Abstract
Purpose
Posterior circulation perforator aneurysms (PCPAs) are a rare type of intracranial aneurysms whose natural history and optimal clinical management are still largely unexplored. This study aims to report our experience with treating ruptured PCPAs and to provide a systematic review of the literature to compare the two most established treatment options, endovascular stenting, and conservative management including administration of antifibrinolytic drugs and watchful waiting.
Methods
We performed a systematic review of the literature following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Major databases were searched for case reports and case report series written in the English language between 1995 and 2020. Additionally, we retrospectively reviewed our stroke center database for cases of ruptured PCPAs between January 2014 and July 2020. Endovascular stenting and conservative treatment were compared using endpoints, including favorable outcome rate (mRS 0-2), occlusion rate, mortality rate, periinterventional complication rate, and re-hemorrhage rate.
Results
We identified 31 patients treated endovascularly using stents and 33 patients treated conservatively, with the administration of antifibrinolytic drugs in 3 of them. Our analysis showed no statistically significant difference between the groups, except for the occlusion rate.
Conclusions
The optimal management strategy of PCPAs is still unknown, but stenting can be considered as an effective occlusion method with an acceptable complication rate. Preventive ventricular drainage may be necessary due to the high hydrocephalus rate encountered in ruptured PCPAs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Posterior circulation perforator aneurysms (PCPAs) are a rare subtype of intracranial aneurysms first described by Ghogawala et al. in 1996 [1], with 89 cases reported in the literature. Aboukais et al. defined basilar artery perforator aneurysms (BAPAs) as those whose neck is entirely on a perforator artery without the direct involvement of the basilar trunk [2]. Certain characteristics of these aneurysms, such as small size and dissecting pathogenesis, have previously been highlighted as obstacles leading to diagnostic difficulties [3]. This hinders the understanding of their natural history, which is thought to differ from more common saccular aneurysms [4]. A classification system was proposed by Satti et al., based on the relationship of the aneurysm to the parent perforator artery [5]. However, there is still no clear definition of PCPA in the literature.
Neurosurgical treatment is complicated by location, small size, and connection of the aneurysm to perforator arteries. As a result, an increasing number of patients are recently treated using an endovascular approach, with an evident shift toward using stents as a mean of flow-diversion-mediated aneurysm occlusion. Furthermore, some authors appraise conservative treatment as the safest with outcome comparable to active treatment [6,7,8]. As of yet, no clear consensus has been achieved on the optimal treatment approach to ruptured PCPAs.
The objective of this article was to provide a systematic review of conservative and endovascular treatment of ruptured PCPAs using stents. The two treatment approaches were compared focusing on mortality rate, re-hemorrhage rate, hydrocephalus rate, and long-term functional outcome quantified using the modified Rankin Scale (mRS) score. Additionally, we present a case series of our single-center experience of endovascular treatment of ruptured PCPAs and a single case of conservatively treated ruptured PCPA.
Materials and methods
A comprehensive literature search of PubMed (Medline), Scopus, and Directory of Open Access Journals (DOAJ) was conducted to identify publications related to ruptured PCPAs published between January 1995 and July 2020. The search was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9]. The following keywords were queried singly and in combination with Boolean operators: “basilar artery perforator aneurysms,” “posterior circulation perforator aneurysms,” “perforator aneurysms,” and “tiny aneurysms.” The search was limited to studies written in the English language. Inclusion criteria were as follows: (1) studies reporting ruptured PCPAs, such as case reports and case series, and (2) case reports or case series with reported follow-up, either clinical or radiographic, and a follow-up outcome score. Exclusion criteria were as follows: (1) review articles, letters to the editor, and cases missing angiographic or clinical follow-up; (2) cases of PCPAs associated with other vascular malformations, such as arteriovenous malformation (AVM) or pure arterial malformation; (3) cases of posttraumatic PCPAs; (4) cases described in multiple articles; and (5) Satti type I aneurysms. The included studies were selected and independently reviewed by two of the authors (D.G., T.H.), with discrepancies in the obtained data resolved by consensus. Additional manual cross-checking of references was performed until no new articles were found.
Data extracted from the studies included patient age, sex, Hunt and Hess scale, Fisher score, subarachnoid hemorrhage (SAH) location, diagnostic imaging modality and post-SAH time of diagnosis, the number of vascular imaging examinations needed to establish the diagnosis, location and size of the aneurysm, treatment modality, presence of hydrocephalus, presence of re-hemorrhage, presence of ischemia, mortality, duration and type of follow-up, and functional outcome quantified using modified Rankin Scale (mRS) score.
