Abstract
Background
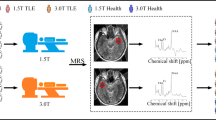
Magnetic resonance spectroscopy (MRS) provides non-invasive information about metabolic features in different regions of the brain affected by mesial temporal sclerosis (MTS).
Purpose
To review articles analyzing the most common alterations in biochemical parameters in MTS and the applications of MRS in presurgical assessment.
Methods
We undertook a systematic literature search for MRS in MTS in PubMed, SCOPUS, and Cochrane based on the MESH terms ““Magnetic Resonance Spectroscopy”, “Proton Magnetic Resonance Spectroscopy”, “Carbon-13 Magnetic Resonance Spectroscopy”, “1H-MRS”, “31P-MRS”, “mesial temporal sclerosis”, “hippocampal sclerosis”, “mesial temporal seizure”, and “mesial temporal epilepsy”.
Results
Of the initial 134 articles found, 30 were selected after the exclusion process. Of these, 13 detected a decrease in N-acetylaspartate (NAA), 9 showed a decreased in the ratio NAA/Cho+Cr, and 8 demonstrated a decreased in the ratio NAA/Cr, all of them in the ipsilateral hippocampus. Nine studies also found reduced NAA levels in extrahippocampal regions.
Conclusions
The main findings were a decrease in NAA in the ipsilateral hippocampus. In addition, NAA levels were low outside the hippocampus so MTS could be a more extensive disease. Patients without MTS also presented a decrease in NAA in the ipsilateral hippocampus although NAA was even lower in the MTS patients. Thus, MRS could be useful in the presurgical evaluation to locate the epileptogenic focus, but not specific for the diagnosis of MTS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About a quarter of patients with epilepsy fail to respond to medical treatment, experiencing an increase in morbidity and mortality compared to the general population [1]. One of the most common forms of epilepsy associated with a poorer response to antiepileptic drugs is temporal lobe epilepsy with mesial sclerosis (MTS).
In most cases of MTS, surgical treatment reduces, either in whole or in part, the frequency of epileptic seizures, improving the quality of life of drug-resistant patients. In addition, resective surgery plus antiepileptic drugs results in a lower probability of seizures than antiepileptic treatment alone [2,3,4].
Hippocampal resection is the most frequent surgical procedure in MTS. However, it requires an exhaustive presurgical evaluation to select patients who could benefit from surgery; reduce the morbidity and mortality associated with the procedure, although this is below 1% [5]; and identify the epileptogenic focus of seizures. Different non-invasive techniques have been developed to help with this assessment, providing structural and functional information about the epileptogenic focus. One such technique is magnetic resonance spectroscopy (MRS).
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy is a non-invasive technique able to evaluate the products of metabolism in a living tissue, detecting possible dysfunctions and supplementing the information provided by MR. To do this, it is necessary to choose a spectrum and the region to study. The different spectra in MRS include.
Proton magnetic resonance spectroscopy (1H-MRS)
This is the most used MRS modality due to the abundance of hydrogen in the tissues of the human body. The metabolites studied in 1H-MRS include [6,7,8,9] (1) N-acetylaspartate (NAA), considered to be a marker of neuronal function as it is a product of the oxidative metabolism of neuronal mitochondria, and NAA has an osmoregulatory function and is absent in glial cells; (2) creatine (Cr) and choline (Cho), which are present in neurons and, in higher concentrations, in astrocytes, and Cr and Cho are related to intracellular bioenergetic functions; (3) Myo-inositol (mI), associated with osmoregulation and intracellular signaling; and (4) Glutamate+Glutamine (Glx), the most important excitatory neurotransmitters in the neuronal synapse. Furthermore, NAA/(Cho+Cr), NAA/Cr, NAA/Cho, and Cr/NAA are used in the evaluation of bioenergetic and neuronal functions.
