Abstract
Background and Purpose
Radiation therapy is commonly utilized in the majority of solid cancers and many hematologic malignancies and other disorders. While it has an undeniably major role in improving cancer survival, radiation therapy has long been recognized to have various negative effects, ranging from mild to severe. In this manuscript, we review several intracranial manifestations of therapeutic radiation, with particular attention to those that may be encountered by radiologists.
Methods
We conducted an extensive literature review of known complications of intracranial radiation therapy. Based on this review, we selected complications that had salient, recognizable imaging findings. We searched our imaging database for illustrative examples of these complications, focusing only on patients who had a history of intracranial radiation therapy. We then selected cases that best exemplified expected imaging findings in these entities.
Results
Based on our initial literature search and imaging database review, we selected cases of radiation-induced meningioma, radiation-induced glioma, cavernous malformation, enlarging perivascular spaces, leukoencephalopathy, stroke-like migraine after radiation therapy, Moyamoya syndrome, radiation necrosis, radiation-induced labyrinthitis, optic neuropathy, and retinopathy. Although retinopathy is not typically apparent on imaging, it has been included given its clinical overlap with optic neuropathy.
Conclusions
We describe the clinical and imaging features of selected sequelae of intracranial radiation therapy, with a focus on those most relevant to practicing radiologists. Knowledge of these complications and their imaging findings is important, because radiologists play a key role in early detection of these entities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiation therapy (RT) is commonly used to treat most solid tumors and many hematologic malignancies. In the modern era, RT plays a central role in the management of a wide variety of intracranial disease, especially metastatic and primary brain malignancy. While RT has undeniably played a central role in improving cancer survival in recent decades, its potential side effects have long been recognized. The first recognized ill effect was radiation-induced malignancy in a radiation worker reported in 1902, only 7 years after the discovery of X-rays by Wilhelm Röntgen [1].
Despite advances in radiation technology and techniques that limit high-dose exposure to adjacent normal tissues, toxicities still occur. Many adverse intracranial effects related to radiation therapy can be diagnosed or at least suggested on imaging. Therefore, it is important for radiologists to be aware of these complications. Our goal is to review selected sequelae of RT in the brain, with a focus on entities that are either common or can be detected on imaging.
The included entities were chosen using a systematic approach. We first conducted a comprehensive review of the literature, focusing on original research and review articles discussing intracranial complications of radiation therapy. Next, using information from the literature and our own experience, we identified specific entities that were either common or readily diagnosed on imaging. Finally, we further reviewed the literature pertaining to these specific entities and selected representative cases from our institution’s database.
Radiation-induced tumors
In 1948, radiation-induced malignancy was formally defined by Cahan et al. [2], and modifications of Cahan’s criteria are still used to assess for radiation-induced tumors [3]. There are four primary elements necessary for a radiation-induced tumor to be diagnosed. First, a tumor must arise within previously irradiated tissue. Second, a sufficient latent period, at least several years, must have elapsed between the initial irradiation and the development of the secondary tumor. Third, the treated tumor and the subsequent tumor must both have been biopsied and shown to differ histologically. Fourth, the tissue in which the secondary tumor arose must have been metabolically and genetically normal prior to radiation therapy. The majority of radiation-induced tumors are meningiomas or gliomas.
Meningioma
Meningiomas are the most common primary brain tumor and the most common type of radiation-induced intracranial neoplasm. In the seminal publications describing the long-term health consequences encountered by atomic bomb survivors, an increased rate of meningioma was demonstrated, which varied with distance from the bomb blast site [4]. Many of these tumors were asymptomatic and discovered only at autopsy. Historically, meningiomas have also been seen at a higher rate in patients exposed to previously high-dose dental X-rays [5] or following superficial irradiation for benign conditions such as acne and tinea capitis [6, 7].
In contemporary clinical practice, most patients with radiation-induced meningioma are likely to have undergone previous high-dose RT for malignancy. A recent epidemiologic review of radiation-induced meningioma following RT for neoplasm reported a mean latency period of 22.9 ± 11.4 years between initial radiation and meningioma diagnosis [8]. Most of the patients were children at the time of initial exposure (mean age 13.0 ± 13.5 years), consistent with numerous reports that children are at increased risk of radiation-induced tumors relative to adults. Most tumors (88.1%) were solitary, but the rate of multiple meningiomas (11.9%) was higher than that seen in the population at large. Another study found up to a 9.5-fold increased incidence of meningiomas in patients treated with 1–2 Gy during childhood [9].
Radiation-induced meningiomas have an identical imaging appearance to sporadic cases, presenting as a dural-based extra-axial mass with enhancement, sometimes with calcification on CT. Though generally benign, these tumors can be large with substantial mass effect. Importantly, radiation-induced meningiomas tend to be of higher grade than their sporadic counterparts (Fig. 1). In one large study of 205 radiation-induced meningiomas, 68.3% were WHO grade I, 26.8% grade II, and 4.9% grade III. Overall, the rate of grade II and III tumors was higher than seen in sporadic cases of meningioma [8]. Sometimes, features such as low tumor ADC values can suggest these higher grade meningiomas [10]. Aggressive meningiomas are also more common in patients with a history of high-dose radiation. Treatment is similar to that of sporadic meningiomas and varies depending on tumor size, location, and multiplicity. Surgical resection is commonly employed for symptomatic meningiomas, and paradoxically, radiotherapy may be used as adjunctively [9].
Radiation-induced meningioma. A 45-year-old man with transtentorial WHO grade III meningioma diagnosed approximately 40 years after radiation for cerebellar medulloblastoma. Coronal (a) and axial (b, c) post-contrast T1WI show an avidly enhancing dural-based extra-axial mass (a–c, arrows) with internal non-enhancing areas of necrosis. Axial T2W images (e, d) demonstrate hyperintensity in the adjacent brain parenchyma (d, e, arrows) compatible with a combination of edema and invasion
Infiltrating glioma
Glioma is the second most common radiation-induced intracranial tumor after meningioma. In one analysis of patients treated with sellar radiation for pituitary adenomas, the relative risk of glioma was 7.9 times higher than that of the normal population [11]. The vast majority of radiation-induced gliomas are WHO grade III astrocytoma (anaplastic astrocytoma) or grade IV astrocytoma (glioblastoma; Fig. 2) [12]. In a review of 176 cases of radiation-induced glioma, Elsamadicy and colleagues reported a 9-year median latency period, with over 80% of cases occurring within 15 years of radiation [13]. As with meningioma, most radiation-induced gliomas occurred in young adults who were children at the time of initial radiation (Fig. 2).