Additionally, we performed a retrospective search of our prospectively maintained tertiary stroke center database for patients treated for ruptured PCPAs between January 1, 2014, and July 1, 2020. For all identified cases, the same dataset was extracted as for the cases from the literature review. Ethics approval was obtained from the Ethics Committee of our Institution.
The cases were divided into two groups to compare treatment approaches. The first group included patients treated actively using stents as a means of flow diversion, including sole stenting, stent-in-stent technique, and flow diverters. The second group included patients treated conservatively, including both watchful waiting and administration of antifibrinolytic therapy.
To detect any differences between the two groups, they were compared using the following baseline characteristics: (a) patient sex; (b) patient age; (c) higher Fisher grade, i.e., Fisher grade 3 or 4; and (d) aneurysm size. For each group the following endpoints were evaluated and compared between the two groups: (a) follow-up duration; (b) favorable outcome rate, defined as mRS 0–2; (c) mortality rate, defined as mRS 6 or otherwise stated in the article; (d) occlusion rate, defined as RRC (Raymond Roy Classification) 1 or otherwise stated in the article; (e) peri-interventional complication rate, defined as any neurovascular complication in the 30 days following the intervention; (f) hydrocephalus rate, present either at presentation or as a late complication of SAH; and (g) re-hemorrhage rate, defined as re-hemorrhage after endovascular treatment or during and after conservative treatment.
The baseline characteristics and endpoints were analyzed to identify potential statistically significant differences between the two groups. Continuous variables were presented as means and ranges, categorical variables as rates or ratios. Continuous variables were compared using the Mann-Whitney U test and categorical variables using the Fisher exact test, given the small sample size and small presumed frequencies. The statistical tests were performed using SPSS v26.0 (IBM, Armonk, New York, USA). Results with p < 0.05 were considered statistically significant.
To assess the risk of bias, we used a modification of the Newcastle Ottawa Scale. The methodology was taken from Granja et al. [8] with a few alterations. Each study was independently assessed by the two authors using the following criteria: (1) clear demographic data, (2) severity on presentation, (3) treatment effectiveness, (4) radiological follow-up, and (5) clinical follow-up. Studies lacking either radiological or clinical follow-up were considered high risk of bias. For the remaining criteria, studies were considered low risk of bias if they missed none or one of the remaining criteria, medium risk if they missed two, and high risk if they missed all three remaining criteria.
Results
The retrospective search of our database yielded six cases of ruptured PCPAs (male = 4), age range 46–65 years, with a median Hunt and Hess scale (HHS) and a Fisher score of 2 and 4, respectively. Five patients presented with a diffuse SAH pattern (83%), one with perimesencephalic SAH (17%). The aneurysms were identified during the initial angiography in four cases (67%), their sizes were between 1.2–4 mm (2.1 ± 1 mm). In five patients, a distal third basilar perforator was harboring the aneurysm (83%), and in one patient, the aneurysm was located on a V4 segment of the vertebral artery (VA) perforator (17%). Remarkably, in three patients a significant reduction of SAH was demonstrated on repeat pre-embolization CT exams with a remaining persistent clot in the interpeduncular/prepontine cistern. The clot was visible up to post-SAH day 12 in Patient #2, post-SAH day 19 in Patient #5, and post-SAH day 10 in Patient #6. In the rest of the patients, no follow-up pre-embolization CT exams were available due to shorter diagnosis delay.
Out of five endovascularly treated patients, four were treated using the stent-in-stent technique and one patient using sole stenting with clinical and imaging follow-up ranging from 4 to 18 months (average 13 months). In one patient who underwent emergency stenting, a bolus half-dose of i.v. eptifibatide was administered immediately before stent deployment, with the remaining half-dose administered by 12-h infusion. All other patients were pre-procedurally started on 75 mg of clopidogrel. Platelet reactivity was routinely checked with a point-of-care platelet reactivity device (VerifyNowTM PRU Test). If the therapeutic range was reached, an additional 500 mg ASA bolus was administered intravenously immediately before the procedure. Otherwise, the patient was put on ticagrelor instead of clopidogrel. The postprocedural antiplatelet regime consisted of clopidogrel 75 mg plus ASA 100 mg daily or ticagrelor 90 mg twice daily plus ASA 100 mg daily. Clopidogrel and ticagrelor were discontinued after a variable time period, depending on individual circumstances, and ASA was continued for up to a year. Re-hemorrhage occurred in one of the endovascularly treated patients. Three patients had an excellent outcome (modified Rankin Scale (mRS) 0), one had a slight right-sided hemiparesis without significant disability (mRS 1), and one ended up with severe disability (mRS 5). One patient was treated conservatively despite re-hemorrhage and had an excellent outcome (mRS 0). Five out of six patients (83%) developed hydrocephalus requiring external ventricular drainage (EVD) or ventriculoperitoneal (VP) shunt. General data regarding these six patients can be found in Table 1. Detailed presentations of two patients are presented as captions under Fig. 1 (Patient 1) and Fig. 2 (Patient 4).