Phosphorus magnetic resonance spectroscopy (31 P-RM-e) [7, 10]
This allows the measurement of phosphorus metabolites related to the energy state and membrane composition of human brain cells. This includes products of membrane synthesis such as phosphodiesters (PDE) or phosphomonoesters (PME). ATP (α, β, γ), phosphocreatine (PCr), and inorganic phosphate reflect the energy demand of the brain region studied.
Other spectra that can be used in MRS are 13C, 7Li, and 19F. However, they are only available in a few specialized research centers.
Single voxel and multiple voxel magnetic resonance spectroscopy
Once we have chosen the spectrum we then select a brain region to study [7, 9].
Single voxel magnetic resonance spectroscopy
This consists of selecting a unique voxel, which can acquire multiple shapes. The voxel must be placed in regions, which are not affected by air or water interphases. Single voxel is especially indicated in brain diseases in which imaging abnormalities are shown on MR, such as MTS.
Multiple voxel magnetic resonance spectroscopy
This allows spectroscopic data from several voxels to be obtained at the same time. It is commonly used in diseases without MR abnormalities, such as temporal epilepsy with no signs of mesial sclerosis (n-MTS)
The purpose of our systematic review was to collect the main findings obtained by MRS in MTS patients and to determine its usefulness in presurgical evaluation.
Materials and methods
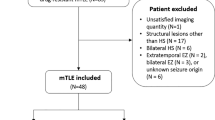
A systematic literature search for MRS in MTS was made in the databases PubMed, SCOPUS, and The Cochrane Library Plus, based on the MESH terms ““Magnetic Resonance Spectroscopy”, “Proton Magnetic Resonance Spectroscopy”, “Carbon-13 Magnetic Resonance Spectroscopy”, “1H-MRS”, “31P-MRS”, “mesial temporal sclerosis”, “hippocampal sclerosis”, “mesial temporal seizure”, “mesial temporal epilepsy”, and the Boolean operators (AND, OR). We did not use additional filters. After removing 23 duplicates, there were 134 articles. We chose articles according to the PRISMA scale [11] (Preferred Reporting Items for Systematic Reviews and Meta-analyses), which is composed of phases summarized in Fig. 1.
First, we performed a critical reading of titles and abstracts. Our inclusion criteria were articles in which patients with MTS were evaluated with MRS in its different modalities. In addition, we included publications in which MRS was correlated with other tests (Video-EEG, neuropsychological tests, volumetry, or histopathology).
Our exclusion criteria were (1) studies of other epilepsies, (2) studies which only included temporal epilepsy without sclerosis (n-MTS), (3) articles without an English version, (4) reviews, (5) animal studies, (6) case series, (7) studies with fewer than 10 patients with MTS, and (8) studies involving children.
After screening by title and abstract, we read the full text of the remaining 42 articles. Five articles were rejected because it was not specified that the temporal epilepsy was due to sclerosis. Three articles were excluded because there were fewer than ten cases of MTS. Two articles in which no MRS was performed and two articles in which spectroscopic evaluation was made on resected brain tissue were also excluded. Thus, 30 studies were finally included in our systematic review.
Results and discussion
We undertook a systematic review of articles reporting the spectroscopic data obtained from patients with MTS in order to examine the application of MRS in presurgical evaluation. The review finally included 30 studies, summarized in Table 1, which specifies author and year of publication, objectives, design, brain region studied, MRS characteristics, additional tests, and results. Of these 30, 23 included MRS as part of the presurgical evaluation of patients with MTS [10, 12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33], while 2 studies performed MRS after surgery [34, 35]. The remaining 5 did not specify whether MRS was included for presurgical or postsurgical assessment [36,37,38,39,40].