Radiation-induced glioblastoma. A 30-year-old man with a right cerebellar hemisphere glioblastoma approximately 20 years after treatment of cerebellar medulloblastoma. Axial post-contrast T1-weighted image (a) and FLAIR image (b) demonstrate a ring-enhancing mass with surrounding T2 hyperintensity.
Imaging findings of radiation-induced gliomas are similar to those of sporadic tumors and depend on the specific tumor type. Thus, while it is important to suggest the diagnosis based on the appearance of a new, aggressive-appearing tumor, biopsy is often ultimately necessary. Median overall survival in this review was less than 1 year, which is worse than malignant glioma overall, especially considering the relatively young age of the radiation-induced malignancy patient population [14].
Radiation-induced cerebral cavernous malformations
Cerebral cavernous malformations (CCM), variably referred to as cavernomas, cavernous hemangiomas, and cavernous angiomas, are benign vascular tumors with thin walls, dilated capillary spaces, and no intervening brain tissue. Most CCMs are congenital and can be either sporadic or familial (typically autosomal dominant). The first publication suggesting a relationship between radiation and CCMs occurred in 1994 [15]. This association has subsequently become well-accepted. As with meningioma and glioma, CCMs are more common in patients irradiated in childhood. In an analysis of 59 consecutive patients aged 21 years or younger treated for medulloblastoma with radiotherapy, Lew and colleagues reported CCM development in 18 patients (26 lesions) over the follow-up period, for cumulative incidences of lesion development of 5.6, 14, and 43% at 3, 5, and 10 years, respectively [16]. Only one patient developed symptoms requiring intervention. Another retrospective review reported a cumulative incidence of 3% (95% CI 1–8%) at 10 years after RT and 14% (95% CI 7–26%) at 15 years [17].
Imaging features of radiation-induced CCMs are indistinguishable from sporadic or familial cases. Findings include circumferential and lobulated hypointensity of hemosiderin (Fig. 3), sometimes with a heterogenous “mulberry” appearance internally due to repeated hemorrhage. Blooming on susceptibility weighted imaging is characteristic. Of note, it is not uncommon to see the brain parenchyma studded with multiple CCMs. Importantly, small CCMs, which may appear as tiny hypointense foci on susceptibility weighted images, may be indistinguishable from microhemorrhages that are unrelated to CCMs. Such microhemorrhages are also a known complication of radiation therapy, occurring in up to 49% of patients [18].
SMART syndrome and cavernous hemangioma. A 50-year-old female with history of radiation treated cerebellar low-grade astrocytoma at age 12 presenting with headache and left visual field symptoms. Axial post-contrast T1-weighted image (a) and FLAIR image (b) demonstrate cortical enhancement and hyperintensity of the right posterior occipital lobe (arrowheads). The patient also developed a left posterior temporal lobe cavernous hemangioma (arrows). The lesion displays intrinsic T1 hyperintensity (a), a T1/T2 hypointense rim (a, b), and marked hypointensity on SWI (c) due to hemosiderin staining
CCMs frequently do not require treatment but should be monitored on serial imaging for interval growth or hemorrhage. Should they become symptomatic, surgical resection can be considered. In such cases, it is important for radiologists to identify associated developmental venous anomalies, which should be preserved to avoid venous infarct.
Enlarging perivascular spaces secondary to radiation
Virchow-Robin or perivascular spaces are benign pial-lined spaces that surround penetrating arteries and arterioles within the brain parenchyma. Giant “tumefactive” perivascular spaces within the basal ganglia or brainstem may be an incidental finding [19]. These are generally stable on serial imaging, enlarging only occasionally [20]. However, in patients with a history of cranial radiation, multiple authors have reported the development of progressively enlarging dilated perivascular spaces within the radiation field but relatively remote from the primary tumor site [21, 22]. The precise incidence of this phenomenon is not well known due to the paucity of reported cases. Previous studies have also described cystic changes at the margin of tumor sites following radiation, which may represent a similar process [23,24,25,26]. Cyst formation after stereotactic radiation to arteriovenous malformations has also been described, though this is likely a separate phenomenon [27].
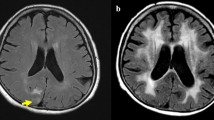
Imaging features include T2 hyperintense, circumscribed parenchymal lesions with suppression on FLAIR (Fig. 4). Rarely, perilesional T2 hyperintensity may be seen. Enlargement of these spaces after radiation therapy should not be mistaken for a more sinister process. No treatment is typically necessary for these benign, incidental lesions.
Enlarging perivascular spaces. Axial T1W post-contrast (left), FLAIR (middle), and T2W (right) images of a 50-year-old man with enlarging left basal ganglia perivascular spaces (arrows) 3 years (a–c) and 6 years (d–f) after whole-brain radiation therapy for adult-onset medulloblastoma. No enlarged perivascular spaces were present at the time of initial diagnosis and treatment
Radiation-induced Leukoencephalopathy
Radiation-induced leukoencephalopathy (RIL) is a rare but life-threatening delayed complication of radiotherapy that may develop months to years after cranial radiation [28]. RIL can range from mild forms with limited clinical effect to a severe clinical syndrome characterized by confusion, memory decline, ataxia, and in rare cases death. The radiologic manifestation of this condition is diffuse white matter abnormality, the severity of which is often associated with clinical severity of the syndrome. However, the degree of involvement on imaging does not necessarily correlate to the severity of imaging findings; for example, patients with extensive white matter changes can have mild symptoms [29]. In advanced stages, severe dementia and hydrocephalus may occur [30]. RIL is most commonly associated with whole-brain radiation (WBRT); however, it has been reported in patients receiving high-dose (30–40 Gy) radiosurgery to large volumes of the brain [29, 31, 32]. The primary challenges in estimating RIL incidence are that nonspecific white matter changes are common in most WBRT patients and that most patients requiring WBRT have a median survival of less than 6 months, limiting available data. Furthermore, additional patient comorbidities, concurrent chemotherapy, tumor-associated neurologic decline, and other risk factors complicate the picture [33]. Severe RIL, typically defined by confusion, memory decline, ataxia, or death, is estimated to occur in fewer than 5% of patients.