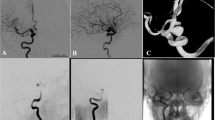
Patient 1 presented with a Hunt Hess grade 2 subarachnoid hemorrhage (SAH). Initial non-contrast head CT (a) demonstrated diffuse SAH spreading through lateral apertures into the fourth ventricle (arrow). Cerebral digital subtraction angiography (DSA) (b) showed a small 2.2-mm aneurysm (arrow) arising from a V4 segment of the left vertebral artery (VA) perforator. Severe isolated vasospasm of the left V4 segment is also noticeable. The aneurysm (arrow), as well as its relation to the perforator and the left VA, could be better appreciated on a 3D shaded-surface images (c). The patient underwent stenting. However, a severe vasospasm with stent shortening and migration occurred during the procedure and it was impossible to place the second stent optimally. Therefore the second stent was placed proximally to the first one. A follow-up non-enhanced head CT (d) performed 7 days later, after clinical deterioration showed a larger amount of SAH (arrows) suggestive of repeat hemorrhage. Repeat hemorrhage most likely occurred because of suboptimal placement of the stents, creating a risk for non-coverage of the perforator origin or endoleak in a patient treated with dual antiplatelet therapy. A follow-up DSA (e) showed persistent filling of the aneurysm at post-SAH month 9
Patient 4 presented with a Hunt Hess grade 2 subarachnoid hemorrhage (SAH). Initial non-contrast head CT (a) demonstrated diffuse SAH extending into the fourth ventricle. A 2-month follow-up DSA (b) demonstrated a small 1.5-mm aneurysm of a distal basilar perforator (arrow). A non-subtracted image (c) shows two stents (LEO Baby 2.5 × 12 mm and LEO Baby 2.5 × 18 mm) (arrow) deployed over the basilar tip 4 days later. A month later the patient developed hydrocephalus requiring ventriculoperitoneal (VP) shunting. A non-enhanced head CT (d) showed dilatation of the temporal horns. In-situ stents can be seen in the basilar artery (arrow). Eighteen months later, a follow-up DSA demonstrated no filling of the aneurysm (e)
Systematic review
In our literature database search, we identified 73 articles. 37 articles were identified as duplicated, and 7 were excluded based on title and abstract alone, as defined by the exclusion criteria (Fig. 3). The remaining 29 articles were evaluated in full text with cross-referencing, resulting in the identification of additional 7 articles. In the 36 articles, 89 cases were identified and assessed for eligibility [1,2,3,4,5,6,7, 10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Eleven cases were excluded. After applying the exclusion criteria, the remaining 78 cases together with 6 cases of our own comprised 84 cases. Data regarding all cases is presented in Supplement Table 1.
The mean patient age was 57 ± 10.5 years, with an age range of 27–82 years. In contrast to the more common saccular cerebral aneurysms, men with ruptured PCPAs outnumbered women by a 1.78:1 ratio. The aneurysms were usually tiny, with a mean diameter of 2.2 ± 1.4 mm (0.5–7 mm). The median Hunt-Hess and Fisher scores were 2 and 3, respectively. The most commonly reported hemorrhage pattern was diffuse SAH (68%). Perimesencephalic hemorrhage was reported in 19% and prepontine hemorrhage in the remaining 13% of cases. First cerebral catheter angiography was negative in 61% of cases, with the aneurysms first visualized on repeat vascular imaging. The rate of hydrocephalus was 34%.
Patients treated endovascularly using stents and patients treated conservatively, including watchful waiting and administration of drugs, were extracted and divided accordingly into two groups to analyze and compare the two treatment modalities. These were described in 23 studies that were assessed for risk of bias (Table 2). Of the included studies, 15 had a low risk of bias (65%), and 8 had a high risk of bias (35%).
