Abstract
Since the early days of molecular biology, organ and tissue regeneration represents a challenging medical goal. However, only recently the advances in the understanding of the cellular components have enabled the promise to become a reality. In this vast panorama of new technologies, stem cells have progressively established themselves as the most effective and user-friendly regenerative therapeutic tool. Scientific meetings, workshops, conferences, and forums focused on translational science of regenerative technologies are today blooming all over the world. The audience questions and, even more, the very often controversial and conflicting explanations highlight the great deal of confusion regarding this new discipline that should be considered today a real independent medical specialty, requiring long-term studies and dedication. All the technologies able to separate and concentrate the adipose tissue (AT) and the stromal vascular fraction (SVF) and their related clinical applications need to comply with a complex but still unclear regulatory frame, becoming everyday more severe and restrictive, this limiting their practical use. The aim of this manuscript is to overview the current status of the regulatory frame and few related ethical considerations and to describe the evolution in the way the adipose-derived stromal vascular fraction (SVF) is isolated, extracted, and concentrated, as well as, of the ongoing researches and related future perspectives. Considerations on the most controversial and still unclear points related to the regenerative medicine and surgery, seen from the perspective of a research group who dedicated their entire professional life to this field, are also provided.
Level of evidence: Not ratable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the early days of molecular biology, organ and tissue regeneration represents a challenging medical goal. However, the scientific progress is only recently starting to realize the hypotheses advanced. As technology and research have improved, the use of regenerative therapies to address different medical challenging situations and diseases has significantly increased.
The regenerative techniques, which have appeared and consolidated in recent years thanks to the contribution of numerous international studies and research protocols supported by a consistent and elegant bibliography, are essentially based on the use of stem cells, tissue engineering, cell reprogramming, and genetic therapy. These new therapeutic lines, originating from the correlation between the results of molecular biology and bioengineering research and their practical and clinical applications, can often be synergistically combined in strict compliance with the fundamental criteria of translational medicine, which provides for a close correlation between research, clinics, and industry.
In this vast panorama of new technologies, stem cells have progressively established themselves as the most effective and user-friendly regenerative therapeutic tool.
In an initial phase, the focus of the research was mainly on embryonic stem cells, of undoubted therapeutic effectiveness but unfortunately burdened by the well-known ethical and legislative implications that have in fact limited their use.
At the end of the 1980s, however, research was fortunately able to demonstrate the existence of adult stem cells of mesenchymal origin identified by the acronym MSCs (mesenchymal stem cells), which have demonstrated a great ability to repair and regenerate damaged tissues.
The initial discovery of this new type of stem cell in 1987 began a frantic research activity that allowed isolating MSCs from various types of adult tissues, such as the bone marrow, umbilical cord blood, placenta, amniotic fluid, synovial fluid, periosteum, and, more recently, adipose tissue.
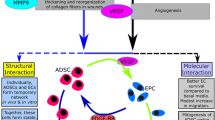
At the present day, the role of the adipose tissue is widely accepted, acting not only as energetic depot but also as real and well-organized organ rich in mesenchymal stem cell (MSC) precursors. MSCs can differentiate and self-replicate to repair damaged tissue and organs via a paracrine activity, by utilizing a variety of known and yet unspecified proteins and peptides. They are crucial in the transfer of exosomes or microvesicles containing mRNA and other signaling molecules and direct transfer of mitochondria.
Adipose tissue physiology and pathophysiology
One point still at the origin of great confusion regards the existing difference between a simple whole adipose tissue transfer (WATT) and a real regenerative procedure (RRP). Whereas the first method is mainly used for volumetric replacements, the second approach is able to repair and regenerate damaged tissues and organs.
In the first case, the harvested fat is simply re-implanted after little to no manipulation. This procedure is limited to a quick cycle of washing and decantation, as in old-style lipofilling [1, 2] or, in the best scenario, after a low-speed centrifugation, as in lipostructure [3, 4]. Conversely, to obtain a strong regenerative effect, the AT needs to undergo the second specific engineering approach able to separate, extract, and concentrate the stromal vascular fraction (SVF) containing extracellular matrix (ECM), mesenchymal stem cell (MSC) precursors, pericytes (assumed to be MSCs), a significant pool of specific and unspecific growth factors (GFs), bioactive proteins, hormones, exosomes, and other microvesicles. As a matter of fact, all these fundamental components of the AT were almost unknown until very recently.
Curiously, after that Wells [5] introduced in 1940 the capacity of the adipose tissue to act not only as energetic depot but also as a real adipose organ (AO), nothing of really innovative was published for over 60 years. Only at the beginning of the third millennium, a new interest in this line of research arose and in less than 10 years, we assisted to a real blooming of new revolutionary studies.
In 2000, Cinti [6] was the first to identify two different components in the AO: the white adipose tissue (WAT) and the brown adipose tissue (BAT), with two totally different metabolic networks and receptorial pathways. Meanwhile, the studies on AT pathophysiology of Lafontan [7] were subverting the traditional concept of cellularity. Until this moment, the concept was assessed “a posteriori” by considering the size and number of non-proliferating adipocytes, which do not proliferate in the post-natal life, and not considering precursors and progenitors, which instead can proliferate and differentiate. These precursors stay as “sleeping precursors” in a quiescent status in the AT niche for the lifetime until a specific metabolic or mechanic switch turns them from the quiescent status to the activated one, able to proliferate and differentiate. At the same time, Caplan [8,9,10] opened in 1989 a new pathway for tissue and organ repair to a new line of researches on regenerative technologies, by identifying and describing adult MSCs. Among the first consequences of these works, we recall the change in the scientific terminology of the term “autologous collagen,” until then considered compound layer of collagen and fibrous tissue from lipocyte cell walls. Due to the heterogeneous composition of the cellular layer, the term was considered obsolete and reductive and was replaced by the compound term “stromal vascular fraction.”
The early studies on MSCs were mainly focused on their isolation, expansion, and characterization in vitro. Great efforts were dedicated for identifying and localizing this population of new cells in situ.
Mesenchymal cells have then been observed in perivascular locations in almost every blood vessel in the body, on both arterial and venous nets. These cells, called pericytes for convenience, are in intimate contact with the basement membrane and surrounding endothelial cells of the microvascular net, from precapillary to small venules, and retain the expression of some specific pericyte markers, such as NG2b and CD146, that are identical to those expressed by isolated MSCs [11].
During localized injury, MSCs are released from the perivascular location, become activated by secreting bioactive molecules, and regulate the local immune response, establishing a following regenerative microenvironment. These and other observations allowed Caplan to speculate by considering all the MSCs as pericytes and opening new physiological and therapeutical perspectives, visualizing the role of MSCs acting as in vivo site-regulated “drugstores” [12]. However, until that moment, all the works conducted on adult stem cells (ASCs) were mainly focused on MSCs, derived from the bone marrow and from few other sources such as blood, the umbilical cord, and placental stroma, without considering that not only the bone marrow, but also the AT originate from the embryonic mesenchyme and contains an SVF full of MSC precursors which can be easily separated and extracted.
Focusing its attention on the implicit potential of this embryogenic evidence, in 2002, Zuk [13] identified the SVF of the human AT as a great source of undifferentiated multipotent MSCs, similar and more abundant compared with the bone marrow. These cells presented a great potential of spontaneous differentiation into the adipogenic, chondrogenic, and osteogenic lineages, all originated from the mesenchymal mesodermal layer. This has been the beginning of a new era in the way to harvest, treat, and use the AT: for the first time the adult mature adipocyte (AMA) was no more in the high spot of the stage and the term “autologous collagen” was becoming out of date. Progressively, a new star was taking its place: the stromal vascular fraction, a very heterogeneous mixture of extracellular matrix (ECM) and cells including erythrocytes, lymphocytes, fibroblasts, monocytes, macrophages, endothelial cells, and pericytes, representing an ideal source to isolate MSCs for tissue regeneration.