The pathophysiology of RIL is thought to be multifactorial and related to vascular compromise, demyelination, and direct neuronal damage [34]. However, the exact mechanism is unclear. Histologically, involved regions demonstrate reactive glial processes, demyelination, endothelial damage, and capillary occlusion [35]. Age, hypertension, diabetes, and underlying leukoaraiosis may all increase the risk of developing RIL lesions [35]. While white matter changes are salient on imaging, it is important to note that recent studies have also demonstrated gray matter volume loss, as described in one study of patients who underwent radiation therapy for glioma treatment [36].
On imaging, RIL is characterized by diffuse parenchymal atrophy and extensive T2 FLAIR hyperintensity within the cerebral white matter (Fig. 5), with preferential anterior involvement and lack of focal lesions [37, 38]. Importantly, parenchymal atrophy can occur as an independent sequela of intracranial radiation therapy. Thus, atrophy in these patients may be due to a combination of changes related to RIL and separate radiation-induced atrophy [28]. Early disease stages may be limited to symmetric “capping” of the frontal and occipital horns of the lateral ventricles, while advanced disease progresses to confluent abnormalities through the periventricular, deep, and subcortical white matter. Subcortical U-fibers are classically spared, as are the posterior fossa, internal capsule, and basal ganglia [39, 40]. Involved areas will appear hypoattenuating on CT. Intraparenchymal cysts may be present in the minority of patients [28]. Communicating hydrocephalus, responsive to CSF shunting, may also be occurred [41]. The precise mechanism for this hydrocephalus is not known, but clinically, this complication resembles normal pressure hydrocephalus. It also has a similar appearance to normal pressure hydrocephalus on imaging, with ventricular enlargement out of proportion to cerebral atrophy [34].
Radiation-induced leukoencephalopathy. A 65-year-old female with breast cancer status post WBRT for central nervous system metastases. Axial FLAIR image (a) demonstrates diffuse cerebral volume loss with confluent T2 hyperintense signal throughout the white matter. Note the characteristic sparing of the subcortical U-fibers (arrow)
Treatment of RIL patients can improve symptoms. A clinical trial evaluating the prophylactic use of the N-methyl-D-aspartate (NMDA) receptor antagonist memantine with WBRT reduced neurocognitive decline, likely through reduced NMDA receptor stimulation [42]. More recently, a clinical trial evaluating hippocampal avoidance during WBRT, with a goal of reducing irradiation of neural stem cells within the subgranular zone of the hippocampus, demonstrated less neurocognitive decline than patients with standard WBRT without hippocampal avoidance [43]. While memantine use and hippocampal avoidance techniques during WBRT reduced neurocognitive decline, it is unclear what impact this will have on RIL risk.
Stroke-like migraine after radiation therapy
The syndrome of stroke-like migraine after radiation therapy (SMART syndrome) is a recently recognized late complication of RT of uncertain pathophysiology [44, 45]. As the name suggests, SMART occurs in patients with a history of RT, often a decade or more after treatment, and manifests with prodromal symptoms of migraine followed by focal neurological deficits. The incidence of SMART syndrome is difficult to estimate, as it is an exceedingly rare entity with a very limited number of reported cases. Still, this diagnosis can be suggested on imaging due to its classic features on MR. The characteristic MR manifestation of SMART is striking gyriform cortical enhancement in the region corresponding to the clinical deficit (Fig. 3). Though initial reports regarding SMART suggested that the clinical manifestations were reversible, it is now known that a subpopulation of patients develop cortical laminar necrosis and subsequent irreversible neurological deficits [46]. The spectrum of disease has further expanded to include other episodic late-radiation complications with associated cerebral enhancement such as acute late-onset encephalopathy after RT and peri-ictal pseudoprogression [47]. Fortunately, SMART syndrome is often self-limiting, with treatment usually being centered on management of symptoms.
Radiation-induced moyamoya
Radiation-induced vasculopathy is a well-recognized late complication of RT which can occur in any vascular bed. The most commonly reported sites include the carotid, coronary, and peripheral arteries [48]. However, intracranial radiation-induced vasculopathies can also occur. For example, cranial telangiectasia occurred in 20% of patients after radiation therapy in one study [49]. Radiation-induced intracranial aneurysms were seen in 1.8% of patients in another study [50]. Mild vascular injury from radiation may lead to lacunar infarcts, with one study finding a higher incidence of pediatric brain tumor patients developing such infarcts when treated with radiation [49]. However, perhaps the most profound intracranial manifestation of radiation-induced vasculopathy, particularly from an imaging standpoint, is moyamoya syndrome. A large retrospective study of children who had undergone cranial irradiation for primary brain tumors found that 3.5% developed evidence of moyamoya, and risk factors for the disease included younger age at time of radiation, higher doses to the optic chiasm, and neurofibromatosis type 1 [51]. In a review of 54 patients with radiation-induced moyamoya, the majority were children, but 4.2% were over 40 years of age at the time of radiation [52]. Median time from radiation to moyamoya diagnosis was 40 months. The most common primary tumor was low-grade glioma in 37 patients (69%), of which 29 (54% overall) were optic pathway gliomas and 26% of patients were diagnosed with neurofibromatosis type 1. Moyamoya has also more specifically been seen following proton beam radiotherapy [53].