The endovascular stenting group consisted of 31 patients (Table 3). The patients were treated as follows: 14 stent-in-stent (45%), 13 flow-diverter stent (42%), 3 sole stenting (10%), and 1 using both stent and flow diverter (3%). In 8 cases, periprocedural complications were reported (26%). Hydrocephalus was reported in 10 cases (32%), repeat hemorrhage in one case (3%), and ischemia in six cases (20%). The occlusion of the aneurysm was verified in 29 cases (94%). There were no fatal outcomes. The average follow-up period was 14.2 months, ranging from 1 to 72 months. The mean clinical outcome score was mRS 1 with values between mRS 0 and mRS 5. A favorable outcome (mRS 0–2) was reported in 90% of cases.
The conservative group consisted of 33 patients (Table 4). Three patients were treated using antifibrinolytic drugs (9%) [7]; watchful waiting was employed in rest. In eight cases there was a failed endovascular approach (24%). Hydrocephalus was reported in 10 cases (32%), repeat hemorrhage in three cases (10%), and ischemia in nine cases (32%). The occlusion of the aneurysm was verified in 23 cases (70%). A fatal outcome occurred in 2 patients (6%). The follow-up ranged from 1 to 78 months, with an average of 15.1 months. In three cases, patients were reported as lost to follow-up (LTFU) (9%). The mean outcome score was mRS 1.50 (range mRS 0–mRS 6) with a favorable outcome (mRS 0–2) reported in 77% of cases.
Our statistical analysis showed no significant difference between the two groups in baseline characteristics, including patient sex and age, higher Fisher grade rate, and aneurysm size (p < 0.05). Regarding the endpoints, no significant difference was found for follow-up duration, favorable outcome rate, mortality rate, re-hemorrhage rate, hydrocephalus rate, and ischemia rate (p < 0.05). The aneurysm occlusion rates were 94% vs. 70%, with a statistically significant difference between the two groups (p = 0.023). The results of these tests are presented in Table 5.
Discussion
Aneurysms arising from posterior circulation perforator arteries are very rare. Among them, the most common are basilar artery (BA) perforator aneurysms, first described by Ghogawala et al. in 1996 [1]. They are defined as aneurysms whose neck is entirely located on a perforator artery without the direct involvement of the basilar trunk [2]. Satti et al. further proposed a classification scheme, analog to that of lenticulostriate aneurysms and based on their relationship with the parent vessel and perforating arteries [5]. Type I aneurysms arise from the basilar trunk adjacent to the perforator origin without direct involvement, Type IIa aneurysms incorporate the origin of the perforator, Type IIb aneurysms have the perforator arising from the dome of the aneurysm, and type III aneurysms are fusiform aneurysms arising beyond the basilar artery. As Satti type I aneurysms do not involve perforator arteries, they diverge from Aboukais’ definition and as such were excluded from this review.
BA perforator arteries are divided into three groups based on their origin: rostral (mesencephalic perforator arteries originating distal to the superior cerebellar artery (SCA)), middle (pontine perforator arteries originating between the anterior inferior cerebellar artery (AICA) and SCA), and caudal (proximal to AICA). There are between 2 and 5 rostral and 5 to 9 middle perforator arteries [37]. According to our literature analysis, most PCPAs arose from rostral BA perforators (61%), as was the case with five of our patients. The second most common location was the middle BA perforators (31%). Perforator branches of the vertebral artery range in number from 1 to 3 [38]. To the best of our knowledge, Patient #1 is the first reported case so far. We found three cases of ruptured aneurysms located on a perforator artery arising from the P1 segment of the posterior cerebral artery (PCA) [15, 26, 33] and two cases of SCA perforator aneurysms [15, 28].
The most commonly reported hemorrhage pattern was a diffuse SAH (68%), with contained hemorrhage in the interpeduncular and prepontine cistern seen in the rest. Several authors noted the latter pattern to resemble benign non-aneurysmal perimesencephalic hemorrhage (BNPH) and hypothesized that a percentage of BNPH could be related to undetected PCPAs [1, 6, 11]. In BNPH, the center of hemorrhage is directly anterior to the midbrain with no extension to the lateral Sylvian fissures or the anterior hemispheric fissure [39]. Although BNPH is considered a well-defined entity, some reports suggested a limited intra- and interobserver agreement on CT [40]. Rinke et al. found that in 11 out of 12 patients with BNPH, a repeat 1-week post-SAH CT exam showed complete disappearance of the cisternal blood [39]. On the contrary, we identified a persistent clot in the interpeduncular or prepontine cistern in three of our Patients, who had repeat CT exams with persistent clot up to post-SAH day 19. However, we found no similar reports in the literature.