Since that moment, a consistent and elegant literature on this topic has been produced by many excellent colleagues including among others the following: Aust [14], von Heimburg [15], Yoshimura [16, 17], Rubin [18], Rigotti [19], and Zocchi [20,21,22,23].
The new revolutionary concepts merging from this consistent line of research totally subverted the way to approach, treat, and use the AT: not only as a natural filler for volumetric replacement but also as an excellent source, maybe the best, of regenerative components. This principle should always be used as guideline whenever it is planned and realized a fat derivate transfer.
Regulatory overview
Another important and often neglected aspect that generates concerns is the almost total lack of knowledge or, even worst, the indifference to the regulatory framework concerning the use of these new technologies. Scientists and medical professionals have the moral obligation to fully understand and comply with the existing laws before approaching any type of these new procedures.
The current status of the regulatory guidelines of regenerative technologies is fairly standard in most developed countries and, more or less, pattered on the American guidelines. Early exceptions are represented by Malaysia, Japan, Vietnam, Cambodia, Thailand, Germany, Switzerland, San Marino, and few others. After attracting unwanted charlatans eager to take advantage of their regulatory oversight, these countries have begun to re-evaluate their laissez-faire approach to regulations. In the USA, cellular therapies are currently regulated by the Food and Drug Administration (FDA), the Office of Cellular, Tissue, and Gene Therapies (OCTGT) within the FDA Center for Biologics Evaluation and Research (CBER).
Since the time the author was involved in this field of research, many changes are continuing to occur to the regulatory and compliance guidelines and are continuing to evolve over the time and thus still are not sufficiently clear. What is a clear is that a regulatory framework must exist if there is to be an organized, methodical, and scientifically based approach to ensure the safe and effective application of these new and exciting technologies. Physician scientists should be at the forefront of and leading these discussions with lawmakers and regulatory organizations and should take on part of the burden in policing rouge physicians and industries that fail to adhere to accepted standards.
At the very beginning and for a long time, there were very little FDA guidelines, except for the Section 361 of the Public Health Service (PHS) Act. This document was intended to be an inspectional tool to assist FDA investigators in distinguishing between the human cells, tissues, and cellular- and tissue-based products (HCT/Ps), commonly known as 361 products, that are regulated by the Center for Devices and Radiological Health (CDRH) as simple medical devices and those that are regulated by the Center for Biologics Evaluation and Research (CBER) as drugs and biological products, commonly known as 351 products.
In the Section 361 of the PHS Act is also outlined the basic concepts relied to the HTC products of homologous, autologous, same-day procedure, operating room (OR) facility, time frame. This is with the intent of drawing a precise dividing line between the limits of minimal-grade manipulation (MGM) and high-grade manipulation (HGM). Among the techniques listed as MGM, we can find decantation, filtration, centrifugation, mechanical disruption, enzymatic digestion, and few others, but only if the tissue is harvested, treated, and re-implanted during the same surgical session and inside the same operating room.
Among the HGM techniques, we can find characterization, expansion, cultivation, and all the other complementary laboratory techniques routinely used for cell manipulation. In this category should also fall all the above-listed MGM techniques whenever used outside the OR or in subsequent time.
Practically speaking, whatever can be obtained with a technique staying within the limits of MGM should be considered 361 Products and therefore allowed and exempted from Section 351 of the PHS Act. This grouping includes all the products that FDA has determined as not meeting the above-listed criteria and are regulated as drugs and/or biological products.
The use of all these cell derivates is not allowed in private practice and outside of strict protocols approved and supervised by ethic committees. This definition although on the surface apparently clear has been the origin of many ongoing problems related to its practical interpretation and enforcement as these simple definitions fail to consider the complexity that is medicine and biology.
Under the constant pressure of manufacturers, media, and, last but not least, scientists, FDA has tried many times to clarify and amend the Section 361 of the PHS Act, unfortunately minimal success and a worsening of the sense of confusion. Likely this concern over the increasing difficulty in interpreting and enforcing such unclear rules as well as the immediate need to place some degree of control on a rapidly expanding and unregulated stem cell industry, the FDA recently decided that it was quicker and easier to take the determination that all the autologous adipose-derived stem cell procedures and anything implying the use of MSCs should fall under the Section 351 of the PHS Act.
As a consequence of this unenlightened decision, all these 351 products require clinical trials to demonstrate their safety and efficacy in a process that is nearly identical to that required for pharmaceutical products to enter the marketplace.
The situation is comparable (but not exactly the same) to the one existing between an injectable dermal filler and a drug. The first one is considered a medical device and can hit the market very easily and with minimal or none clinical trials; the second one requires long and very expensive clinical trials, compliance with a burden of limitations, and an endless number of checks and approvals. There is no reasonable or cost-effective way to further studies or to have any hope of eventually proving clinical indications if even autologous adipose SVF is to be defined as a drug. Fortunately, this FDA resolution has currently not yet been adopted by regulatory agencies in other countries; however, there is the consistent risk that sooner or later they will adopt a similar approach.
On the top of these already very restrictive rules, AT derivates for clinical applications need to fulfill additional specific requirements according to current good manufacturing practice (cGMP) compared with those used for research applications and involve the use of certified and validated instruments and components. Moreover, in case of ASC enzymatic digestion, few additional points have to be considered.
The AT has to be harvested by a validated procedure in a certified facility. This means that, in addition to applied standard surgery room requirements, the physician’s rooms also need to be checked for suitability. All the equipment needs to be certified, and medical and nursing personnel have to receive appropriate training. To avoid contamination, the graft needs to be stored and kept in a closed sterile container, with suitable temperature maintenance and monitoring. The guidelines are set by the regulatory agencies in each individual country and are finalized to ensure the highest possible patients’ safety [22].
Faced with this complex and foggy legislative structure, a considerable laxity in the enforcement of the rules can be found. This is due either to a lack of regulatory enforcement, manpower, like in the USA, or to a certain degree of laxity in pursuing the infringement it is observed in other countries, mainly in Central and South America and in the Eastern Europe. The only exception is probably the European Community where, even if the regulatory main frame is obsolete (mostly dating from 2007), a great diligence in enforcing such regulation is usually found. The difference is probably due to the fact that in many European countries, there is an overlapping of spheres of competence between the various public bodies and police forces, this resulting in a redundancy of manpower for focusing on the enforcement of the rules.
Ethical concerns
As a consequence of this chaotic regulatory situation, several medical specialists (e.g., general practitioners, dentists, anesthesiologists, osteopaths), most of the time without an appropriate surgical training, are dangerously ignoring existing rules. Currently, there are probably thousands of doctors, clinics, or institutes in the USA, in South America, in the Far East, and in East Europe actively promoting “stem cell procedures” with MSCs, BMAC treatments, amniotic products (which are not actually “living cells” but only HGF), and cord blood–derived cells. Many of these doctors or entities are participating in “dubious” Institutional Review Board (IRB) studies promoted by commercial companies relying on “scientific networks” of practitioners participating in these so called safety studies. Meanwhile, commercial companies, often under the umbrella of newly born “Scientific Societies” bearing fancy and bombastic names, organize and sell “low-level” instructional courses of 1 or 2 days for training doctors, nurses, and office managers in order to “certify” them not only in how to use the equipment but also in how to perform the surgical procedures. This “intensive training” allows participants to choose the necessary equipment to prepare bioderivates and to build their own hands-on experience at the expense of the health of their patients, often without a consistent clinical result and leading, sometimes, to true disasters. Recently in the USA, a 77-year-old lady went blind after the injection of a “magic cocktail” of stem cells in the anterior chamber of her eyes to treat her macular degeneration.
It is very disappointing to see how many of these doctors are eager to embrace this new expanding line of therapies only as a marketing tool without really knowing much behind the science of what they are doing. A great number are approaching this new medical field pushed by media and industry for promotional and commercial reasons only, using false and unethical claims in their aggressive advertising just looking to make quick money by “cashing in” on unproven procedures not being dependent upon medical insurance reimbursement.
This explosion of “stem cell” clinics and centers or institutes all over the world is now reaching critical levels, and every day the number of “colleagues” promoting PRP or amniotic derivates as “miraculous stem cell therapies” for treating everything from back pain to erectile dysfunction is increasing.
Because of this situation, not only the medical practitioners but also the patients are often confusing what is legitimate and what is not. Unfortunately, the fall out of this situation is jeopardizing the overall reputation of this whole field of research. Those colleagues, eager to jump on this running train, should be warned about the huge potential risks related to all these new procedures, especially when performed without the appropriate training and in a total lack of scientific knowledge. This chaotic situation is unfortunately existing for every aspect of the regenerative procedures starting from the most basic and common component: the platelet-rich plasma commonly known as PRP.
A consistent literature demonstrates that platelets play a fundamental role in hemostasis and are a natural source of growth factors including platelet-derived growth factor (PDGF), insulin growth factor (IGF), vascular endothelial growth factor (VEGF), platelet-derived angiogenic factor (PDAF), and transforming growth factor beta (TGF-β) (Table 1). The release of growth factors is triggered by the activation of platelets and initiated by different substances such as thrombin, calcium chloride, and collagen.
The GFs are involved in key stages of wound healing and regenerative processes including chemotaxis, proliferation, differentiation, and angiogenesis. According to the definition of PRP, it is supposed that these growth factors are at high concentrations in the PRP. In addition to growth factors (GFs), platelets release different substances (e.g., fibronectin, vitronectin, sphingosine 1-phosphate), extremely important in wound healing. An advantage of PRP in respect to using single recombinant human growth factor delivery is the release of multiple growth factors and differentiation factors upon platelet activation [24, 25]. Most of the doctors decide to approach this new therapeutic field to boost their practice and having little or no knowledge at all of the science behind it. Many of these tools are “push button machines” supported by economic and “ready-to-use” kits. What most of these doctors know about PRP is where to buy the tools, how to open the kit, and where the “on” button must be pressed. Many of these tools manufacturers have misloaded the doctors selling them the PRP as a stem cell procedure, increasing consequently the marketing hype and worsening consumer confusion.
Regrettably, once a doctor makes an investment in a specific automated machine that produces a specific PRP type, is all he or she usually has to use not even taking into consideration that are existing many different types of PRP each one offering different features, different concentrations of GF, and different therapeutic actions for different clinical applications.
Unfortunately, most of the doctors are still using red PRP, cheaper and easier to be obtained, often with some fancy artisanal techniques, without considering that its great charge of white cells is inducing a huge cytokine activation resulting in additional inflammatory response. This is higher interfering with the regenerative action and often voiding advantages and benefits and helping to feed the false idea that “PRP often fail”. Yellow PRP (YPRP), lower concentration amber PRP (LCAPRP), high-concentration amber PRP (HCAPRP), plasma rich in GF (PRGF), and platelet lysate (PL) are offering a higher concentration of specific and unspecific GF (from 20 to 45% more) and less side effects and risk of complications.
The PL, for example, seems to offer an immediate release of the GF and an important anti-inflammatory action and therefore is particularly suitable to be added to freshly insulated SVF whenever is required to use a higher concentration of regenerative components in a very small volume, like for treating hip and shoulder joints, enthesis, and vocal cords. At present, just few automated and very expensive machines can produce this type of PRP, so its clinical use is still less common. This is a real shame because the PRP, if correctly used with the appropriate indication, can be a very powerful tool in regenerative medicine, especially in combination with other bioderivates, as better described in the final part of this article. Unfortunately, still few physicians possess the scientific knowledge and technical expertise to understand and master regenerative procedures in order to safely and effectively approach this challenging discipline.
Everyone coming from a practical surgical field without a solid background in molecular biology, including the author, has to accept to humbly bend the head and dedicate a lot of time and energy to build a basic understanding of the scientific rational behind these new technologies and every new related commercial proposal. Fortunately, an ever-increasing number of surgeons are performing regenerative procedures with seriousness and dedication, following the most updated protocols in “state-of-the art” facilities with excellent results, strictly remaining within the existing legal limits in a total respect of patient’s safety and ethic.
Authors’ approach to SVF
These preliminary considerations on the most relevant legal and ethical concerns are crucial for a better understanding of the technical aspects related to AT manipulation. Due to the existing situation in the daily practice, it is very difficult to find a compromise between the ideal technical solution and the current legal limits. Hereafter, the authors will summarize his their experience-based point of view after executing a countless number of tests, trials, experiments, successes, and failures, without any ambition to state universal guidelines or dogmas.
The authors’ group is working with the AT as a medical treatment for more than 30 years. By removing the AT in excess, they developed a new revolutionary body-contouring technique based on ultrasonic energy, in order to safely remove up to 30 l of AT in a single surgical session (ultrasonic-assisted lipoplasty (UAL)) [26,27,28]. Engineering the AT for separation and extracting its noble extracellular components had been in the authors’ mind since a long time, even before the first publications of Cinti [6] and Lafontan [7] regarding the new concepts of AT pathophysiology and AT cellularity. At the time, authors considered the middle layer as the most interesting and with a high-density ECM cellular emulsion. Curiously, the authors employed almost 15 years to understand that the most important fraction rich in MSC precursors was in the lowest layer of the syringe (Fig. 1a, b). After a deep investigation of the physical principles and the effect of the US energy on biological tissues [29], in 1988, the author’s group developed and presented their personal technique at many international meetings. They presented and published the related patented instruments (Fig. 2a, b), including the very first manual surgical centrifuge for fat ever designed, able to insulate, extract, and concentrate the autologous collagen (the previous denomination of the SVF) from the adipose tissue for facial rejuvenation [20, 21, 30] (Fig. 3a, b).
By successfully using this technique on hundreds of patients, it has been possible to detect and appreciate, on the top of the volumetric persistence, also a consistent improvement of skin texture and elasticity. Considering the AT a powerful regenerative agent, our group started to fully dedicate his activity by investigating the AT physiology and cellularity, in order to develop new techniques and protocols able to separate and extract the regenerative components.
In July 2006, at the ISAPS International Congress in Rio de Janeiro, authors were the first to present his studies on adipose tissue–derived stem cells and a 4-year follow-up on its clinical applications for breast reconstruction and facial rejuvenation [31]. Until that moment, the overall understanding of the importance of the SVF, the AT, and related intrinsic components was, among plastic surgeons, still very poor. The authors’ conference arose a long and heated debate but also positive and constructive comments [20].
Since that time, the authors’ group dedicated a great deal of energy and resources to constantly improve the technique for extracting, separating, and concentrating the SVF from AT in the aim of increasing patients’ safety and results.
Hereafter, a short compendium about the Authors evolution. The first technical step is to treat the freshly harvest AT with a process of lipocondensation [23].
Lipocondensation
Lipocondensation separates and removes the oily contents from the AT through an in vitro preliminary treatment (before the re-implantation) of the adult mature adipocytes with low survival rates (TGs—80% of its total volume) (Fig. 4) preserving SVF integrity and viability. Subsequently, the harvested decanted fat is transferred in fat processing units (FPU) for the condensation phase. The FPU are then placed in a special machine named as Lipokit (Medi-Khan, Seoul, Korea). Here they undergo to a cycle of high-speed centrifugation (2500g) for 9 min. Due to centrifugal force, the metal plunger of the FPU, weighting 32 g, applies a pressure of 120 kg/cm2 on the fat’s surface. Under this high mechanical stress, the larger portion of adult adipocytes is destroyed and the oily content (TGs) deposits in the upper part of the FPU, totally separated from the other components by the plunger (Fig. 5a–c).
a, b After the condensation process, 60 cm3 of already decanted fat is reduced to less than 30 cm3. Adult mature adipocytes are destroyed while stromal vascular fraction (SVF) is preserved. The tissue is now ready for re-implantation. c Triacylglycerols (TGs) are collected in the upper layer and easily discarded
After the condensation process, 60 cm3 of already decanted fat is reduced to less than 30 cm3. While adult mature adipocytes are destroyed, SVF integrity is totally preserved. At this point, the SVF must be separated and extracted from the condensed fat. For many years, the author’s group was treating the condensed fat exploiting the enzymatic digestion with collagenase. The handling protocols and the different washing cycles have been constantly improved (from just one cycle with Ringer’s lactate in the first phase of his experience up to four cycles with Ringer, AB, and Warton solution at the end) to get a final product theoretically free from any remnant enzymatic content. Spectrophotometric, fluorometric, and chemiluminescence assays can randomly test the residual enzymatic activity by assessing the quantity of active enzyme in the processed tissue. Very few groups can count on the cooperation of an in-house enzymologist for conducting those tests in total safety. Therefore, the current data are still controversial and discontinuous.
In addition, even the use of collagenase in the operating room is controversial: its borderline situation regarding laws and regulations, the difficult manipulation, its potential (but never confirmed) mutagenic activity, and its high costs are important issues that need to be considered. The only reason, and seldomly an advantage, of using collagenase is represented by its intrinsic ability to break down the AT to the single adipocyte. This result cannot be reached by any of the different mechanical devices present on the market today able to generate cell clusters only. It is important to highlight that there is not a strict scientific correspondence between the isolation methods and the dimensional subdivision of the different cell clusters. Names like millifat, microfat, nanofat, and recently picofat are only labels used for commercial strategies without any scientific rational.
For all the above reasons even in a lack of precise laws, since March 2014, the authors stopped the use of collagenase in the operating room. The authors tried every possible technical tool to continue to insulate and condensate the SVF from the adipose tissue. The main point was to elaborate a technique able to preserve and respect the SVF integrity and vitality still remaining within the limits of an MGM. Alternative enzymatic approaches with trypsin and lecithin were first tried with little advantage in the manipulation and without consistent benefits.
Afterwards, authors tried different means with modest results to mechanically disrupt the AT with choppers, emulsifiers, bead crushers, disruptors, infrared and led light, and shock waves. In June 2015, after a long experimentation and using his long-term and extensive experience on the ultrasonic energy for body contouring, authors started to use a closed and sealed process of sonication to emulsify the adipose tissue in vitro.
AT sonication
The instrumentation and the protocols used in the past for ultrasonic-assisted lipoplasty were modified to adapt them to the new technical needs. Using a special titanium probe, the condensed fat has to be emulsified inside the same FPU with ultrasonic energy (30 s every 60 cm3) in order to disrupt any remnant adult adipocytes, preserving the stromal vascular fraction integrity and vitality.
Then, the separation of the SVF from all the other cellular debris is obtained by a low-speed centrifugation (2 min at 1000 rpm or 1300 g850g) (Fig. 6a, b). At the end of the process, the SVF deposits in the lower part of the FPU (red pellet) and can be carefully removed with a micropipette. This fraction represents no more than 3% of the total volume of the processed fat. It must be weighed that also the sonication process allows obtaining only microclusters of cells and no breakdown to the AT to the single cell. Consequentially, the cell sorting of the treated tissue after expansion and cultivation can result 15–20% poorer. This technique is rapid, efficient, and reliable and if well performed is ensuring the preservation of the SVF integrity and vitality (Table 2). The only negative aspect is the necessity to have an expensive setup not easy to be found in all medical facilities and an ultrasonic generator specially adapted to this specific application and approved for surgical use (class 2A).
This is certainly a limit to its routine use besides those doctors with high-end practices where costs of the surgical equipment are not a major concern.
Any surgical technique should respect the well-known “FRT Gold Principle” (feasible, repeatable, teachable) and if it is too expensive to be universally used is not falling in this category. Therefore, authors are still evaluating and testing easier and more affordable systems in cooperation with labs and industries for merging with new consistent alternatives. At present, it is possible to find in the market more than 30 licensed and registered systems able to insulate and separate the SVF from the AT. This at least is the producers’ claim often not supported by consistent data. Nineteen of them are enzymatic-based systems: while seventeen are using collagenase or collagenase as agents, two of them use trypsin and lecithin. On the other hand, there are twelve non-enzymatic-based systems using mechanical means and different mechanical forces. Unfortunately, not all the systems are closed systems, which should be a basic prerequisite for ensuring a sterile insulation of the derivates unless performed in a clean-room facility which, theoretically, is not falling in the MGM criteria. Probably the best ever published article on this topic, by Oberbauer [32], was analyzing in detail many of these systems, both enzymatic and mechanic, offering an excellent synthesis of the existing situation. Each method or system presents different advantages and disadvantages, and probably, the ideal solution has not been found yet.
Many groups are today working on the same line of research a great step forwards have been recently done with the manual microfragmentation of the AT with microblades.
Microlyzers: a new non-enzymatic SVF insulation technique
To date, still remaining within the frame of freshly isolated SVF, we have been able to obtain very promising results by using a technique of microfragmentation with a new system using special sharpened edge microblades with honeycomb design (Microlyzers from T-Lab) (Fig. 7a–d).
a–d As shown in these figures, the cartridge of Microlyzers has two female-to-female luer-lock adapters (a), able to connect two syringes of 10 cm3 volume. Inside of these adapters, there are particular blades of different sizes, 2400, 1200, and 600 μm (b). The tissue must be transferred between the two syringes from 3 to 5 times to cross the blades, in order to fragment the sample (c). The blades must be used from largest to smallest ones until the desired consistency and viscosity are achieved
Microlyzers are closed filtration systems used for the microfragmentation of adipose tissue. The cartridge has 2 female-to-female luer-lock adapters to connect two 10-cm3 syringes for adipose tissue transfer and can be used with blades of different sizes, 2400, 1200, and 600 μm. The tissue is manually transferred back and forth between syringes from 3 to 5 times. The blades must be used in the order from largest to smallest size until the desired consistency and viscosity are achieved.
We first conducted experimental studies in vitro to check cell counts and vitality of the biomaterial obtained.
From April 2018 to June 2019, we performed more than 200 tests on AT samples harvested from different body areas.
We compared 3 different types of protocols using microlyzation alone or associated to vibration and/or shaking and testing the components with conical and tubing approaches.
The tubing approach led us to have similar results around 2.7–2.8 million/ml of nucleated cells using a Luna-Stem counter from South Korea. Dilution factor was set at 1.11 due to sampling material (18 ml) and dye (2 ml) ratios for cell counting, and the viability data has been determined to be close to 90% in all samples.
The pellet formation, as expected, showed a higher count even in re-suspended milieu, respectively 5.5 million and 7.92 million of nucleated cells per milliliter.
With the characterization studies conducted with flow cytofluorometry on freshly insulated samples, 3.8% of CD90- and CD105-positive cells (mesenchymal cells) have been found.
We also conducted some test of expansion and cultivation of the obtained material, and the cell population showed high activity reaching, after the 3-day cycle in 20 days, levels of 60% of adherent cells.
These findings are very promising and supportive for the efficiency and viability of this new technique that is offering a very cost-effective option, desired consistency, and viscosity of the adipose tissue eliminating the fibrotic remnants and even high nucleated cell yields.
At present, this is our approach of choice for all cases where small/medium volume of fresh SVF is needed especially combined with other bioactive components.
New perspectives in regenerative surgery
A great deal of ongoing researches is constantly opening new perspectives to expand and improve practical and clinical applications of regenerative procedures. The authors’ group is currently investigating several promising options.
The bioactive composite grafts
A very interesting new perspective for improving transplanted cell survival is represented by the bioactive composite grafts (BACG), a revolutionary strategy to approach and realize regenerative therapies. The main concept behind this new philosophy is to focus the attention on the recipient site that can be activated to become a real bioreactor. A “Taylor-Made Regenerative Mixture,” containing multiple components that has been proven to be way more effective in inducing and supporting regenerative processes if compared to a single component graft.
The protocols of this new therapeutic strategy containing pericytes and MSC precursors are based on the use of freshly insulated AT SVF, GF from blood derivates (not simple PRP but PRGF or PRF), and other specific and unspecific biocatalyzers, including bioactive proteins, amino acids, and vitamins (Table 3). The rational for the proposed use of biocatalyzers is that all cell culture media contain a multitude of components and, in most cases, a complete medium consists of a chemically well-defined basal medium with the addition of a variable quantity of defined additives. Although the basal medium culture contains low molecular weight substances such as inorganic ions, amino acids, vitamins, and other components (e.g., glucose and pyruvate), this composition is often not enough for the in vitro growth of mammalian cells. In addition, high molecular weight supplements as proteins have to be added to fulfill cell requirements. Besides proteins (serum), these supplements may also contain peptides, lipoproteins, phospholipids, or lipids. Therefore, the addition of some specific or unspecific biocatalyzers to the regenerative mixture helps to the natural stimulation and acceleration of cells’ recruitment towards some specific cellular lines or supports the cell’s intake in the recipient site. At present, the most valuable biocatalyzers are represented by bioactive proteins (BP), amino acids (AA), and vitamins (VIT).
Bioactive proteins
To stimulate cells’ recruitment (MSC progenitors) in the AT towards a specific cellular line, it is possible to add to the regenerative mixture a specific morphogenetic protein able to induce the formation of some specific mesenchymal cells. For example, the addition of 1/2000 IU of bone morpho protein 2 (BMP2) can stimulate the recruitment into the chondrogenic lineage and consequently treat osteoarthritis [33].
Amino acids
Amino acids are the raw material that support protein synthesis and have a crucial role in the cultivation of mammalian cells. This importance of amino acids was soon realized after the development of the first cell lines. From that moment, a mixture of amino acids has been always supplied to cultured cells. Cells need twelve essential l-amino acids: arginine, cystine, leucine, isoleucine, lysine, methionine, phenylalanine, threonine, tryptophan, histidine, tyrosine, and valine. The addition to the graft of a variable quantity of these components inside of an amino acidic cocktail can actively support cells’ intake and growth [34].
Vitamins
Vitamins mainly act as cofactors or protein groups for many enzymes. They are essential for cell function and metabolism. The absence of vitamins in culture may lead to decrease cell growth, death, or loss of function. The most active vitamins are as follows: biotin, folate, nicotinamide, pantothenic acid, riboflavin, thiamine, and vitamin B12 [35]. Although these components have very low concentrations, the availability of vitamins is extremely important for cells, which are usually not able to synthesize them. Adding all or some of these components to the regenerative mixture is helping to overcome the metabolic stress and supporting cell survival. All the components are mixed following different proportions and percentages. The two biological derivates, SVF and enriched PRP, constitute the most relevant part of remix regeneration mixtures (RRM). These two main components are usually mixed with a ratio of 5:1 (e.g., for 10 cm3 of RRM, 8 cm3 of adipose-derived SVF and 2 cm3 of PL). However, this proportion depends on the clinical situation, on the therapeutic needs, and on the volume of the recipient site. If necessary, biocatalyzers are added only on a small proportion to support a stronger regenerative action and currently not in post-oncologic applications. Due to the poor understanding of the oncogenic role of cancer’s stem cells, authors are limiting the use of this powerful regenerative media. These findings are supported by a consistent cohort of testing trials that assess the recruiting activity, sorting and cellularity, and especially the improved regenerative action on the recipient sites. All these components are mimicking the action of an “in vivo DMEM medium,” and the recipient site in this case is acting as a real bioreactor. In parallel with the development of the therapeutic strategies, it has also been structured an international platform finalized to identify, analyze, and select cutting edge technologies in each specific field of regenerative medicine, merging with the most efficient protocols and guidelines tailored for every specific clinical need and every anatomic district, called REMIX (REgenerative MIXture).
The multilineage-differentiating stress-enduring cells
The multilineage-differentiating stress-enduring (MUSE) cells are another component of the adipose tissue newly discovered in 2010 by scientists of the Tohoku University (Sendai, Japan). They are currently classified as endogenous non-cancerous pluripotent stem cells and represent around 1–3% of MSCs [36]. Since the beginning, they were considered an alternative source of human-induced pluripotent cells (iPS cells) with high generation efficiency naturally present inside of mesenchymal tissues. In addition to their capacity to self-renew and to differentiate into cells representative of all three germ layers, MUSE cells can differentiate themselves into tissue-specific cells and repair damaged tissues. Because of all of these capacities, MUSE cells efficiently home into damaged tissues and lead to soft tissue recovery and regeneration [37]. During their biological life, stem cells undergo different intrinsic (e.g., DNA damages) and extrinsic (e.g., chemical and physical) stresses. As seen in different primitive organisms, MUSE cells are stress-tolerant cells which present highly conserved cellular mechanisms. In physiological conditions, MUSE cells exist in a quiescent state with effective DNA damage checkpoints and DNA repair mechanisms. An alteration of the local cellular microenvironment can activate these stress-tolerant cells individually and generate different downstream effects. In contrast to embryonic stem and induced pluripotent stem cells, MUSE cells exhibit low telomerase activity, a normal karyotype, and do not undergo tumorigenesis once implanted in SCID mice [37]. Furthermore, the level of senescence and apoptosis is markedly lower compared with non-MUSE cells.
At present, there is great scientific interest in understanding the mechanisms underlying the activation and the different capacities of these cells. Probably, the ability of MUSE cells to act in difficult microenvironment conditions is due to their efficient and constant cooperation with monocytes and macrophages, their quick and efficient sensing of DNA damage, and the following activation of DNA repair systems. Lastly, MUSE cells could also be involved in immunomodulation and antigen response, which could contribute to tissue regeneration and functional repair in vivo.
The adipose tissue as the main source of MUSE cells can be considered especially when harvested from specific anatomic areas like the anterior and lateral faces of the tights.
For these reasons, there is the necessity to better comprehend the mechanism of actions of MUSE cells in tissue regeneration and the stimuli that could lead differentiation of MUSE cells. To try to clarify this aspect, a preliminary proteomic analysis of MUSE cells isolated from different adipose depots was performed. This study confirmed that MUSE cells are sensible to the stress and especially to oxidative stress occurring in adipose tissue, but also to the inflammation with high stimulation of the immune system. Surely inflamed or damaged tissues are characterized by a higher presence of MUSE cells, but their role and the differentiation pathways are still poorly understood.
More accurate studies of MUSE cells’ genome and proteome are necessary to promote a future treatment in which these cells could be selectively isolated and injected. Their pluripotency associated with low telomerase activity and low tumorigenicity, compared with the multipotent mesenchymal stem cells, ensures the regeneration of higher number of tissues with also higher safety. At the same time, researchers must focus their studies also on the interaction among MUSE cells and other cells, particularly monocytes and macrophages that could be playing the role of first mediators in the recruitment and the activation of MUSE cells, when tissues are damaged.
The regenerative medicine could be highly improved by the comprehension of the above-described mechanisms by stimulating the MUSE cell population in counteracting the tissue aging and inflammation processes, at the base of many different pathologies.
The current role of exosomes in regenerative medicine and surgery
In recent years, exosomes have become increasingly popular in the medical field and scientific community due to their widespread distribution, their possible functions as biomarkers of disease, and their great potential to be applied as therapeutic agents. Exosomes are nanovesicles measuring from 50 to 100 nm in diameter and have recently been identified as vital mediators of paracrine communication [38, 39]. It has also been discovered that these nanovesicles are widely biologically distributed: they have already been found in almost all types of body fluids, including saliva [40], milk [41], amniotic fluid [42], blood [43], and urine [44]. Exosomes are small membrane vesicles that originate from the inside budding of the late endosomal membrane. These nanovesicles are typically spherical or have a dish morphology; they are enveloped by a lipid bilayer enriched in cholesterol, sphingomyelin, and ceramide; they have a density of 1.13–1.19 g/ml; and their membrane is enriched by protein markers (tetraspanins, TSG101, Hsp70) [45]. Exosomes are secreted by almost all of the living cells that have been examined so far, including normal epithelial cells, tumor cells [46], T and B cells [47], mast cells, and dendritic cells [47, 48].
Exosomes contain special biomolecules, functional proteins, and nucleic acids, including microRNAs (miRNAs), messenger RNAs (mRNAs), and even DNA [49].
Initially, exosomes were regarded as useless cellular waste, but it has been recognized that they have many important cellular functions. After being released, exosomes can act upon target cells in the vicinity of the parent cells in a paracrine manner, and they can also enter biological fluids, to be delivered to target cells far from the secreting cells, similar to an endocrine process [50]. When exosomes are absorbed by specific target cells, the exosomal contents, especially miRNAs, will mediate numerous biological processes [51]. Exosomes have been predominately studied in their roles in cancer progression and immunoregulation [52]. Also, biomolecules in exosomes might be applied as biomarkers for disease diagnosis, prognosis, and even injury conditions since because their levels or contents might change following the occurrence of some diseases or injuries [53]. Their functions have already been widely explored, and lately their potential for regulating tissue repair and regeneration has gained attention, particularly in cutaneous repair and regeneration [54]. The main evidence supporting this originates from investigations focusing on mesenchymal stem cell (MSC) transplantation for tissue regeneration. Currently, it is believed that MSCs achieve a therapeutic effect in vivo mainly through paracrine signaling [54, 55]. They can release biologically active molecules that affect the proliferation, migration, and survival of the neighboring cells. Many studies have reported that MSC-derived conditioned medium promotes cutaneous regeneration [56]. Exosomes are the main bioactive vesicles responsible for the paracrine effects of MSCs; they in fact regulate many physiological and pathological processes by affecting the survival, proliferation, migration, and gene expression of recipient cells and by reprograming targeted cell behaviors.
On this evidence it could be possible to adopt a cell-free therapy utilizing paracrine factors, such as exosomes, to promote tissue repair and regeneration, which would avoid the risks associated with direct stem cell transplantation, such as teratomas, immune rejection, and the reduced regenerative capacity of engrafted cells [57]. Numerous preclinical studies have confirmed that MSC exosomes play a key role in tissue regeneration and repair, particularly in cutaneous wound healing [58]. MSC exosomes participate in each phase of the cutaneous wound healing processes by delivering various molecules, such as trophic factors, functional proteins, and RNAs, including mRNA and miRNAs. However, their full functions remain still unclear. It is anyway thoroughly believed that MSC exosome–mediated therapy could play an essential role in providing cutaneous regeneration and repair after pathological damage.
The 430-nm LED photobiomodulation: cellularity and replication effects
As mentioned before, Lafontan [59] subverted the consolidated pathophysiology of non-proliferating adipocytes. Considering the local vascular network of the AT, the in vitro studies of Lafontan demonstrate that cell progenitors stay as quiescent “sleeping precursors” within the adipose tissue niches until a specific metabolic or mechanical trigger turns them from the quiescent status to the activated one, able to trigger cell proliferation and differentiation. At present, there are cell processing procedures leaded by characteristic devices (e.g., AdiLight 1®; Adistem Ltd., Hong Kong) able to activate the quiescent adipose stem cells through a 430-nm LED photobiomodulation (PBM). After harvesting, centrifuging, and modulating the lipoaspirated fat sample with AdiLight 1®, the isolated stem cells become fully functional and can immediately return to the patient through an IV [60].
To date, there is great interest in the use of photobiomodulation therapy (PBMT), defined as a non-ionizing photonic energy able to induce photochemical changes within cellular structures. RA Brochetti et al. [36] are currently investigating the possibility of reducing chronic lung fibrosis (LF) by treating the damaged tissue with PBMT. Their results showed that PBMT significantly reduces the number of inflammatory cells in the alveolar space, interstitial thickening, collagen production, and both static and dynamic pulmonary elastance. From the molecular point of view, authors observed reduced inflammatory parameters (e.g., IL-6) and CXCL1/KC released from pneumocytes and fibroblasts in culture. The same group reached, the year after, encouraging conclusions regarding the stimulation of neuroprogenitor cells by PBMT in the treatment of neurological disorders. Their results indicate that PBM significantly enhances neurogenesis and synaptogenesis after ischemic stroke. In addition, there is a robust suppression of reactive gliosis and production of pro-inflammatory cytokines. Altogether, PBM could improve positively the neuronal microenvironment, representing a promising therapeutic tool for neuronal repair following ischemic stroke.
From the cellular and molecular point of view, visible red and near-infrared light energies (wave length 430 nm) are mainly absorbed by mitochondria. The mitochondrial enzyme cytochrome oxidase c, acting as a chromophore, accepts the photonic energy deriving from photobiomodulation, causing different downstream effects (e.g., production of ATP, NO, and mild oxidants, activation of cellular repair and healing mechanisms) with significant impact on cellularity and differentiation. Considering the importance of photobiomodulation in the course of evolution, we expect a fundamental role also within the different molecular mechanisms involved in our laboratory and clinical experiments.
The acellular adipose matrix
The injectable tissue-engineered adipose tissue substitute, ready to be used “off the shelves,” delivers stem cells as an etheric implant, offering meanwhile the capability of filling irregular defects and stimulating a natural soft tissue regeneration. This technology can reach great value in plastic, reconstructive, and esthetic surgeries and in all the medical specialties where a strong regenerative action is required. In this direction, a promising option is represented by the extracellular matrix (ECM), a naturally derived proteinaceous biomaterial largely free of any potential immunogenic cellular content. This substance preserves its tissue-specific structural and functional components and mediates tissue regeneration without any artifacts. In order to be suitable for clinical applications, the AT must be decellularized by which cells are discharged from tissues and purified from DNA and viral charges. It is crucial to preserve all of the essential cues for cell homeostasis in its three-dimensional structure and its extracellular matrix components. An example of acellular matrix is the acellular dermal matrix (ADM), a soft tissue substitute derived from decellularized donated human skin introduced in the market in 2007 [61]. Even if its utility has been largely demonstrated in breast reconstructions, burns, and wound management, the very high costs still constitute a problem for the routine use in plastic surgery. Moreover, due to lack of pericytes and growing factors, its regenerative activity is very limited. The adipose tissue represents the ideal source of extracellular matrix (ECM) containing collagen (type I, II, IV, and VI), laminin, and fibronectin. In addition, its stromal vascular fraction (SVF) contains a huge number of pericytes and a rich pool of specific and unspecific GF. Because of the high content of triglycerides (TGS) and their low density, the decellularization of the AT is consistently different from any other tissues and requires a preliminary step of delipidation [62]. This process avoids the foaming of TGS, an inconvenience that interferes with the subsequent technical steps of decellularization.
Deeply involved in adipose-derived stem cell (ADSC) research and technologies and after a fair experience with acellular dermal matrix (ADM) for breast reconstruction, in February 2014, authors started to better investigate the possibility of insulating a similar material from the adipose tissue with a reliable and efficient technique [62]. In October 2014, he visited the Institute of Bio Materials of the Vietnam National University of Science (VNUHCM) in Ho Chi Minh City. This Institute is currently directed by Prof. Tran Le Bao Ha, a world-renowned molecular biologist with a great deal of experience on decellularization protocols of different types of tissues for therapeutic use [63,64,65]. In January 2015, they joined their mutual expertise and started a common research project in this field under the coordination and direction of this author. After 3 years of intensive common work, a new reliable, efficient, and affordable mechanic/detergent-based method of decellularization of the AT able to produce a very pure acellular adipose matrix (AAM) free from cellular components and with minimal quantities of DNA was ready (Figs. 6 and 7). After this partnership, the laboratory phase is now completed, and the project is ready for the industrial phase, in order to standardize the production and comply with registration and laws. Altogether, AAM seems to be a very promising tool, able to implement and improve fat transfer outcomes. The constant research and innovation will allow in a short future to make this procedure as a stand-alone alternative for tissue filling with great regenerative potential.
Conclusions and outlook
The considerations expressed in this manuscript, based on the 30-year authors’ group experience in this specific field, should allow a better understanding of the most important, controversial, and still unclear points related to this very promising and challenging discipline.
Today, regenerative medicine and surgery are an irreplaceable therapeutic device for restoring function and integrity to damaged tissues and organs, exploiting the extraordinary potential for regeneration inherent in the body itself in a biologically active, autologous, and minimally invasive way.
It is rightly considered the most innovative therapeutic solution of the twenty-first century, and for its peculiarities, it is a discipline of last generation certainly placeable in the broad and current concepts of “well-being” and eubiotics, basic rights that must be ensured to every human being.
The initial reservations of researchers on therapies with mesenchymal stem cells have been largely overcome by a systematic review of clinical studies that could unequivocally confirm that MSCs are safe and that they can regenerate damaged tissues in the microenvironment of the receiving site, thus constituting a new and irreplaceable tool for healing, not for curing multiple diseases including chronic ones.
The goal that regenerative medicine sets itself can be achieved in a very short time if there is the will and the ability to create a “governance of research” focused to stimulate an interdisciplinary collaboration that involves all the main actors of the scientific community, from medical professionals to those of basic science, molecular biology, bioengineering, economics, sociology, and communication where the plastic surgeons, as historic experts in supplying the most abundant, reliable, and efficient source of regenerative components, are playing a predominant role.
Today physicians and scientists are fortunate to have this unique opportunity to take part in this age of the regenerative medicine epoch coinciding at a time when tools such as translational research and genomics are providing a greater understanding of cellular-based medicine. The following decades will see changes that will forever alter medicine and the historical role of the physician. Technology has been shown to rapidly transform our social and information management systems, and physicians must prepare themselves to be leaders in this revolution to ensure that the appropriate ethical and scientifically based approaches are pursued and rewarded. It is an exciting opportunity to help usher in this new age, and hopefully, such advances will be embraced not feared and physicians will find a new sense of professional invigoration and a reigniting of their youthful sense of awe and wonder in the pursuit of this latest medical milestone.
References
Illouz Y-G (1986) The fat cell “graft”: a new technique to fill depressions. Plast Reconstr Surg 78(1):122–123
Fournier P (1985) Microlipoextraction et microlipoinjection. Rev Chir Esthet Lang Franc 10:36–40
Coleman SR (2006) Facial augmentation with structural fat grafting. Clin Plast Surg 33(4):567–577
Coleman SR (2006) Structural fat grafting: more than a permanent filler. Plast Reconstr Surg 118(3S):108S–120S
Wells HG (1940) Adipose tissue, a neglected subject. J Am Med Assoc 114(22):2177–2183
Cinti S (2000) Anatomy of the adipose organ. Eat Weight Disord 5(3):132–142
Lafontan M (2016) The adipose tissue: development, physiology and pathophysiology. Intern Stand Press Book Chapt. 6
Caplan AI (2005) Mesenchymal stem cells: cell–based reconstructive therapy in orthopedics. Tissue Eng 11(7–8):1198–1211
Caplan AI (1994) The mesengenic process. Clin Plast Surg 21(3):429–435
Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9(5):641–650
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Bűhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3(3):301–313
Caplan AI (2008) All MSCs are pericytes? Cell Stem Cell 3(3):229–230
Zuk PA, Zhu M, Ashjian P, de Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295
Aust L, Devlin B, Foster SJ, Halvorsen YDC, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM (2004) Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 6(1):7–14
von Heimburg D, Hemmrich K, Haydarlioglu S, Staiger H, Pallua N (2004) Comparison of viable cell yield from excised versus aspirated adipose tissue. Cells Tissues Organs 178(2):87–92
Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K (2006) Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol 208(1):64–76
Yoshimura K, Suga H, Eto H (2009) Adipose-derived stem/progenitor cells: roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen Med 4(2):265–273
Rubin JP, DeFail A, Rajendran N, Marra KG (2009) Encapsulation of adipogenic factors to promote differentiation of adipose-derived stem cells. J Drug Target 17(3):207–215
Rigotti G, Marchi A, Galié M, Baroni G, Benati D, Krampera M, Pasini A, Sbarbati A (2007) Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 119(5):1409–1422
Zocchi M (1989) Remodelage facial complet par implants de collagene autologue. Revue Française de Chirurgie Esthétique 14:63
Zocchi M (1990) Méthode de production de collagène autologue par traitement du tissu graisseux. Journal de Médecine Esthétique et de Chirurgie Dermatologique 17(66):105–114
Zocchi M, Zuliani F (2008) Bicompartmental breast lipostructuring. Aesthet Plast Surg 32(2):313–328
Zocchi ML, Zocchi L (2017) Large-volume breast fat transfer: technical evolutions and safety aspects based on over 800 cases and 26 years of follow-up. Eur J Plast Surg 40(5):367–382
Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR (1998) Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85(6):638–646
Wang H-L, Avila G (2007) Platelet rich plasma: myth or reality? Eur J Dent 1(4):192–194
Zocchi M (1992) Ultrasonic liposculpturing. Aesthet Plast Surg 16(4):287–298
Zocchi M (1993) Clinical aspects of ultrasonic liposculpture. Perspect Plast Surg 7(02):153–172
Zocchi M (1995) The ultrasonic assisted lipectomy, instructional course. In: Annual meeting of the American Society for Aesthetic Plastic Surgery, San Francisco
Zocchi ML (1999) Basic physics for ultrasound-assisted lipoplasty. Clin Plast Surg 26(2):209–220 vii
Zocchi M (1989) Les implants mixtes de collagène autologue dans le remodelage facial. Journal de Médecine Esthétique et de Chirurgie Dermatologique 16(63):191–201
Zocchi M (2006) New perspectives in plastic surgery: Adiposederived stem cells (ADSC.). In: Abstract Book of the ISAPS World Congress of Rio de Janeiro, August
Oberbauer E et al (2015) Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. Cell Regen 4(1):7
Khan SN, Lane JM (2004) The use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in orthopaedic applications. Expert Opin Biol Ther 4(5):741–748
Salazar A, Keusgen M, von Hagen J (2016) Amino acids in the cultivation of mammalian cells. Amino Acids 48(5):1161–1171
Büntemeyer H, Lehmann J (2001) The role of vitamins in cell culture media. In: Animal cell technology: from target to market. Springer, Berlin, pp 204–206
Fisch SC, Gimeno ML, Phan JD, Simerman AA, Dumesic DA, Perone MJ, Chazenbalk GD (2017) Pluripotent nontumorigenic multilineage differentiating stress enduring cells (Muse cells): a seven-year retrospective. Stem Cell Res Ther 8(1):227
Kuroda Y, Wakao S, Kitada M, Murakami T, Nojima M, Dezawa M (2013) Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat Protoc 8(7):1391–1415
Collino F, Pomatto M, Bruno S, Lindoso RS, Tapparo M, Sicheng W, Quesenberry P, Camussi G (2017) Exosome and microvesicle-enriched fractions isolated from mesenchymal stem cells by gradient separation showed different molecular signatures and functions on renal tubular epithelial cells. Stem Cell Rev Rep 13(2):226–243
Zhang Y, Yu M, Dai M, Chen C, Tang Q, Jing W, Wang H, Tian W (2017) miR-450a-5p within rat adipose tissue exosome-like vesicles promotes adipogenic differentiation by targeting WISP2. J Cell Sci 130(6):1158–1168
Palanisamy V, Sharma S, Deshpande A, Zhou H, Gimzewski J, Wong DT (2010) Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS One 5(1):e8577
Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M, Neve EPA, Scheynius A, Gabrielsson S (2007) Exosomes with immune modulatory features are present in human breast milk. J Immunol 179(3):1969–1978
Keller S, Rupp C, Stoeck A, Runz S, Fogel M, Lugert S, Hager HD, Abdel-Bakky MS, Gutwein P, Altevogt P (2007) CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int 72(9):1095–1102
Caby M-P, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C (2005) Exosomal-like vesicles are present in human blood plasma. Int Immunol 17(7):879–887
Pisitkun T, Shen R-F, Knepper MA (2004) Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci 101(36):13368–13373
Théry C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9(8):581–593
Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L (2001) Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 7(3):297–303
Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, Boudaly S, Mécheri S (2003) Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol 170(6):3037–3045
Théry C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1999) Molecular characterization of dendritic cell-derived exosomes: selective accumulation of the heat shock protein hsc73. J Cell Biol 147(3):599–610
Zhou Y, Zhou G, Tian C, Jiang W, Jin L, Zhang C, Chen X (2016) Exosome-mediated small RNA delivery for gene therapy. Wiley Interdiscip Rev RNA 7(6):758–771
Mead B, Tomarev S (2017) Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl Med 6(4):1273–1285
Stahl PD, Barbieri MA (2002) Multivesicular bodies and multivesicular endosomes: the “ins and outs” of endosomal traffic. Sci Signal 2002(141):pe32
Rong L et al (2016) Immunosuppression of breast cancer cells mediated by transforming growth factor-β in exosomes from cancer cells. Oncol Lett 11(1):500–504
Panich T, Chancharoenthana W, Somparn P, Issara-Amphorn J, Hirankarn N, Leelahavanichkul A (2017) Urinary exosomal activating transcriptional factor 3 as the early diagnostic biomarker for sepsis-induced acute kidney injury. BMC Nephrol 18(1):10
Caplan AI, Correa D (2011) The MSC: an injury drugstore. Cell Stem Cell 9(1):11–15
Pittenger M (2009) Sleuthing the source of regeneration by MSCs. Cell Stem Cell 5(1):8–10
Kusindarta DL, Wihadmadyatami H, Fibrianto YH, Nugroho WS, Susetya H, Musana DK, Wijayanto H, Prihatna SA, Wahyuni AETH (2016) Human umbilical mesenchymal stem cells conditioned medium promote primary wound healing regeneration. Vet World 9(6):605–610
Jing H, He X, Zheng J (2018) Exosomes and regenerative medicine: state of the art and perspectives. Transl Res 196:1–16
Wu P, Zhang B, Shi H, Qian H, Xu W (2018) MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy 20(3):291–301
Lafontan M (2014) Adipose tissue and adipocyte dysregulation. Diabetes Metab 40:16–28
Illouz Y-GS, Stereodimas A (2011) Adipose stem cells and regenerative medicine. Springer, Berlin
Wu I, Nahas Z, Kimmerling KA, Rosson GD, Elisseeff JH (2012) An injectable adipose matrix for soft tissue reconstruction. Plast Reconstr Surg 129(6):1247–1257
Fu R-H, Wang YC, Liu SP, Shih TR, Lin HL, Chen YM, Sung JH, Lu CH, Wei JR, Wang ZW, Huang SJ, Tsai CH, Shyu WC, Lin SZ (2014) Decellularization and recellularization technologies in tissue engineering. Cell Transplant 23(4–5):621–630
Bao H (2009) Decellularized human amniotic membrane. J Biotech 7
Bao H (2014) Method of decellularization of porcine cancellous bone. Int J Life Sci Med Res 4:38–45
Bao H (2016) Decellularized porcine pericardium for clinical applications. Turk J Biol 10(3906):1510–1544
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding information
No funds have been received.
Conflict of interest
Michele L. Zocchi, Vincenzo Vindigni, Andrea Pagani, Ortensia Pirr, Giamaica Contio, Andrea Sbarbati, and Franco Bassetto declare that they have no conflict of interest.
Ethical approval
No need of any ethical approval because the topic of the article is mainly related to basic science behind regenerative surgery and no direct action on patients has been taken and referred in the article.
Informed consent
For the abovementioned reasons, no informed consent has been necessary.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zocchi, M.L., Vindigni, V., Pagani, A. et al. Regulatory, ethical, and technical considerations on regenerative technologies and adipose-derived mesenchymal stem cells. Eur J Plast Surg 42, 531–548 (2019). https://doi.org/10.1007/s00238-019-01571-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-019-01571-5
Keywords
- Regeneration
- Cell derivates
- PRP
- Adipose tissue (AT)
- Stromal vascular fraction (SVF)
- Mesenchymal stem cells (MSCs)
- Pericytes
- Growth factors (GFs)
- Minimal-grade manipulation (MGM)
- High-grade manipulation (HGM)
- Lipocondensation
- Sonication
- Bioactive composite grafts (BACG)
- Biocatalyzers
- MUSE cells
- Exosomes
- Photobiomodulation
- Acellular adipose matrix (AAM)