Radiation-induced moyamoya imaging features are similar to the sporadic form of the disease. Conventional and cross-sectional angiographic imaging show steno-occlusive changes in the internal carotid arteries and circle of Willis with small collateral vessels (Fig. 6). MR may show acute or chronic infarctions and secondary findings like the “ivy sign” on post-contrast T1-weighted or FLAIR images indicating slow flow in leptomeningeal vessels. Treatment depends on the extent of disease, but anastomotic bypass of the affected vessels may be considered [48].
Radiation-induced moyamoya. A 3-year-old male presenting with a third ventricular primitive neuroectodermal tumor (a), treated with resection and radiation to 5400 cGy. MRA performed 6 years later (b) shows narrowing of the distal right ICA and near-occlusion of the right M1 segment (arrow). Catheter angiogram (c) performed shortly thereafter demonstrates right M1 near-occlusion (arrow) and shows several small collateral moyamoya vessels and robust leptomeningeal collaterals from the anterior cerebral artery territory to the middle cerebral artery territory
Radiation necrosis
Radiation necrosis (RN) is a possible delayed consequence after RT to either intracranial or head and neck tumors that may occur a few months to many years after radiotherapy completion [54, 55]. The incidence of RN is difficult to estimate but is likely in the range of 5–25% [56]. Symptoms mimic those of tumor recurrence and include headache, fatigue, nausea, weakness, neurologic deficits, and somnolence. Symptoms are usually mild, but in rare cases, RN may be severe enough to result in death [12].
Two major hypotheses have been posited to explain the pathogenesis of RN. The first is that RN arises from regional vascular injury, with a cascade of insults leading to small vessel necrosis and subsequent cell death. The second centers around glial cell damage, resulting in hypoxia and demyelination. Both vascular and glial cell damage induce vascular endothelial growth factor production and upregulation of inflammatory markers. These changes disrupt the blood-brain barrier, which may explain the observed edema and contrast enhancement [56].
Imaging characteristics of RN have been extensively described. The typical appearance is a lesional, or mass-like abnormality with central necrosis, often with internal cysts and associated mass effect. Enhancement is nearly always present and has been described as resembling “Swiss cheese” or “soap bubble.” These characterizations describe foci of enhancement intermixed with necrosis within the core; whereas a “spreading wavefront” is used to describe the feathery and ill-defined periphery (Fig. 7) [57, 58]. Irregular, linear areas of T1 shortening are observed in the majority of cases and may represent blood products or calcification associated with radiation-induced mineralizing angiopathy [58]. RN can also be seen in conjunction with other sequelae of radiation therapy. One report described a patient who developed RN and hypertrophic olivary degeneration following surgical resection of a posterior fossa tumor and radiation treatment, with radiation potentially contributing to both findings [59]. The co-occurrence of multiple sequelae of radiation can help corroborate a diagnosis of RN.
Radiation necrosis. A 63-year-old male with a history of glioblastoma status-post-resection and subsequent chemoradiation therapy. Axial (a) and coronal (b) T1W post-contrast images demonstrate “soap bubble” type enhancement within the treatment bed, consistent with presumed radiation necrosis (arrows)
Due to multiple overlapping imaging features, however, RN is notoriously difficult to distinguish from tumor recurrence. It is usually located near the primary tumor site, enhances, and may grow over time. Furthermore, none of the aforementioned enhancement patterns are able to reliably differentiate necrosis from tumor [60]. Perfusion imaging is often employed to distinguish between these entities, as RN tends to have lower relative cerebral blood volume (rCBV) than recurrent tumors. This is likely because recurrent or progressive tumor tends to exhibit neovascularity, which elevates rCBV. One recent study found that with modern MRI perfusion techniques, patients with tumor recurrence had a statistically significant elevated rCBV compared with patients with radiation necrosis [61]. PET imaging will similarly show FDG uptake in areas of tumor recurrence, while necrosis is hypometabolic [62]. Calculation of the lesion quotient, too, may be done, in which the area of an intralesional T2 hypointense nodule is divided by the total enhancing region. Lesion quotient values of ≤ 0.3 and ≥ 0.6 suggest RN and recurrent tumor, respectively, though these have not been validated as reliable markers [63, 64]. Finally, intralesional diffusion restriction is sometimes used to evaluate treatment response. Studies regarding the effectiveness of diffusion-weighted imaging have previously been contradictory: low ADC values have been shown to represent recurrent tumor, coagulative necrosis, or a combination of both [65,66,67]. However, recent studies have suggested that diffusion imaging combined with arterial spin labeling or perfusion imaging may be effective in discriminating tumor from RN. Overall, RN remains a challenging mimicker for recurrent tumor that can sometimes, though not always be differentiated from tumor [68, 69].
Radiation-induced labyrinthitis
Radiation-induced labyrinthitis refers to injury to the labyrinth in an irradiated field, believed to be mediated by microvascular ischemic changes which can occur in a delayed fashion. One study reported radiation-induced labyrinthitis at a mean of 12 years after treatment [70], while hearing deterioration has been shown to begin as early as 3 months following completion of RT [71]. Patients with head and neck cancers treated with radiation have been shown to have a higher incidence of hearing loss and greater debility from that loss [72]. Radiographs and CT are typically unremarkable; however, at MRI, cochlear enhancement may be observed, potentially extending to the internal auditory canal fundus (Fig. 8). Anti-inflammatory medications have shown benefit in treatment, with 9 of 15 patients showing some recovery of hearing following treatment in one study [70].
Radiation-induced labyrinthitis. A 55-year-old male with a history of metastatic lung adenocarcinoma treated with two cycles of WBRT presenting with worsening left-sided hearing loss. Axial post-contrast fat-saturated T1-weighted images (a, b) demonstrate enhancement within the fundus of both internal auditory canals (arrows in a) and diffuse left-sided intra-cochlear enhancement (arrow in b) new from an MRI performed 3 months prior (not shown)
Radiation-induced optic neuropathy
Radiation-induced optic neuropathy (RION) is an uncommon but devastating form of delayed radionecrosis (Fig. 9). A 3-month to 9-year range of latency has been reported, with most events occurring 10–20 months after radiation exposure. Patients commonly present with rapid onset of painless vision loss which is severe and irreversible, but transient episodes of visual disturbance or loss in the weeks prior to complete visual loss may occur. The pathogenesis of RION is thought to be related to chronic microvascular and glial damage of the optic tracts [73, 74]. Predisposing factors include prior radiation, concurrent chemotherapy, optic nerve compression, hypertension, and smoking [75, 76].