These aneurysms are characteristically tiny, with a mean diameter of 2.2 mm derived from our literature analysis. Due to slower blood flow in the harboring perforator artery, they demonstrate slow filling leading to difficult visualization [1, 3, 11, 23]. PCPAs are believed to be of dissecting origin [11] and often partially thrombosed. This was demonstrated by Mathieson et al., who presented a case with a macroscopic aneurysm diameter of 6 mm and an observable angiography filling of only 1 mm [23]. In our review, initial cerebral angiograms were negative in 61% of cases. A rigorous angiographic technique with proper patient immobilization, adequate injections, and repeat examinations are of most importance for successful visualization [11]. Some authors recommended a vigorous injection into the dominant vertebral artery, with contrast reflux into the contralateral vertebral artery, thereby minimizing the artifact from basilar artery contrast washout [7]. In the case of negative initial angiography, repeat imaging is required. However, the exact timing is still a matter of debate [2, 4, 7, 11, 23].
Hydrocephalus is a common complication of SAH, reported in approximately 20% of SAH patients [41, 42]. Acute hydrocephalus requiring preoperative EVD, ventilation on admission, aneurysms in the posterior circulation, and aneurysms size > 2.5 cm was identified as predictors of shunt-dependent hydrocephalus in an independent cohort of 3120 cases of aneurysmal SAH [43]. Cagnazzo et al. showed that the administration of antiplatelet drugs during the endovascular treatment of acutely ruptured intracranial aneurysms increases the risk of EVD-related hemorrhage, which can be minimized if EVD is placed before the procedure [44]. Furthermore, some authors recommended preprocedural EVD insertion when signs of obstructive hydrocephalus, intraventricular blood, or raised intracranial pressure are present before initiation of antiplatelet therapy [35]. Our limited case-series showed an unusually high rate of hydrocephalus (5/6 patients) compared with previously reported cases (34%). This could be due to differences in the definition and reporting of hydrocephalus. Therefore, it is our opinion that preventive ventricular drainage should be considered in patients with a large amount of intraventricular blood or early asymptomatic dilatation of ventricles.
Once identified, the optimal treatment of ruptured PCPA is still a matter of debate. We believe that coiling should be avoided as previous reviews reported it as the least successful first treatment approach [8]. Additionally, coiling and liquid embolization should be viewed as treatment options with inherent occlusion of the parent artery in an eloquent region with an otherwise potentially benign course when treated conservatively [8]. Some authors postulated that stent-within-stent placement across the aneurysm neck would divert flow away and impede blood outflow from the aneurysm thus promoting thrombosis [14], with a preserved flow within branches arising from the aneurysm itself or adjacent perforators due to continuous physiological demand [24]. The emersion of flow diverters provided a sufficient mean of PCPA occlusion, first employed in 2014 [36]. It was observed, however, that the risk of re-rupture is enhanced by the initiation of dual antiplatelet therapy, required after the placement of a flow diverter or stent [45]. Philips et al. reported a 14% rate of perforator territory infarctions and a 9,4% rate of permanent neurological complications when placing a flow diverter stent in the treatment of posterior circulation aneurysms [46]. Stenting represents a highly effective treatment option, as aneurysmal occlusion was verified in 94% of cases according to our analysis. The only two cases without demonstrated occlusion regarded a patient treated with flow diverter who suffered from subacute pontine ischemia with basilar artery enlargement and stent shortening [26] and Patient #1 from Our series, where a failed procedure occurred.
On the other hand, conservative management of PCPAs is favored by some authors, proposing that these aneurysms have a common benign course with a low re-hemorrhage rate [6, 18]. Buell et al. recommended initial conservative management with administration of antifibrinolytic therapy to patients with no absolute contraindications and consideration of endovascular treatment in case of PCPA enlargement on repeat imaging [7]. However, in the vast majority of conservatively treated patients watchful waiting was employed (91%). Our analysis showed an unusually high rate of ischemia among conservatively treated patients (32%), with reported failed endovascular approach in 56% of them [15, 18, 32]. Further studies evaluating the natural history of ruptured PCPAs are necessary because no safe active treatment within the 24-hour time period is currently available, unlike for other ruptured aneurysms. It is our opinion that this could be the main reason leading some clinicians towards a watchful waiting approach.