MRS studies in ipsilateral hippocampus
The main findings were found in the ipsilateral hippocampus, showing a decrease in NAA (13/30 studies) [12, 13, 15, 19, 21, 23,24,25, 28, 29, 31, 32, 38], a decrease in the ratio NAA/Cho+Cr (9/30 studies) [17, 18, 30, 33, 34, 36, 37, 39, 40], and a decrease in the ratio NAA/Cr (8/30 studies) [14, 16, 20, 22, 26, 27, 35, 39]. MTS was traditionally associated with a neuron loss in the hippocampus [23]. More recent studies hypothesized that there is a relationship between MTS and neuronal mitochondrial dysfunctions [41]. NAA is a product of the Krebs Cycle, which takes place in the mitochondria, so the alterations observed in these 29 articles seem to be consistent with the mitochondrial dysfunction theory. In addition, as NAA has a role in the osmoregulation of neurons [8], low NAA levels cannot maintain the integrity of these cells, resulting in neuron loss.
The decrease in NAA can be detected in different ways, for example, measuring just NAA or determining its different ratios. The ratios that are most altered are NAA/Cho+Cr and NAA/Cr. However, when a decrease is detected in NAA/Cho+Cr or NAA/Cr, it is questioned whether the ratio is reduced due to theoretical high levels of Cr or Cho (which are in the denominator of the ratio, so an increase in their levels could result in a decrease of the ratio) instead of altered NAA levels. Accordingly, it is necessary to obtain the individual results of Cr and Cho, as did the studies summarized in Table 1.
In most studies, patients with MTS did not show a statistically significant alteration in Cr or Cho levels [12, 13, 16,17,18,19,20,21,22, 27, 30,31,32,33,34,35,36,37, 40]. The only exception was the study by Riederer et al. [15], in which Cr was decreased, contrary to what was expected. However, this study admitted a few limitations in spectroscopic data acquisition due to artifacts of bone and air from areas adjacent to the selected voxel.
Comparative studies in extrahippocampal regions
MTS may affect other brain regions outside the hippocampus, as seen in several of the studies included in our systematic review. A decrease in NAA levels was detected in frontal and occipital lobes [36], thalamus [39], temporal operculum, cerebellum, insula [21], and temporal lobe white matter of the ipsilateral side [13, 27]. In addition, Park et al. [10] noted that additional mitochondrial products such as inorganic phosphate and the PCr/γ-ATP were decreased in the insula and basal ganglia using 31-MRS. In 9 studies, NAA was decreased on the contralateral side, and the most altered region on this side was the hippocampus [12, 15, 18, 20, 31, 32, 34, 35, 39].
Studies in which MRS was performed for surgical evaluation
Eleven studies found a decrease in NAA, NAA/Cho+Cr, and NAA/Cr ratios in presurgical evaluation [12, 14,15,16, 18,19,20,21,22, 25, 40].
MRS was performed as postsurgical evaluation in 2 studies, which concluded that the damage produced by MTS could be caused by functional and dynamic mechanisms. Spencer et al. [35] only included postsurgical MRS, while Lantz et al. [34] analyzed spectroscopic data obtained both before and after surgery.
Lantz et al. [34] noted that patients with MTS who responded to surgical treatment showed MRS normalization of the contralateral abnormalities detected in the preoperative evaluation, with NAA values almost reaching those found in healthy subjects. This suggests that the decrease in NAA could be reversible and modified by such treatment as hippocampal resection. Also, postoperative normalization was more pronounced for patients showing an extensive decrease in NAA [34]. The explanation for this is still unknown. However, postsurgical normalization in MRS could be more appreciable in patients who have instability in their clinical condition and their metabolic state, so they have more room for improvement as compared to patients with disease restricted mainly to the hippocampus. If this association is correct, presurgical MRS could allow the selection of patients who could benefit more from hippocampal resection.
Comparative MRS studies with clinical characteristics and epileptiform activity on EEG
Concerning the possible association between MRS and seizures, 6 studies showed that lower NAA correlated with an early onset age of the symptoms [32], longer duration of epilepsy [20, 32, 37], greater frequency of seizures [30], and epileptogenic activity on EEG on the ipsilateral and contralateral sides [20].
Focusing on studies which evaluated the response to surgery, Spencer et al. [35] noted that patients with a higher frequency of seizures after surgery presented more brain regions with low NAA in postsurgical MRS. Therefore, postsurgical MRS could predict the long-term response in patients with MTS, although comparative studies with MRS evaluation before and after surgery are necessary.
MRS studies that included neuropsychological evaluation
One of the most common and disabling symptoms in MTS is dysfunction in verbal memory, more severe in patients with left MTS. Four studies detected a decrease in NAA [24, 28, 29] and a decrease in NAA/Cho+Cr [37] in patients with a worse performance in verbal memory tests.
Regarding patients with right MTS, no cognitive deficiencies related to verbal memory could be detected, except in the study of Mantoan et al. [37], in which patients with right MTS who had low NAA/Cho+Cr levels in the left hippocampus had worse scores in verbal memory.
Finally, Martin et al. [29] included facial recognition tests, with the worst scores detected in patients with a decrease in NAA in the right hippocampus.
Comparative studies of patients with and without sclerosis
Several comparative studies included in this review noted that, as well as MTS, n-MTS patients also presented a decrease in NAA [12, 19, 38] and a decrease in NAA/Cho+Cr [40], in the ipsilateral hippocampus, although NAA was even lower in the MTS patients.
According to some studies, to distinguish between MTS and n-MTS, we should focus on other metabolites. The most striking difference was in the level of Glx, which was significantly increased in 2 studies. Woermann et al. [31] found this in the ipsilateral hippocampus and Simister et al [22] detected it on the contralateral side. However, Doelken et al. [38] and Riederer et al. [15] did not find any significant alteration in Glx in n-MTS patients. Though more studies including this metabolite are necessary, Glx could possibly differentiate between MTS and n-MTS.
Regarding mI, the results from 6 studies were conflicting; 4 found no statistically significant differences between MTS and n-MTS [12, 13, 22, 38]. On the other hand, Riederer et al. [15] noted that n-MTS patients presented a lower mI/Cr ratio than MTS patients, while Mueller et al. [19] only detected low mI levels in MTS patients.
Single voxel and multiple voxel MRS
Single voxel MRS was performed in 18 studies [12,13,14,15,16,17,18, 20, 25, 27, 28, 30,31,32,33, 37,38,39], proving technically easier to analyze the hippocampus. Patients with MTS were evaluated using multiple voxel in the remaining 12 studies [10, 19, 21,22,23,24, 26, 29, 34,35,36, 40], mainly used to study extrahippocampal regions in which it could be difficult to place the voxel due to the absence of structural abnormalities on MR [9]. However, none of these studies compared single and multiple voxel MRS in patients with MTS.
Limitations and future lines
The main limitations of the studies analyzed in this review were the heterogeneity in the MRS technique, the sample sizes, and the characteristics of the population. We included only two studies performed MRS after surgery, so it is not possible to conclude the usefulness of MRS in post-surgical evaluation. Accordingly, this field requires further studies that include presurgical and postsurgical MRS evaluation in a larger sample size to understand the main reasons for the postoperative normalization in the contralateral hippocampus. In addition, it is necessary to establish a protocol for MRS evaluation, using the same ratio of NAA in order to accurately compare between different studies. Future studies all need to include quantitative and qualitative variables such as the frequency of seizures, onset age, duration of epilepsy, and epileptogenic activity on EEG. Only then will we be able to determine the possible association between these variables and MRS. In addition, measurement of other metabolites such as Glx and mI is also required in the MRS evaluation.
Conclusions
This systematic review shows that, in most MRS studies, patients with MTS presented a decrease in NAA in the ipsilateral hippocampus. Furthermore, the decrease in NAA could be detected in extrahippocampal regions on the ipsilateral and the contralateral sides. Additionally, comparative studies also presented NAA decreased in the ipsilateral hippocampus in n-MTS patients. Thus, MRS could be useful but not specific for the diagnosis of MTS and could have a role in the presurgical evaluation to locate the epileptogenic focus. However, further research in this field is needed.
Abbreviations
- Cr:
-
Creatine
- Cho:
-
Choline
- Glx:
-
Glutamine
- MRS:
-
Magnetic resonance spectroscopy
- MTS:
-
Mesial temporal sclerosis
- NAA:
-
N-acetylaspartate
- n-MTS:
-
No signs of mesial sclerosis
- 1H-MRS:
-
Proton magnetic resonance spectroscopy
- mI:
-
Myo-inositol
- 31 P-RM-e:
-
Phosphorus magnetic resonance spectroscopy
- PCr:
-
Phosphocreatine
- PDE:
-
Phosphodiesters
- PME:
-
Phosphomonoesters.
References
Jacoby A, Snape D, Baker GA (2009) Determinants of quality of life in people with epilepsy. Neurol Clin 27(4):843–863
Engel J, Wiebe S, French J, Sperling M, Williamson P, Spencer D, Gumnit R, Zahn C, Westbrook E, Enos B (2003) Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia 44(6):741–751
Engel J Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G, Fried I, Jacobs M, Vinters HV, Mintzer S, Kieburtz K (2021) Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 307(9):922–930
Wiebe S, Blume WT, Girvin JP, Eliasziw M (2001) A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 345(5):311–318
Chapell R, Reston J, Snyder D, Treadwell J, Treager S, Turkelson C (2003) Management of treatment-resistant epilepsy. Evid Rep Technol Assess (Summ) 77:1–8
Duncan JS (1997) Imaging and epilepsy. Brain 120(2):339–377
Pérez-Gómez M, Junqué C, Mercader JM, Berenguer J (2000) Application of magnetic resonance spectroscopy in the study of brain disease. Rev Neurol 30(2):155–160
Martinez MA, Florenzano NV, Macchia EA (2016) Metabolism of N-acetyl-L-aspartate: its diagnostic and prognostic value. Rev Neurol 62(8):361–370
Londoño A, Arbeláez A, Ascencio JL (2006) Applications of magnetic resonance spectroscopy in the study of central nervous system illnesses. Revisión Acta Neurolo Colomb 22(1):42–54
Park EJ, Otaduy MC, de Lyra KP, Andrade CS, Castro LH, Passarelli V, Valerio RM, Jorge CL, Tsunemi MH, Leite Cda C (2016) Extratemporal abnormalities in phosphorus magnetic resonance spectroscopy of patients with mesial temporal sclerosis. Arq Neuropsiquiatr 74(2):93–98
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7):e1000100
Doelken MT, Richter G, Stefan H, Doerfler A, Noemayr A, Kuwert T, Ganslandt O, Nimsky CH, Hammen T (2007) Multimodal coregistration in patients with temporal lobe epilepsy - results of different imaging modalities in lateralization of the affected hemisphere in MR imaging positive and negative subgroups. AJNR Am J Neuroradiol 28(3):449–454
Briellmann RS, Mark Wellard R, Masterton RA, Abbott DF, Berkovic SF, Jackson GD (2007) Hippocampal sclerosis: MR prediction of seizure intractability. Epilepsia 48(2):315–323
Imbesi SG (2006) Proton magnetic resonance spectroscopy of mesial temporal sclerosis: analysis of voxel shape and position to improve diagnostic accuracy. J Comput Assist Tomogr 30(2):287–294
Riederer F, Bittsanský M, Schmidt C, Mlynárik V, Baumgartner C, Moser E, Serles W (2006) 1H magnetic resonance spectroscopy at 3 T in cryptogenic and mesial temporal lobe epilepsy. NMR Biomed 19(5):544–553
Lee SK, Kim DW, Kim KK, Chung CK, Song IC, Chang KH (2005) Effect of seizure on hippocampus in mesial temporal lobe epilepsy and neocortical epilepsy: an MRS study. Neuroradiology 47(12):916–923
Düzel E, Kaufmann J, Guderian S, Szentkuti A, Schott B, Bodammer N, Hopf M, Kanowski M, Tempelmann C, Heinze HJ (2004) Measures of hippocampal volumes, diffusion and 1H MRS metabolic abnormalities in temporal lobe epilepsy provide partially complementary information. Eur J Neurol 11(3):195–205
Zubler F, Seeck M, Landis T, Henry F, Lazeyras F (2003) Contralateral medial temporal lobe damage in right but not left temporal lobe epilepsy: a 1H magnetic resonance spectroscopy study. J Neurol Neurosurg Psychiatry 74(9):1240–1244
Mueller SG, Laxer KD, Suhy J, Lopez RC, Flenniken DL, Weiner MW (2003) Spectroscopic metabolic abnormalities in mTLE with and without MRI evidence for mesial temporal sclerosis using hippocampal short-TE MRSI. Epilepsia 44(7):977–980
Park SA, Kim GS, Lee SK, Lim SR, Heo K, Park SC, Chang JW, Kim DI, Lee BI (2002) Interictal epileptiform discharges relate to 1H-MRS-detected metabolic abnormalities in mesial temporal lobe epilepsy. Epilepsia 43(11):1385–1389
Capizzano AA, Vermathen P, Laxer KD, Matson GB, Maudsley AA, Soher BJ, Schuff NW, Weiner MW (2002) Multisection proton MR spectroscopy for mesial temporal lobe epilepsy. AJNR Am J Neuroradiol 23(8):1359–1368
Simister RJ, Woermann FG, McLean MA, Bartlett PA, Barker GJ, Duncan JS (2002) A short-echo-time proton magnetic resonance spectroscopic imaging study of temporal lobe epilepsy. Epilepsia 43(9):1021–1031
Kuzniecky R, Palmer C, Hugg J, Martin R, Sawrie S, Morawetz R, Faught E, Knowlton R (2001) Magnetic resonance spectroscopic imaging in temporal lobe epilepsy: neuronal dysfunction or cell loss. Arch Neurol 58(12):2048–2053
Sawrie SM, Martin RC, Knowlton R, Faught E, Gilliam F, Kuzniecky R (2001) Relationships among hippocampal volumetry, proton magnetic resonance spectroscopy, and verbal memory in temporal lobe epilepsy. Epilepsia 42(11):1403–1407
Chang KH, Kim HD, Park SW, Song IC, Yu IK, Han MH, Lee SK, Chung CK, Park YH (2000) Usefulness of single voxel proton MR spectroscopy in the evaluation of hippocampal sclerosis. Korean J Radiol 1(1):25–32
Feichtinger M, Pauli E, Schäfer I, Eberhardt KW, Tomandl B, Huk J, Stefan H (2000) Ictal fear in temporal lobe epilepsy: surgical outcome and focal hippocampal changes revealed by proton magnetic resonance spectroscopy imaging. Arch Neurol 58(5):771–777
Meiners LC, Van Der Grond J, Van Rijen PC, Springorum R, De Kort GAP, Jansen GH (2000) Proton magnetic resonance spectroscopy of temporal lobe white matter in patients with histologically proven hippocampal sclerosis. J Magn Reson Imaging 11(1):25–31
Sawrie SM, Martin RC, Gilliam FG, Faught RE, Maton B, Hugg JW, Bush N, Sinclair K, Kuzniecky RI (2000) Visual confrontation naming and hippocampal function: a neural network study using quantitative (1)H magnetic resonance spectroscopy. Brain 123(Pt 4):770–780
Martin RC, Sawrie S, Hugg J, Gilliam F, Faught E, Kuzniecky R (1999) Cognitive correlates of 1H MRSI-detected hippocampal abnormalities in temporal lobe epilepsy. Neurology 53(9):2052–2058
Namer IJ, Bolo NR, Sellal F, Nguyen VH, Nedelec JF, Hirsch E, Marescaux C (1999) Combined measurements of hippocampal N-acetyl-aspartate and T2 relaxation time in the evaluation of mesial temporal lobe epilepsy: correlation with clinical severity and memory performances. Epilepsia 40(10):1424–1432
Woermann FG, McLean MA, Bartlett PA, Parker GJ, Barker GJ, Duncan JS (1999) Short echo time single-voxel 1H magnetic resonance spectroscopy in magnetic resonance imaging-negative temporal lobe epilepsy: different biochemical profile compared with hippocampal sclerosis. Ann Neurol 45(3):369–376
Duc CO, Trabesinger AH, Weber OM, Meier D, Walder M, Wieser HG, Boesiger P (1998) Quantitative 1H MRS in the evaluation of mesial temporal lobe epilepsy in vivo. Magn Reson Imaging 16(8):969–979
Connelly A, Van Paesschen W, Porter DA, Johnson CL, Duncan JS, Gadian DG (1998) Proton magnetic resonance spectroscopy in MRI-negative temporal lobe epilepsy. Neurology 51(1):61–66
Lantz G, Seeck M, Lazeyras F (2006) Extent of preoperative abnormalities and focus lateralization predict postoperative normalization of contralateral 1H-magnetic resonance spectroscopy metabolite levels in patients with temporal lobe epilepsy. AJNR Am J Neuroradiol 27(8):1766–1769
Spencer DC, Szumowski J, Kraemer DF, Wang PY, Burchiel KJ, Spielman DM (2005) Temporal lobe magnetic resonance spectroscopic imaging following selective amygdalohippocampectomy for treatment-resistant epilepsy. Acta Neurol Scand 112(1):6–12
Mueller SG, Ebel A, Barakos J, Scanlon C, Cheong I, Finlay D, Garcia P, Weiner MW, Laxer KD (2011) Widespread extrahippocampal NAA/(Cr+Cho) abnormalities in TLE with and without mesial temporal sclerosis. J Neurol 258(4):603–612
Mantoan MA, Caboclo LO, de Figueiredo Ferreira Guilhoto LM, Lin K, da Silva Noffs MH, de Souza Silva Tudesco I, Belzunces E, Carrete H Jr, Bussoletti RT, Centeno RS, Sakamoto AC, Yacubian EM (2009) Correlation between memory, proton magnetic resonance spectroscopy, and interictal epileptiform discharges in temporal lobe epilepsy related to mesial temporal sclerosis. Epilepsy Behav 16(3):447–453
Doelken MT, Stefan H, Pauli E, Stadlbauer A, Struffert T, Engelhorn T, Richter G, Ganslandt O, Doerfler A, Hammen T (2008) 1H-MRS profile in MRI positive- versus MRI negative patients with temporal lobe epilepsy. Seizure 17(6):490–497
Fojtiková D, Brázdil M, Skoch A, Jírů F, Horký J, Marecek R, Mikl M, Krupa P (2007) Magnetic resonance spectroscopy of the thalamus in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Epileptic Disord 9(Suppl. 1):S59–S67
Lu JJ, Ren LK, Feng F, You H, Zhang LH, Li ML, Sun F, Fu HH, Jin ZY (2006) Metabolic abnormalities in mesial temporal lobe epilepsy patients depicted by proton MR spectroscopy using a 3. 0t MR scanner. Chin Med Sci J 21(4):209–213
Waldbaum S, Patel M (2010) Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Res 88(1):23–45
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fernández-Vega, N., Ramos-Rodriguez, J.R., Alfaro, F. et al. Usefulness of magnetic resonance spectroscopy in mesial temporal sclerosis: a systematic review. Neuroradiology 63, 1395–1405 (2021). https://doi.org/10.1007/s00234-021-02704-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02704-z