Radiation-induced optic neuropathy. A 72-year-old woman 14 months after receiving 54 Gy fractionated radiation for a left clinoid meningioma. Axial (a) and coronal (b) post-contrast T1-weighted images demonstrate optic nerve gadolinium enhancement (arrows) and associated T2 hyperintensity (c, arrows)
Most commonly, RION is reported in patients with treatment directed towards anterior or skull base brain tumors or those of the nasopharynx and paranasal sinuses, as high-dose radiation is directed immediately adjacent to the optic pathways. The risk of RION with conventionally fractionated radiation is thought to be “near zero” and “unusual” with optic apparatus doses below 50 Gy or between 50 and 55 Gy, respectively. As doses exceed 60 Gy, the risk likewise increases with estimates between 8 and 20% [77]. In the setting of single-fraction radiosurgery, doses of up to 12 Gy are considered safe with low potential for RION. While these are best estimates from the general population, some patients may develop RION despite exposure to doses below these “safe” thresholds [75].
Imaging findings of RION include enlargement, T2 hyperintensity, and enhancement of any portion of the optic nerves. While these findings are not readily distinguishable from other causes of optic neuritis, the history of radiotherapy and selective involvement of the radiation field can be helpful in making this diagnosis. Treatment remains incompletely understood, but some studies have found bevacizumab to provide benefit [78].
Radiation-induced retinopathy
Radiation-induced retinopathy is a rare yet disabling complication related to ocular irradiation. Unlike the previously mentioned entities, radiation-induced retinopathy is usually occult on imaging. Nonetheless, it can present similarly to RION clinically and the two can be challenging to distinguish. Radiation-induced retinopathy, like RION, is seen in patients with visual acuity changes in the setting of past anterior cranial irradiation. The presence of comorbidities such as uncontrolled diabetes and hypertension increases the radiation retinopathy risk. In contrast to RION, retinopathy does not have a radiographic correlate but it can be directly visualized through fundoscopic evaluation, making diagnostic certainty more evident.
In contrast to RION, patients with radiation-induced retinopathy typically present with slow and gradual visual loss [79, 80]. Latency of radiation retinopathy is reported from 2 months to more than a decade after RT, with most cases occurring between 6 months and 3 years [81, 82]. Vasculopathy develops from endothelial insult leading to a proliferative response from migrating adjacent endothelial cells. Ultimately, the clotting cascade is activated resulting in telangiectasia, hemorrhage, retinal and macular edema, microaneurysms, and cotton-wool spots, which may be seen on fundoscopic examination.
The incidence of radiation-induced retinopathy is unclear but the condition is quite rare. Usually, retinopathy is not uniquely collected as a toxicity in most clinical trials, and the majority of data are limited to case reports. Radiation-induced retinopathy is uncommon with maximal doses of 45 to 50 Gy with conventionally fractionated radiation and up to 24 Gy of small volume in the setting of single-fraction radiosurgery [83,84,85,86,87,88].
Conclusion
We have reviewed numerous complications of intracranial radiation therapy, the majority of which can be detected on imaging. Fortunately, radiation-related neurotoxicity has been declining due to advances in radiation technology and techniques that limit high-dose radiation exposure to adjacent normal tissues. However, a strong understanding of potential complications is necessary for radiologists to appropriately interpret imaging in the setting of prior RT. Although imaging findings of radiation-induced pathology are not always unique, careful attention to clinical history and knowledge of these complications can allow one to suggest these diagnoses.
Abbreviations
- CCM:
-
Cerebral cavernous malformations
- RION:
-
Radiation-induced optic neuropathy
- RIL:
-
Radiation-induced leukoencephalopathy
- RN:
-
Radiation necrosis
- RT:
-
Radiation therapy
- SMART:
-
Stroke-like migraine after radiation therapy
- WBRT:
-
Whole-brain radiation therapy
References
Frieben A (1902) Demonstration eines cancroids des rechten handruckens, das sich nach langdauernder einwirkung von roentgenstrahlen entwickelt hatte. Fortschr Roentgenstr 6:106–111
Cahan WG, Woodard HQ, Higinbotham NL, Stewart FW, Coley BL (1948) Sarcoma arising in irradiated bone; report of 11 cases. Cancer 1(1):3–29
Schrantz JL, Araoz CA (1972) Radiation induced meningeal fibrosarcoma. Arch Pathol 93(1):26–31
Sadamori N, Shibata S, Mine M, Miyazaki H, Miyake H, Kurihara M, Tomonaga M, Sekine I, Okumura Y (1996) Incidence of intracranial meningiomas in Nagasaki atomic-bomb survivors. Int J Cancer 67(3):318–322
Claus EB, Wiemels J, Wrensch M (2013) Dental x-rays and risk of meningioma: response to Drs. Calnon, Jorgensen, and white. Cancer 119(2):465–466
Albright EC, Allday RW (1967) Thyroid carcinoma after radiation therapy for adolescent acne vulgaris. Jama 199(4):280–281
Modan B, Baidatz D, Mart H, Steinitz R, Levin SG (1974) Radiation-induced head and neck tumours. Lancet 1(7852):277–279
Yamanaka R, Hayano A, Kanayama T (2017) Radiation-Induced Meningiomas: An Exhaustive Review of the Literature. World Neurosurg 97:635–644 e8