Some previous reports noted a lack of reporting of objective scales regarding aneurysm occlusion using stenting as a method of flow diversion [8], but this could be due to the inability to properly image such small aneurysms with the available resolution of current imaging equipment. Another reason could be the unknown value of such scales for further treatment choices. It is our opinion that in case of incomplete occlusion after treatment, no further active treatment should be pursued to avoid complications. On the contrary, re-hemorrhage and significant regrowth following stent treatment should prompt evaluation for further treatment.
The results of this study revealed no statistically significant difference in favorable outcome rate, mortality rate, re-hemorrhage rate, hydrocephalus rate, and ischemia rate between conservatively and endovascularly treated patients using stents. This shows that both treatment approaches can be used with a similar outcome. A significant difference in occlusion rates was found between the two groups. This result can be interpreted by the proportion of conservatively treated patients lost to follow-up, which was 9% according to our analysis.
We would like to stress the importance of careful evaluation of follow-up non-contrast CT exams additional to meticulous DSA and 3D-angiography of the posterior circulation, in search of a remnant blood clot in the interpeduncular or prepontine cistern. The presence of a persistent clot in a patient with angiography-negative perimesencephalic hemorrhage could indicate a possibility of a ruptured distal or mid-basilar PCPA. Considering the limited number of patients included in this study, further research is needed to validate this finding.
Limitations
The main limitations of this review are pertaining to the retrospective nature of case reports and case series. The data analysis is furthermore limited by the paucity of published cases and small sample sizes. Inconsistent reporting of information regarding aneurysm morphology, location, severity on presentation, hydrocephalus, treatment management, complications, follow-up duration and modality, and functional outcome resulted in several studies with a high risk of bias. Finally, we found a high rate of LTFU patients and patients with no follow-up vascular imaging in the conservative group, which possibly hinders the conclusiveness of the results.
Conclusion
In summary, PCPAs are potentially underdiagnosed pathology with imaging characteristics similar to BPNH and a generally favorable outcome following conservative treatment. Stenting has been shown as a safe and effective active treatment option; therefore, it is our opinion that the decision of treatment modality should be made on a case-by-case basis. We believe this rare disease needs to be further evaluated through new studies involving larger sample sizes and possibly a specific registry of PCPA cases designed by involved medical specialties.
References
Ghogawala Z, Shumacher JM, Ogilvy CS (1996) Distal basilar perforator artery aneurysm: case report. Neurosurgery 39:393–396. https://doi.org/10.1097/00006123-199608000-00034
Aboukais R, Zairi F, Estrade L, Quidet M, Leclerc X, Lejeune JP (2016) A dissecting aneurysm of a basilar perforating artery. Neurochirurgie 62:263–265. https://doi.org/10.1016/j.neuchi.2016.03.003
Peschillo S, Caporlingua A, Cannizzaro D, Resta M, Burdi N, Valvassori L, Pero G, Lanzino G (2016) Flow diverter stent treatment for ruptured basilar trunk perforator aneurysms. J Neurointerv Surg 8:190–196. https://doi.org/10.1136/neurintsurg-2014-011511
Sanchez-Mejia RO, Lawton MT (2007) Distal aneurysms of basilar perforating and circumferential arteries. J Neurosurg 107:654–659. https://doi.org/10.3171/JNS-07/09/0654
Satti SR, Vance AZ, Fowler D, Farmah AV, Sivapatham T (2017) Basilar artery perforator aneurysms (BAPAs): review of the literature and classification. J Neurointerv Surg 9:669–673. https://doi.org/10.1136/neurintsurg-2016-012407
Park SQ, Kwon O-K, Kim SH, Oh CW, Han MH (2009) Pre-mesencephalic subarachnoid hemorrhage: rupture of tiny aneurysms of the basilar artery perforator. Acta Neurochir (Wien) 151:1639–1646. https://doi.org/10.1007/s00701-009-0416-0
Buell TJ, Ding D, Raper DMS, Chen CJ, Hixson HR, Crowley RW, Evans AJ, Jensen ME, Liu KC (2018) Posterior circulation perforator aneurysms: a proposed management algorithm. J Neurointerv Surg 10:55–59. https://doi.org/10.1136/neurintsurg-2016-012891
Granja MF, Monteiro A, Agnoletto GJ, Jamal S, Sauvageau E, Aghaebrahim A, Hanel R (2020) A systematic review of non-trunk basilar perforator aneurysms: is it worth chasing the small fish? J Neurointerv Surg 12:412–416. https://doi.org/10.1136/neurintsurg-2019-015311
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535–b2535. https://doi.org/10.1136/bmj.b2535
Chau Y, Sachet M, Sédat J (2018) Should we treat aneurysms in perforator arteries from the basilar trunk? Review of 49 cases published in the literature and presentation of three personal cases. Interv Neuroradiol 24:22–28. https://doi.org/10.1177/1591019917734531
Chavent A, Lefevre P-H, Thouant P, Cao C, Kazemi A, Mourier K, Ricolfi F (2014) Spontaneous resolution of perforator aneurysms of the posterior circulation. J Neurosurg 121:1107–1111. https://doi.org/10.3171/2014.7.JNS132411
Chen L, Chen E, Chotai S, Tian X (2012) An endovascular approach to ruptured aneurysms of the circumferential branch of the basilar artery. J Clin Neurosci 19:527–531. https://doi.org/10.1016/j.jocn.2011.04.049
Daruwalla VJ, Syed FH, Elmokadem AH, Hurley MC, Shaibani A, Ansari SA (2016) Large basilar perforator pseudoaneurysm: a case report. Interv Neuroradiol 22:662–665. https://doi.org/10.1177/1591019916659261
Deshaies EM, Jacobsen W, Krishnamurthy S (2011) Enterprise stent-within-stent embolization of a basilar artery perforator aneurysm. World J Neurosci 01:45–48. https://doi.org/10.4236/wjns.2011.13007
Ding D, Starke RM, Jensen ME, Evans AJ, Kassell NF, Liu KC (2013) Perforator aneurysms of the posterior circulation: case series and review of the literature. J Neurointerv Surg 5:546–551. https://doi.org/10.1136/neurintsurg-2012-010557
Finitsis S, Derelle A-L, Tonnelet R, Anxionnat R, Bracard S (2017) Basilar perforator aneurysms: presentation of 4 cases and review of the literature. World Neurosurg 97:366–373. https://doi.org/10.1016/j.wneu.2016.10.038
Fiorella D, Albuquerque FC, Deshmukh VR, Woo HH, Rasmussen PA, Masaryk TJ, McDougall CG (2006) Endovascular Reconstruction with the Neuroform Stent as Monotherapy for the Treatment of Uncoilable Intradural Pseudoaneurysms. Neurosurgery 59:291–300. https://doi.org/10.1227/01.NEU.0000223650.11954.6C
Forbrig R, Eckert B, Ertl L, Patzig M, Brem C, Vollmar C, Röther J, Thon N, Brückmann H, Fesl G (2016) Ruptured basilar artery perforator aneurysms—treatment regimen and long-term follow-up in eight cases. Neuroradiology 58:285–291. https://doi.org/10.1007/s00234-015-1634-1
Gross BA, Puri AS, Du R (2013) Basilar trunk perforator artery aneurysms. Case report and literature review. Neurosurg Rev 36:163–168. https://doi.org/10.1007/s10143-012-0422-1
Hamel W, Grzyska U, Westphal M, Kehler U (2005) Surgical treatment of a basilar perforator aneurysm not accessible to endovascular treatment. Acta Neurochir (Wien) 147:1283–1286. https://doi.org/10.1007/s00701-005-0615-2
Jiang Y, Li Y (2017) Treatment of tiny intracranial aneurysms with guidewire manipulation. Chinese Neurosurg J 3:39. https://doi.org/10.1186/s41016-017-0103-6
Kim Y-J, Ko JH (2014) Sole stenting with large cell stents for very small ruptured intracranial aneurysms. Interv Neuroradiol 20:45–53. 10.15274/INR-2014-10007
Mathieson CS, Barlow P, Jenkins S, Hanzely Z (2010) An unusual case of spontaneous subarachnoid haemorrhage—a ruptured aneurysm of a basilar perforator artery. Br J Neurosurg 24:291–293. https://doi.org/10.3109/02688690903572095
Nyberg EMK, Chaudry MI, Turk AS, Spiotta AM, Fiorella D, Turner RD (2013) