfGGGPS) and the full-length and N-terminal-half sequences of HisF from T. maritima (TmHisF and TmHisF-N) are shown. Secondary structural elements are colored according to their respective half-barrels, consistent with the colour scheme in Fig. 2. Conserved catalytic residues (D) are highlighted in red and the “standard phosphate binding motifs” are shaded in blue. The black arrows and orange shading indicate the special positions of the four-fold sequence hallmarks which are considered to be G-X-D, X-X-D, or G-X-X (Nagano et al. 2002). The sequence identity and similarity of AfGGGPS are ∼17 and 45% against full-length TmHisF or ∼12 and 33% when also considering TmHisF-N as shown. Note that Asp13 (D13) is conserved within all known GGGPSs and implicated in substrate binding (Payandeh et al. 2006); it occupies the position of an absolutely essential Asp residue in HisA and HisF (Leopoldseder et al. 2004).
A DALI search (Holm and Sander 1996) reveals that the structure of GGGPS is closely related to HisA and HisF (Table 1). When considering their different enzymatic functions and the unique adaptations which are found in GGGPS, i.e., GGGPS is a dimeric protein with a modified TIM-barrel fold (Fig. 2A) (Payandeh et al. 2006), the overall Cα-RMSD between these proteins is remarkably small (Table 1 and Figs. 2D–F) (Leopoldseder et al. 2004), especially for their half-barrels (Figs. 2D–H) (Höcker et al. 2001; Lang et al. 2000). Beyond these structural comparisons (Table 1 and Figs. 2A–H) (Höcker et al. 2001; Lang et al. 2000; Leopoldseder et al. 2004), it is striking that the sequence identity between GGGPS and HisA (not shown) or GGGPS and HisF (Fig. 3) is even higher than that observed between HisA and TrpF or between HisF and TrpF (Leopoldseder et al. 2004). Said explicitly, HisA and HisF appear to be more closely related to GGGPS at the sequence and structural level than either protein is to TrpF (or TrpC). Moreover, the “standard phosphate-binding motif” (Nagano et al. 2002) is highly conserved among these TIM-barrel proteins (Fig. 3), and an essential catalytic residue of HisA and HisF is also conserved within GGGPS (Fig. 3) (Leopoldseder et al. 2004; Payandeh et al. 2006). This residue, implicated in binding the Mg2+-GGPP substrate (Payandeh et al. 2006), is required for optimal GGGPS activity. Specifically, the GGGPS D13A mutant shows only 67% ± 8.0% activity relative to the wild-type protein (see Materials and Methods). Finally, upon noting the four-fold HisF-like sequence hallmarks (Nagano et al. 2002) which are present within GGGPS (Fig. 3), it becomes clear that GGGPS shares a common ancestry with HisA and HisF.
The HisA/HisF TIM-Barrel Family Establishes a Time Line for the Advent of GGGPS
Histidine and tryptophan were presumably among the last amino acids to be recruited into the universal set of 20 (Jordan et al. 2005, 2006). Irrespective of controversy (Hurst et al. 2006; McDonald 2006), it can be reasoned that this had to predate the LUA in order for any of the amino acids to get a proper foothold (Jordan et al. 2005; Vetsigian et al. 2006). Considering that histidine and tryptophan are biosynthetically “expensive” amino acids (Akashi and Gojobori 2002), and therefore more recent (Hurst et al. 2006), this approximates a time period for the existence of (βα)4 half-barrels and the creation (or evolution) of new (βα)8 TIM-barrels (Fig. 4). Hence, the circumstances that gave rise to HisA, HisF, TrpF, and TrpC function would seem to coincide with (but predate) the appearance of GGGPS. Thus, we purpose that GGGPS’s remarkable relatedness to HisA and HisF can establish a time period for the invention of GGGPS (Fig. 4). This implies the “transfer” of GGGPS from the LUA to the first progenitors of Archaea. Since this will serve to pinpoint the divergence of Archaea, the above arguments begin to illustrate the notion of a lipid-based divergence (Glansdorff 2000; Hemmi et al. 2004; Koga et al. 1998; Martin and Russell 2003; Peretó et al. 2004; Wächtershäuser 2003) or GGGPS-driven speciation event.
Schematic time line for the emergence of Archaea. A time line supported by evidence in the fossil record is illustrated along the left-hand side (Byr = 109 years) (Nisbet and Sleep 2001; Tice and Lowe 2004). The relative order and position of GDH-like, UbiA-like, HisA, HisF, TrpF, and TrpC proteins within the LUA remain somewhat arbitrary; however, the advent of HisA, HisF, TrpF, and TrpC can be presumed to be “late” events (see main text). Our arguments imply the invention of GGGPS prior to the “crystallization” (Woese 1998) of Archaea. The order and position of G1PDH and DGGGPS within the first archaeal ancestor remain arbitrary; however, our enzyme-driven lipid capture hypothesis remains valid even if G1PDH and/or DGGGPS predates GGGPS (see main text). If the LUA had bacterial-like membrane lipids that were heterochiral with respect to a glycerophosphate-like backbone, perhaps the advent of G3PDH (or another downstream enzyme) might have similarly led to the “crystallization” of Bacteria (see main text). Regarding the first appearance of Archaea, our ∼3.4-Byr estimate might actually be fairly conservative (Nisbet and Sleep 2001; Tice and Lowe 2004), perhaps by as much as ∼0.4–0.6 Byr (Nisbet and Sleep 2001). Also, please note our intentional use of the term LUA over “last universal common ancestor” (LUCA). This is intended to indicate the possibility (perhaps the likelihood) that “bacterial” lineages began to radiate from the LUA prior to our proposed GGGPS-driven “archaeal” speciation event; more generally, we assume that the LUA itself must have been a heterogeneous and evolving entity—by definition.
GGGPS: Nature’s Only Known TIM-Barrel Prenyltransferase
Nature has clearly exploited the TIM-barrel structural scaffold with a large number of enzymatic functions (Nagano et al. 2002; Sterner and Höcker 2005). However, since GGGPS is the only known TIM-barrel prenyltransferase (Payandeh et al. 2006), the generation of this function seems to be a uniquely archaeal invention (Boucher et al. 2004; Payandeh et al. 2006). Furthermore, with over 23,000 structurally diverse metabolites, the isoprenoid biosynthetic pathway is unrivaled and ubiquitous in Nature (Liang et al. 2002; Sacchettini and Poulter 1997). Given the unique and highly specialized folds which are known for other prenyltransferases (Kuzuyama et al. 2005; Liang et al. 2002; Sacchettini and Poulter 1997), the limited distribution of the GGGPS TIM-barrel implies that it is the sole (but not the only) invention of the direct predecessors of Archaea. Moreover, GGGPS and DGGGPS are entirely absent from the realms of Bacteria and Eukarya (Boucher et al. 2004; Hemmi et al. 2004). By contrast, integral components of the bacterial and eukaryotic lipid biosynthetic apparatus can be identified within archaeal genomes (Daiyasu et al. 2005; Peretó et al. 2004; Sakasegawa et al. 2004), and phospholipid fatty acids have been documented in Archaea (Gattinger et al. 2002). For these reasons, we are inclined to conclude that the distribution of GGGPS, DGGGPS, and the AMLs have not been dispersed nor infiltrated by bacteria or eukaryotes since their invention. These arguments likely rule out the possibility of horizontal gene transfer (HGT), and they help to explain why there is no exception to the backbone stereochemistry of archaeal or bacterial and eukaryotic membrane lipids. As such, it is notable that others have already discussed the physicochemical properties through which Nature may select for homo- vs. heterochiral membrane lipids (Glansdorff 2000; Koga et al. 1998; Peretó et al. 2004; Wächtershäuser 1988, 2003). Wächtershäuser (2003) provides some excellent details (e.g., references within) that can be summarized as follows. Heterochiral lipid bilayers, which were potentially present within the LUA (and now thought to be evolutionarily extinct), are known to be inherently less stable due to packing considerations (Wächtershäuser 2003); this might have favored a mechanism to produce homochiral membrane lipids (Fig. 4). Also, note that the distinction between a heterochiral-to-homochiral transition and (for example) a G3P-to-heterochiral, heterochiral-to-G1P-based transition has already been discussed (Wächtershäuser 2003). We agree with the suggestion that the former would seem evolutionarily more feasible (see below).
Enzyme-Driven Speciation: Encapsulation-Induced Isolation
Since biological membranes display a vertical line of inheritance, and AMLs are a defining characteristic (Woese et al. 1990), our view implicates GGGPS as a prime candidate to have driven the divergence of Archaea from the LUA. Considering our proposed time line (Fig. 4), and as a process of elimination, the above observations imply that bacterial-like lipids were present within the LUA. Among the possibilities, we cannot rule out the existence of “hybrid” lipids within the LUA (Schouten et al. 2000). While new archaeal or bacterial isolates may prove to contain such lipids, it has also been noted that these lipids may have formed abiotically (Koga and Morii 2005). In keeping with the presumed nature of the LUA (Glansdorff 2000; Vetsigian et al. 2006; Wächtershäuser 2003; Woese 1998, 2002), as well as other reports (Morii and Koga 2003; Morii et al. 2000; Peretó et al. 2004; Wächtershäuser 2003; Zhang et al. 2006), our analysis is consistent with the notion that heterochiral lipid headgroup moieties (probably glycerophosphate-like) were present within the LUA. This might suggest the possible lipid-based divergence of Bacteria from the LUA (Peretó et al. 2004; Wächtershäuser 2003), perhaps through the recruitment of G3PDH activity (Fig. 4) (Peretó et al. 2004), or a downstream enzyme, analogous to what is being suggested here for GGGPS and the AMLs. As such, it is interesting that G3PDH was found to be nonessential in a “minimal” bacterium (Glass et al. 2006).
Based on the above considerations, our observations support the “simple” bacterial-like nature of the LUA, as well as the simple-to-complex theory of life, i.e., the divergence of Archaea from a bacterial-like LUA upon completion of the universal genetic and amino acid code (Fig. 4). Thus, borrowing from the theory and terminology of Woese (1998), we propose that the advent of GGGPS was the initiating event leading to the eventual “crystallization” of Archaea.
Our proposal creates an interesting framework. Historically, seven traits have been recognized as being distinctly archaeal, and many of these can be considered “informational genes” (Simonson et al. 2005; Woese 2002). Specifically, members of the domain Archaea share antibiotic resistance (Briones et al. 2005; Kandler and König 1998), rRNA sequence features (Woese et al. 1990), characteristic modified nucleotides in tRNA (Ishitani et al. 2002), unique structures of DNA-dependent RNA polymerase (Langer et al. 1995), novel cofactors (Martin 2004), distinct proteinaceous cell walls (Kandler and König 1998), and G1P-ether-linked isoprenoid lipids (De Rosa et al. 1986; Koga and Morii 2005; Woese et al. 1990). Although most of these genes are not found within the “archaeal genomic signature” (Graham et al. 2000), it is important to note that an analogous situation has been realized for the “photosynthetic” and “cyanobacterial” gene signatures (Mulkidjanian et al. 2006). Although initially unanticipated, these findings may reflect “recent” lineage commitments (Graham et al. 2000) and/or processes of HGT (Mulkidjanian et al. 2006). Nevertheless, since informational genes rarely experience HGT (Simonson et al. 2005; Woese 2002), this implies that every archaeal invention must have remained just that: distinctly archaeal. Given the widespread gene sharing and common gene pool inherent to the ancestral community (Glansdorff 2000; Vetsigian et al. 2006; Wächtershäuser 2003; Woese 1998, 2002), a paradox arises. How does one invent something and not share it with the rest of the community? Encapsulation-induced isolation, or “lipid capture,” could be a relatively simple answer.
Although highly speculative, the following is our proposed model. With the advent of GGGPS, a small portion of the ancestral community became the progenitors of present-day Archaea. Upon recruitment (Jensen 1976) of G1PDH and DGGGPS function, a conversion of the membrane bilayer from bacterial-like lipids to archaeal-like lipids would have occurred (Fig. 4). As such, homochiral G1P-based AMLs were born. Assuming that this created a physical barrier, it would have produced an evolutionary vacuum, a void in which these early “Archaea” were free to explore. As they emerged from or began to fill this evolutionary bubble, perhaps these prokaryotes had developed most (if not all) of their newfound, i.e., distinctly archaeal features.
A “lipid capture” model can easily confine and restrict all distinctly archaeal traits, informational or otherwise; it also avoids the fundamental and paradoxical problems associated with the other available options. Thus, our concept describes the molecular equivalent of erecting a mountain range in order to divide a population, where the new archaeal membrane bilayer initially produces an insurmountable obstacle to genetic “free trade.”
Lipid Capture: Clarifications and Compromise
Our insight has arisen solely from a structural point of view, the TIM-barrel fold of GGGPS (Nemoto et al. 2003; Payandeh et al. 2006). Other observations simply follow. GGGPS catalyzes the committed step of AML biosynthesis and provides the unique G1P-based backbone stereochemistry; GGGPS represents the only known TIM-barrel prenyltransferase in Nature; and, in the absence of our proposed time line (Fig. 4), GGGPS’s exceptional relatedness to HisF and HisA is otherwise perplexing. Hence, our suggestion of a GGGPS-driven speciation event appears to represent the first “concrete” time line for the divergence of Archaea from the LUA. Of course, the apparent simplicity, modularity, stability, and evolvability of the TIM-barrel structural scaffold (Höcker et al. 2001, 2004; Lang et al. 2000; Leopoldseder et al. 2004; Nagano et al. 2002; Silverman et al. 2001; Sterner and Höcker 2005) also make it an attractive component of the LUA.
From our standpoint, the LUA would have been a rather sophisticated “entity.” Therefore, evolutionary intermediates must have existed at many levels. While we have attempted to indicate some possibilities, we believe that our GGGPS-driven archaeal progenitor describes one such intermediate. As such, it is interesting that our view does not necessarily favor (or disfavor) a thermophilic or psychrophilic origin for life. Based on the collective properties of AMLs (Albers et al. 2000; Koga and Morii 2005), and the lipids which presumably preceded them, we might argue the case either way. Our concept also seems compatible with other provocative evolutionary theories. For instance, although we are prone to consider the “genome” of our LUA to be DNA-based (for reasons of complexity), perhaps the GGGPS-driven lipid capture event may have primed this intermediate to accept one of Forterre’s (2006) viruses.
In general, our lipid capture hypothesis is consistent with microbial phylogeny (Ciccarelli et al. 2006) and physiology, e.g., protein domain content (Yang et al. 2005), protein secretion, and cell wall structure (Albers et al. 2006). However, in addition to the informational systems alluded to above, some gaps remain. For example, although the products are universal (Fig. 1), our theory does not immediately account for the discrepancies between the mevalonate and the non-mevalonate isoprenoid biosynthetic pathways found within Archaea and Bacteria (Boucher et al. 2004; De Rosa et al. 1986; Eisenreich et al. 2004). Also, this work does not address an evolutionary origin for the unique outer membrane of the archaeal Ignicoccus species (Näther and Rachel 2004). Finally, since G3PDH enzymes are occasionally found within archaeal genomes, we note the discussion by Sakasegawa et al. (2004) which describes their potential physiological roles (unrelated to lipid biosynthesis) and evolutionary origin.
Nonetheless, our model suggests hypotheses that may be testable within the laboratory. At present, we are most intrigued by the possibility of heterologously reconstituting the AML biosynthetic apparatus within a bacterium (e.g., G1PDH, GGGPS, DGGGPS, and DGGGPR). Recombinant expression of this diagnostic archaeal pathway, perhaps with the conditional knockdown of endogenous lipid production, might give rise to a bacterium whose membrane is comprised solely of AMLs.
Perspectives
The lipid capture hypothesis can provide a convenient framework for the (rapid) expansion and advances that are present within Archaea, but which are absent from their bacterial counterparts. We have pinpointed an initiating event, reconstructed a time line, and offered structural evidence to support this theory. If correct, then in addition to highlighting aspects of molecular evolution (which include the creation of new enzymes and biochemical pathways), our view indicates that GGGPS represents an example of an enzyme-driven speciation event. Given the popular theory that eukaryotes first arose through the symbiosis or fusion of Bacteria and Archaea (Embley and Martin 2006; Glansdorff 2000; Koga et al. 1998; Martin and Koonin 2006; Simonson et al. 2005; Wächtershäuser 2003; Woese 2002), then the evolution of TIM-barrel proteins and GGGPS could arguably have even broader implications.
References
Akashi H, Gojobori T (2002) Metabolic efficiency and amino acid composition in the proteomes of Escherichia coli and Bacillus subtilis. Proc Natl Acad Sci USA 99:3695–700
Albers SV, van de Vossenberg JL, Driessen AJ, Konings WN (2000) Adaptations of the archaeal cell membrane to heat stress. Front Biosci 5:D813–D820
Albers SV, Szabó Z, Driessen AJ (2006) Protein secretion in the Archaea: multiple paths towards a unique cell surface. Nat Rev Microbiol 4:537–547
Boucher Y, Kamekura M, Doolittle WF (2004) Origins and evolution of isoprenoid lipid biosynthesis in Archaea. Mol Microbiol 52:515–527
Briones C, Manrubia SC, Lázaro E, Lazcano A, Amils R (2005) Reconstructing evolutionary relationships from functional data: a consistent classification of organisms based on translation inhibition response. Mol Phylogenet Evol 34:371–381
Chen A, Zhang D, Poulter CD (1993) (S)-geranylgeranylglyceryl phosphate synthase. Purification and characterization of the first pathway-specific enzyme in archaebacterial membrane lipid biosynthesis. J Biol Chem 268:21701–21705
Ciccarelli FD, Doerks T, von Mering C, Creevey CJ, Snel B, Bork P (2006) Toward automatic reconstruction of a highly resolved tree of life. Science 311:1283–1287
Daiyasu H, Hiroike T, Koga Y, Toh H (2002) Analysis of membrane stereochemistry with homology modeling of sn-glycerol-1-phosphate dehydrogenase. Protein Eng 15:987–995
Daiyasu H, Kuma K, Yokoi T, Morii H, Koga Y, Toh H (2005) A study of archaeal enzymes involved in polar lipid synthesis linking amino acid sequence information, genomic contexts and lipid composition. Archaea 1:399–410
De Rosa M, Gambacorta A, Gliozzi A (1986) Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol Rev 50:70–80
Eisenreich W, Bacher A, Arigoni D, Rohdich F (2004) Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell Mol Life Sci 61:1401–1426
Embley TM, Martin W (2006) Eukaryotic evolution, changes and challenges. Nature 440:623–630
Fischer W, Arneth-Seifert D (1998) D-Alanylcardiolipin, a major component of the unique lipid pattern of Vagococcus fluvialis. J Bacteriol 180:2950–2957
Forterre P (2006) Three RNA cells for ribosomal lineages and three DNA viruses to replicate their genomes: a hypothesis for the origin of cellular domain. Proc Natl Acad Sci USA 103:3669–3674
Forterre P, Philippe H (1999) Where is the root of the universal tree of life? Bioessays 21:871–879
Garcia-Gil LJ, Gich FB, Fuentes-Garcia X (2003) A comparative study of bchG from green photosynthetic bacteria. Arch Microbiol 179:108–115
Gaspar JA, Liu C, Vassall KA, Meglei G, Stephen R, Stathopulos PB, Pineda-Lucena A, Wu B, Yee A, Arrowsmith CH, Meiering EM (2005) A novel member of the YchN-like fold: solution structure of the hypothetical protein Tm0979 from Thermotoga maritima. Protein Sci 14:216–223
Gattinger A, Schloter M, Munch JC (2002) Phospholipid etherlipid and phospholipid fatty acid fingerprints in selected euryarchaeotal monocultures for taxonomic profiling. FEMS Microbiol Lett 213:133–139
Glansdorff N (2000) About the last common ancestor, the universal life-tree and lateral gene transfer: a reappraisal. Mol Microbiol 38:177–185
Glass JI, Assad-Garcia N, Alperovich N, Yooseph S, Lewis MR, Maruf M, Hutchison CA III, Smith HO, Venter JC (2006) Essential genes of a minimal bacterium. Proc Natl Acad Sci USA 103:425–430
Graham DE, Overbeek R, Olsen GJ, Woese CR (2000) An archaeal genomic signature. Proc Natl Acad Sci USA 97:3304–3308
Han JS, Ishikawa K (2005) Active site of Zn2+-dependent sn-glycerol-1-phosphate dehydrogenase from Aeropyrum pernix K1. Archaea 1:311–317
Hemmi H, Shibuya K, Takahashi Y, Nakayama T, Nishino T (2004) (S)-2,3-di-O-geranylgeranylglyceryl phosphate synthase from the thermoacidophilic archaeon Sulfolobus solfataricus. Molecular cloning and characterization of a membrane-intrinsic prenyltransferase involved in the biosynthesis of archaeal ether-linked membrane lipids. J Biol Chem 279:50197_50203
Höcker B, Beismann-Driemeyer S, Hettwer S, Lustig A, Sterner R (2001) Dissection of a (βα)8-barrel enzyme into two folded halves. Nat Struct Biol 8:32–36
Höcker B, Claren J, Sterner R (2004) Mimicking enzyme evolution by generating new (βα)8-barrels from (βα)4-half-barrels. Proc Natl Acad Sci USA 101:16448–16453
Holm L, Sander C (1996) Mapping the protein universe. Science 273:595–603
Hurst LD, Feil EJ, Rocha EP (2006) Protein evolution: causes of trends in amino-acid gain and loss. Nature 442:E11–E112
Ishitani R, Nureki O, Fukai S, Kijimoto T, Nameki N, Watanabe M, Kondo H, Sekine M, Okada N, Nishimura S, Yokoyama S (2002) Crystal structure of archaeosine tRNA-guanine transglycosylase. J Mol Biol 318:665–677
Itabashi Y, Kuksis A (1997) Reassessment of stereochemical configuration of natural phosphatidylglycerols by chiral-phase high-performance liquid chromatography and electrospray mass spectrometry. Anal Biochem 254:49–56
Jensen RA (1976) Enzyme recruitment in evolution of new function. Annu Rev Microbiol 30:409–425
Jordan IK, Kondrashov FA, Adzhubei IA, Wolf YI, Koonin EV, Kondrashov AS, Sunyaev S (2005) A universal trend of amino acid gain and loss in protein evolution. Nature 433:633–638
Jordan IK, Kondrashov FA, Adzhubei IA, Wolf YI, Koonin EV, Kondrashov AS, Sunyaev S (2006) Protein evolution: causes of trends in amino-acid gain and loss—Reply. Nature 442:E12
Kandler O, König H (1998) Cell wall polymers in Archaea (Archaebacteria). Cell Mol Life Sci 54:305–308
Kennedy EP, Rumley MK, Schulman H, van Golde LM (1976) Identification of sn-glycero-1-phosphate and phosphoethanolamine residues linked to the membrane-derived oligosaccharides of Escherichia coli. J Biol Chem 251:4208–4213
Kleywegt GJ (1996) Use of non-crystallographic symmetry in protein structure refinement. Acta Crystallogr D Biol Crystallogr 52:842–857
Koga Y, Morii H (2005) Recent advances in structural research on ether lipids from archaea including comparative and physiological aspects. Biosci Biotechnol Biochem 69:2019–2034
Koga Y, Kyuragi T, Nishihara M, Sone N (1998) Did archaeal and bacterial cells arise independently from noncellular precursors? A hypothesis stating that the advent of membrane phospholipid with enantiomeric glycerophosphate backbones caused the separation of the two lines of descent. J Mol Evol 46:54–63
Koga Y, Sone N, Noguchi S, Morii H (2003) Transfer of pro-R hydrogen from NADH to dihydroxyacetonephosphate by sn-glycerol-1-phosphate dehydrogenase from the archaeon Methanothermobacter thermautotrophicus. Biosci Biotechnol Biochem 67:1605–1608
Kraulis PJ (1991) MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J Appl Cryst 24:946–950
Kuper J, Doenges C, Wilmanns M (2005) Two-fold repeated (βα)4 half-barrels may provide a molecular tool for dual substrate specificity. EMBO Rep 6:134–139
Kurland CG, Collins LJ, Penny D (2006) Genomics and the irreducible nature of eukaryote cells. Science 312:1011–1014
Kuzuyama T, Noel JP, Richard SB (2005) Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products. Nature 435:983–987
Lang D, Thoma R, Henn-Sax M, Sterner R, Wilmanns M (2000) Structural evidence for evolution of the β/α barrel scaffold by gene duplication and fusion. Science 289:1546–1550
Langer D, Hain J, Thuriaux P, Zillig W (1995) Transcription in Archaea: similarity to that in Eucarya. Proc Natl Acad Sci USA 92:5768–5772
Leopoldseder S, Claren J, Jürgens C, Sterner R (2004) Interconverting the catalytic activities of (βα)8-barrel enzymes from different metabolic pathways: sequence requirements and molecular analysis. J Mol Biol 337:871–879