Umansky F, Shoshan Y, Rosenthal G, Fraifeld S, Spektor S (2008) Radiation-induced meningioma. Neurosurg Focus 24(5):E7
Toh CH, Castillo M, Wong AMC, Wei KC, Wong HF, Ng SH, Wan YL (2008) Differentiation between classic and atypical meningiomas with use of diffusion tensor imaging. AJNR Am J Neuroradiol 29(9):1630–1635
Brada M, Ford D, Ashley S, Bliss JM, Crowley S, Mason M, Rajan B, Traish D (1992) Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. Bmj 304(6838):1343–1346
Yamanaka R, Hayano A (2015) Radiation-Induced Glioma, in Molecular Considerations and Evolving Surgical Management Issues in the Treatment of Patients with a Brain Tumor, T. Lichtor, Editor. IntechOpen p 1004
Elsamadicy AA, Babu R, Kirkpatrick JP, Adamson DC (2015) Radiation-induced malignant gliomas: a current review. World Neurosurg 83(4):530–542
Johnson DR, Ma DJ, Buckner JC, Hammack JE (2012) Conditional probability of long-term survival in glioblastoma: a population-based analysis. Cancer 118(22):5608–5613
Ciricillo SF, Cogen PH, Edwards MS (1994) Pediatric cryptic vascular malformations: presentation, diagnosis and treatment. Pediatr Neurosurg 20(2):137–147
Lew SM, Morgan JN, Psaty E, Lefton DR, Allen JC, Abbott R (2006) Cumulative incidence of radiation-induced cavernomas in long-term survivors of medulloblastoma. J Neurosurg 104(2 Suppl):103–107
Gastelum E, Sear K, Hills N, Roddy E, Randazzo D, Chettout N, Hess C, Cotter J, Haas-Kogan DA, Fullerton H, Mueller S (2015) Rates and characteristics of radiographically detected intracerebral cavernous malformations after cranial radiation therapy in pediatric cancer patients. J Child Neurol 30(7):842–849
Haller S, Vernooij MW, Kuijer JPA, Larsson EM, Jäger HR, Barkhof F (2018) Cerebral microbleeds: imaging and clinical significance. Radiology 287(1):11–28
Salzman KL, Osborn AG, House P, Jinkins JR, Ditchfield A, Cooper JA, Weller RO (2005) Giant tumefactive perivascular spaces. AJNR Am J Neuroradiol 26(2):298–305
Ogawa T et al (1995) Unusual widening of Virchow-Robin spaces: MR appearance. AJNR Am J Neuroradiol 16(6):1238–1242
Gopinath M, Nagesh C, Kesavadas C (2018) Post radiation evolution of giant Virchow-Robin spaces in a case of pituitary macroadenoma. Indian J Radiol Imaging 28(3):373–374
Mark IT, Carr CM, Ruff MW, Flanagan EP, Johnson DR (2020) Enlarging perivascular spaces following radiation therapy in the brain: a report of 2 cases and literature review. World Neurosurg 138:436–439
Brandsma D, van Helvoirt R, Taphoorn MJ (2001) Multiple cysts in the cerebral white matter: a rare complication of whole brain radiation therapy. J Neuro-Oncol 53(1):51–54
Edmister WB, Lane JI, Gilbertson JR, Brown RD, Pollock BE (2005) Tumefactive cysts: a delayed complication following radiosurgery for cerebral arterial venous malformations. AJNR Am J Neuroradiol 26(5):1152–1157
Wang YX et al (2010) Evolution of radiation-induced brain injury: MR imaging-based study. Radiology 254(1):210–218
Zhou X, Liao X, Ren X, Xiang K, Hu Q, Zhang M, He H, Shen L, Wei Q (2017) Dynamic MRI follow-up of radiation encephalopathy in the temporal lobe following nasopharyngeal carcinoma radiotherapy. Oncol Lett 14(1):715–724
Pomeraniec IJ, Ding D, Starke RM, Liu KC, Mrachek EK, Lopes MB, Sheehan JP (2018) Delayed cyst formation after stereotactic radiosurgery for brain arteriovenous malformations. J Neurosurg 129(4):937–946
Bompaire F, Lahutte M, Buffat S, Soussain C, Ardisson AE, Terziev R, Sallansonnet-Froment M, de Greslan T, Edmond S, Saad M, Nioche C, Durand T, Alamowitch S, Xuan KH, Delattre JY, Renard JL, Taillia H, Chargari C, Psimaras D, Ricard D (2018) New insights in radiation-induced leukoencephalopathy: a prospective cross-sectional study. Support Care Cancer 26(12):4217–4226
Trifiletti DM, Lee CC, Schlesinger D, Larner JM, Xu Z, Sheehan JP (2015) Leukoencephalopathy after stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 93(4):870–878
Bargiotas I et al (2018) Balance impairment in radiation induced leukoencephalopathy patients is coupled with altered visual attention in natural tasks. Front Neurol 9:1185
Monaco EA 3rd et al (2013) Leukoencephalopathy after whole-brain radiation therapy plus radiosurgery versus radiosurgery alone for metastatic lung cancer. Cancer 119(1):226–232
Sarbu N, Shih RY, Jones RV, Horkayne-Szakaly I, Oleaga L, Smirniotopoulos JG (2016) White matter diseases with radiologic-pathologic correlation. Radiographics 36(5):1426–1447
Dietrich J, Winter SF, Klein JP (2017) Neuroimaging of brain tumors: Pseudoprogression, pseudoresponse, and delayed effects of chemotherapy and radiation. Semin Neurol 37(5):589–596
Mayinger M, Kraft J, Lohaus N, Weller M, Schanne D, Heitmann J, Willmann J, Wilke L, Krayenbuehl J, Tanadini-Lang S, Guckenberger M, Andratschke N (2020) Leukoencephalopathy after prophylactic whole-brain irradiation with or without hippocampal sparing: a longitudinal magnetic resonance imaging analysis. Eur J Cancer 124:194–203
Rimkus Cde M et al (2014) Toxic leukoencephalopathies, including drug, medication, environmental, and radiation-induced encephalopathic syndromes. Semin Ultrasound CT MR 35(2):97–117
Nagtegaal SHJ et al (2020) Effect of radiation therapy on cerebral cortical thickness in glioma patients: Treatment-induced thinning of the healthy cortex. Neurooncol Adv 2(1):vdaa060
Cummings M, Dougherty DW, Mohile NA, Walter KA, Usuki KY, Milano MT (2016) Severe radiation-induced leukoencephalopathy: case report and literature review. Adv Radiat Oncol 1(1):17–20
Valk PE, Dillon WP (1991) Radiation injury of the brain. AJNR Am J Neuroradiol 12(1):45–62
Kumar Y, Drumsta D, Mangla M, Gupta N, Hooda K, Almast J, Mangla R (2017) Toxins in brain! Magnetic resonance (MR) imaging of toxic leukoencephalopathy - a pictorial essay. Pol J Radiol 82:311–319
Tsuruda JS, Kortman KE, Bradley WG, Wheeler DC, van Dalsem W, Bradley TP (1987) Radiation effects on cerebral white matter: MR evaluation. AJR Am J Roentgenol 149(1):165–171
Perrini P, Scollato A, Cioffi F, Mouchaty H, Conti R, di Lorenzo N (2002) Radiation leukoencephalopathy associated with moderate hydrocephalus: intracranial pressure monitoring and results of ventriculoperitoneal shunting. Neurol Sci 23(5):237–241
Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, Choucair A, Fox S, Suh JH, Roberge D, Kavadi V, Bentzen SM, Mehta MP, Watkins-Bruner D, for the Radiation Therapy Oncology Group (RTOG) (2013) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro-Oncology 15(10):1429–1437
Brown PD, Gondi V, Pugh S, Tome WA, Wefel JS, Armstrong TS, Bovi JA, Robinson C, Konski A, Khuntia D, Grosshans D, Benzinger TLS, Bruner D, Gilbert MR, Roberge D, Kundapur V, Devisetty K, Shah S, Usuki K, Anderson BM, Stea B, Yoon H, Li J, Laack NN, Kruser TJ, Chmura SJ, Shi W, Deshmukh S, Mehta MP, Kachnic LA, for NRG Oncology (2020) Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J Clin Oncol 38(10):1019–1029
Bartleson JD, Krecke KN, O'Neill BP, Brown PD (2003) Reversible, strokelike migraine attacks in patients with previous radiation therapy. Neuro-Oncology 5(2):121–127
Black DF, Bartleson JD, Bell ML, Lachance DH (2006) SMART: stroke-like migraine attacks after radiation therapy. Cephalalgia 26(9):1137–1142
Black DF, Morris JM, Lindell EP, Krecke KN, Worrell GA, Bartleson JD, Lachance DH (2013) Stroke-like migraine attacks after radiation therapy (SMART) syndrome is not always completely reversible: a case series. AJNR Am J Neuroradiol 34(12):2298–2303
Di Stefano AL et al (2019) Stroke-like events after brain radiotherapy: a large series with long-term follow-up. Eur J Neurol 26(4):639–650
Katras T, Baltazar U, Colvett K, Rush D, Dunn J, Stanton P Jr (1999) Radiation-related arterial disease. Am Surg 65(12):1176–1179
Murphy ES, Xie H, Merchant TE, Yu JS, Chao ST, Suh JH (2015) Review of cranial radiotherapy-induced vasculopathy. J Neuro-Oncol 122(3):421–429
Yang WH, Yang YH, Chen PC, Wang TC, Chen KJ, Cheng CY, Lai CH (2019) Intracranial aneurysms formation after radiotherapy for head and neck cancer: a 10-year nationwide follow-up study. BMC Cancer 19(1):537
Ullrich NJ, Robertson R, Kinnamon DD, Scott RM, Kieran MW, Turner CD, Chi SN, Goumnerova L, Proctor M, Tarbell NJ, Marcus KJ, Pomeroy SL (2007) Moyamoya following cranial irradiation for primary brain tumors in children. Neurology 68(12):932–938
Desai SS, Paulino AC, Mai WY, Teh BS (2006) Radiation-induced moyamoya syndrome. Int J Radiat Oncol Biol Phys 65(4):1222–1227
Zwagerman NT, Foster K, Jakacki R, Khan FH, Yock TI, Greene S (2014) The development of moyamoya syndrome after proton beam therapy. Pediatr Blood Cancer 61(8):1490–1492
Chao ST, Ahluwalia MS, Barnett GH, Stevens GHJ, Murphy ES, Stockham AL, Shiue K, Suh JH (2013) Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys 87(3):449–457
Loganadane G, Dhermain F, Louvel G, Kauv P, Deutsch E, le Péchoux C, Levy A (2018) Brain radiation necrosis: current management with a focus on non-small cell lung cancer patients. Front Oncol 8:336
Vellayappan B, Tan CL, Yong C, Khor LK, Koh WY, Yeo TT, Detsky J, Lo S, Sahgal A (2018) Diagnosis and management of radiation necrosis in patients with brain metastases. Front Oncol 8:395
Kumar AJ, Leeds NE, Fuller GN, van Tassel P, Maor MH, Sawaya RE, Levin VA (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217(2):377–384
Rogers LR, Gutierrez J, Scarpace L, Schultz L, Ryu S, Lord B, Movsas B, Honsowetz J, Jain R (2011) Morphologic magnetic resonance imaging features of therapy-induced cerebral necrosis. J Neuro-Oncol 101(1):25–32
Litkowski P, Young RJ, Wolden SL, Souweidane MM, Haque S, Gilheeney SW (2012) Collision in the inferior olive: hypertrophic olivary degeneration complicated by radiation necrosis in brainstem primitive neuroendocrine tumor. Clin Imaging 36(4):371–374
Verma N, Cowperthwaite MC, Burnett MG, Markey MK (2013) Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro-Oncology 15(5):515–534
Chuang MT, Liu YS, Tsai YS, Chen YC, Wang CK (2016) Differentiating radiation-induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy: a meta-analysis. PLoS One 11(1):e0141438
Chao ST, Suh JH, Raja S, Lee SY, Barnett G (2001) The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer 96(3):191–197
Dequesada IM, Quisling RG, Yachnis A, Friedman WA (2008) Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery 63(5):898–903 discussion 904
Stockham AL et al. (2010) Validation of the ‘Lesion Quotient’ as a Radiographic Tool to Distinguish between Radiation Necrosis and Tumor Progression for Brain Metastases Treated with Stereotactic Radiosurgery. in In: Proceedings of the American Society for Radiation Oncology. San Diego, CA: International Journal of Radiation Oncology biology + physics
Nael K, Bauer AH, Hormigo A, Lemole M, Germano IM, Puig J, Stea B (2018) Multiparametric MRI for differentiation of radiation necrosis from recurrent tumor in patients with treated Glioblastoma. AJR Am J Roentgenol 210(1):18–23
Nguyen HS, Milbach N, Hurrell SL, Cochran E, Connelly J, Bovi JA, Schultz CJ, Mueller WM, Rand SD, Schmainda KM, LaViolette PS (2016) Progressing bevacizumab-induced diffusion restriction is associated with coagulative necrosis surrounded by viable tumor and decreased overall survival in patients with recurrent glioblastoma. AJNR Am J Neuroradiol 37(12):2201–2208
Zakhari N, Taccone MS, Torres C, Chakraborty S, Sinclair J, Woulfe J, Jansen GH, Nguyen TB (2018) Diagnostic accuracy of centrally restricted diffusion in the differentiation of treatment-related necrosis from tumor recurrence in high-grade gliomas. AJNR Am J Neuroradiol 39(2):260–264
Prah MA, al-Gizawiy MM, Mueller WM, Cochran EJ, Hoffmann RG, Connelly JM, Schmainda KM (2018) Spatial discrimination of glioblastoma and treatment effect with histologically-validated perfusion and diffusion magnetic resonance imaging metrics. J Neuro-Oncol 136(1):13–21
Razek A et al (2018) Differentiation of residual/recurrent gliomas from postradiation necrosis with arterial spin labeling and diffusion tensor magnetic resonance imaging-derived metrics. Neuroradiology 60(2):169–177
Young YH, Lou PJ (1999) Post-irradiation sudden deafness. J Laryngol Otol 113(9):815–817
Wang LF, Kuo WR, Ho KY, Lee KW, Lin CS (2004) A long-term study on hearing status in patients with nasopharyngeal carcinoma after radiotherapy. Otol Neurotol 25(2):168–173
Schultz C, Goffi-Gomez MVS, Pecora Liberman PH, Pellizzon ACA, Carvalho AL (2010) Hearing loss and complaint in patients with head and neck cancer treated with radiotherapy. Arch Otolaryngol Head Neck Surg 136(11):1065–1069
Levin LA, Gragoudas ES, Lessell S (2000) Endothelial cell loss in irradiated optic nerves. Ophthalmology 107(2):370–374
Ryu S, Kolozsvary A, Jenrow KA, Brown SL, Kim JH (2007) Mitigation of radiation-induced optic neuropathy in rats by ACE inhibitor ramipril: importance of ramipril dose and treatment time. J Neuro-Oncol 82(2):119–124
Doroslovački P, Tamhankar MA, Liu GT, Shindler KS, Ying GS, Alonso-Basanta M (2018) Factors associated with occurrence of radiation-induced optic neuropathy at "safe" radiation dosage. Semin Ophthalmol 33(4):581–588
Varoquaux A, Rager O, Dulguerov P, Burkhardt K, Ailianou A, Becker M (2015) Diffusion-weighted and PET/MR imaging after radiation therapy for malignant head and neck tumors. Radiographics 35(5):1502–1527
Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J (2010) Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys 76(3 Suppl):S28–S35
Indaram M, Ali FS, Levin MH (2015) In search of a treatment for radiation-induced optic neuropathy. Curr Treat Options Neurol 17(1):325
Danesh-Meyer HV (2008) Radiation-induced optic neuropathy. J Clin Neurosci 15(2):95–100
Reichstein D (2015) Current treatments and preventive strategies for radiation retinopathy. Curr Opin Ophthalmol 26(3):157–166
Kline LB, Kim JY, Ceballos R (1985) Radiation optic neuropathy. Ophthalmology 92(8):1118–1126
Viebahn M, Barricks ME, Osterloh MD (1991) Synergism between diabetic and radiation retinopathy: case report and review. Br J Ophthalmol 75(10):629–632
Emami B, Lyman J, Brown A, Cola L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M (1991) Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21(1):109–122
Jackson TL, Chakravarthy U, Kaiser PK, Slakter JS, Jan E, Bandello F, O'Shaughnessy D, Gertner ME, Danielson L, Moshfeghi DM, INTREPID Study Group (2013) Stereotactic radiotherapy for neovascular age-related macular degeneration: 52-week safety and efficacy results of the INTREPID study. Ophthalmology 120(9):1893–1900
Jackson TL, Chakravarthy U, Slakter JS, Muldrew A, Shusterman EM, O'Shaughnessy D, Arnoldussen M, Gertner ME, Danielson L, Moshfeghi DM (2015) Stereotactic radiotherapy for neovascular age-related macular degeneration: year 2 results of the INTREPID study. Ophthalmology 122(1):138–145
Nakissa N, Rubin P, Strohl R, Keys H (1983) Ocular and orbital complications following radiation therapy of paranasal sinus malignancies and review of literature. Cancer 51(6):980–986
Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR (1994) Radiation retinopathy after external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys 30(4):765–773
Shukovsky LJ, Fletcher GH (1972) Retinal and optic nerve complications in a high dose irradiation technique of ethmoid sinus and nasal cavity. Radiology 104(3):629–634
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Presentation at a meeting
This work has not been presented at any meetings.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Intracranial radiation therapy, though clinically invaluable, has a wide spectrum of potential long-term negative effects.
• Knowledge of sequelae of intracranial radiation, as well as their imaging findings, is therefore important.
• Here, we summarize important clinical and radiologic features of selected side effects of intracranial radiation therapy, focusing mainly on those with recognizable imaging findings.
Rights and permissions
About this article
Cite this article
Carr, C.M., Benson, J.C., DeLone, D.R. et al. Intracranial long-term complications of radiation therapy: an image-based review. Neuroradiology 63, 471–482 (2021). https://doi.org/10.1007/s00234-020-02621-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02621-7