Liang PH, Ko TP, Wang AH (2002) Structure, mechanism and function of prenyltransferases. Eur J Biochem 269:3339–3354
Lin ECC (1976) Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol 30:535–578
Martin W (2004) Pathogenic archaebacteria: do they not exist because archaebacteria use different vitamins? Bioessays 26:592–593
Martin W, Koonin EV (2006) Introns and the origin of nucleus-cytosol compartmentalization. Nature 440:41–45
Martin W, Russell MJ (2003) On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos Trans R Soc Lond B Biol Sci 358:59–83, discussion 83–85
McDonald JH (2006) Apparent trends of amino acid gain and loss in protein evolution due to nearly neutral variation. Mol Biol Evol 23:240–244
Merritt EA, Murphy ME (1994) Raster3D Version 2.0. A program for photorealistic molecular graphics. Acta Crystallogr D Biol Crystallogr 50:869–873
Morii H, Koga Y (2003) CDP-2,3-di-O-geranylgeranyl-sn-glycerol:L-serine O-archaetidyltransferase (archaetidylserine synthase) in the methanogenic archaeon Methanothermobacter thermautotrophicus. J Bacteriol 185:1181–1189
Morii H, Nishihara M, Koga Y (2000) CTP:2,3-di-O-geranylgeranyl-sn-glycero-1-phosphate cytidyltransferase in the methanogenic archaeon Methanothermobacter thermoautotrophicus. J Biol Chem 275:36568–36574
Mulkidjanian AY, Koonin EV, Makarova KS, Mekhedov SL, Sorokin A, Wolf YI, Dufresne A, Partensky F, Burd H, Kaznadzey D, Haselkorn R, Galperin MY (2006) The cyanobacterial genome core and the origin of photosynthesis. Proc Natl Acad Sci USA 103:13126–13131
Nagano N, Orengo CA, Thornton JM (2002) One fold with many functions: the evolutionary relationships between TIM barrel families based on their sequences, structures and functions. J Mol Biol 321:741–765
Näther DJ, Rachel R (2004) The outer membrane of the hyperthermophilic archaeon Ignicoccus: dynamics, ultrastructure and composition. Biochem Soc Trans 32:199–203
Nemoto N, Oshima T, Yamagishi A (2003) Purification and characterization of geranylgeranylglyceryl phosphate synthase from a thermoacidophilic archaeon, Thermoplasma acidophilum. J Biochem (Tokyo) 133:651–657
Nichols DS, Miller MR, Davies NW, Goodchild A, Raftery M, Cavicchioli R (2004) Cold adaptation in the antarctic archaeon Methanococcoides burtonii involves membrane lipid unsaturation. J Bacteriol 186:8508–8515
Nisbet EG, Sleep NH (2001) The habitat and nature of early life. Nature 409:1083–1091
Nishimura Y, Eguchi T (2006) Biosynthesis of archaeal membrane lipids: digeranylgeranylglycerophospholipid reductase of the thermoacidophilic archaeon Thermoplasma acidophilum. J Biochem (Tokyo) 139:1073–1081
Paltauf F (1994) Ether lipids in biomembranes. Chem Phys Lipids 74:101–139
Payandeh J, Fujihashi M, Gillon W, Pai EF (2006) The crystal structure of (S)-3-O-geranylgeranylglyceryl phosphate synthase reveals an ancient fold for an ancient enzyme. J Biol Chem 281:6070–6078
Penny D, Poole A (1999) The nature of the last universal common ancestor. Curr Opin Genet Dev 9:672–677
Peretó J, López-García P, Moreira D (2004) Ancestral lipid biosynthesis and early membrane evolution. Trends Biochem Sci 29:469–477
Ruzheinikov SN, Burke J, Sedelnikova S, Baker PJ, Taylor R, Bullough PA, Muir NM, Gore MG, Rice DW (2001) Glycerol dehydrogenase: structure, specificity, and mechanism of a family III polyol dehydrogenase. Structure 9:789–802
Sacchettini JC, Poulter CD (1997) Creating isoprenoid diversity. Science 277:1788–1789
Sakasegawa S, Hagemeier CH, Thauer RK, Essen LO, Shima S (2004) Structural and functional analysis of the gpsA gene product of Archaeoglobus fulgidus: a glycerol-3-phosphate dehydrogenase with an unusual NADP+ preference. Protein Sci 13:3161–3171
Schouten S, Hopmans EC, Pancost RD, Damsté JS (2000) Widespread occurrence of structurally diverse tetraether membrane lipids: evidence for the ubiquitous presence of low-temperature relatives of hyperthermophiles. Proc Natl Acad Sci USA 97:14421–14426
Segré D, Ben-Eli D, Deamer DW, Lancet D (2001) The lipid world. Orig Life Evol Biosph 31:119–145
Shineberg B, Young IG (1976) Biosynthesis of bacterial menaquinones: the membrane-associated 1,4-dihydroxy-2-naphthoate octaprenyltransferase of Escherichia coli. Biochemistry 15:2754–2758
Silverman JA, Balakrishnan R, Harbury PB (2001) Reverse engineering the (β/α)8 barrel fold. Proc Natl Acad Sci USA 98:3092–3097
Simonson AB, Servin JA, Skophammer RG, Herbold CW, Rivera MC, Lake JA (2005) Decoding the genomic tree of life. Proc Natl Acad Sci USA 102(Suppl 1):6608–6613
Soderberg T, Chen A, Poulter CD (2001) Geranylgeranylglyceryl phosphate synthase. Characterization of the recombinant enzyme from Methanobacterium thermoautotrophicum. Biochemistry 40:14847–14854
Sterner R, Höcker B (2005) Catalytic versatility, stability, and evolution of the (βα)8-barrel enzyme fold. Chem Rev 105:4038–4055
Suvarna K, Stevenson D, Meganathan R, Hudspeth ME (1998) Menaquinone (vitamin K2) biosynthesis: localization and characterization of the menA gene from Escherichia coli. J Bacteriol 180:2782–2787
Tice MM, Lowe DR (2004) Photosynthetic microbial mats in the 3,416-Myr-old ocean. Nature 431:549–552
Vetsigian K, Woese C, Goldenfeld N (2006) Collective evolution and the genetic code. Proc Natl Acad Sci USA 103:10696–10701
Wachtershäuser G (1988) Before enzymes and templates: theory of surface metabolism. Microbiol Rev 52:452–484
Wachtershäuser G (2003) From pre-cells to Eukarya—a tale of two lipids. Mol Microbiol 47:13–22
Woese CR (1998) The universal ancestor. Proc Natl Acad Sci USA 95:6854–6859
Woese CR (2002) On the evolution of cells. Proc Natl Acad Sci USA 99:8742–8747
Woese CR, Kandler O, Wheelis ML (1990) Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 87:4576–4579
Xiong J, Fischer WM, Inoue K, Nakahara M, Bauer CE (2000) Molecular evidence for the early evolution of photosynthesis. Science 289:1724–1730
Yang S, Doolittle RF, Bourne PE (2005) Phylogeny determined by protein domain content. Proc Natl Acad Sci USA 102:373–378
Zardoya R (2005) Phylogeny and evolution of the major intrinsic protein family. Biol Cell 97:397–414
Zhang H, Shibuya K, Hemmi H, Nishino T, Prestwich GD (2006) Total synthesis of geranylgeranylglyceryl phosphate enantiomers: substrates for characterization of 2,3-O-digeranylgeranylglyceryl phosphate synthase. Org Lett 8:943–946
Acknowledgments
Given the nature of this report, we apologize for not citing or discussing many relevant contributions by a great number of authors. J.P. is grateful to Emily Cowan, Wing Lau, and Wanda Gillon for their support. We thank Dr. Masahiro Fujihashi for his assistance, and Drs. Ayeda Ayed, Ahmad Khorchid, and Michael Plevin for their comments on the manuscript. We appreciate the guidance of Drs. Niles Lehman, Martin Kreitman, and our anonymous referees. This work was supported by the Canada Research Chairs Program, the National Sciences and Engineering Research Council of Canada, and the Canadian Institutes of Health Research (E.F.P.).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Reviewing Editor: Dr. Niles Lehman
Rights and permissions
About this article
Cite this article
Payandeh, J., Pai, E.F. Enzyme-Driven Speciation: Crystallizing Archaea via Lipid Capture. J Mol Evol 64, 364–374 (2007). https://doi.org/10.1007/s00239-006-0141-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-006-0141-8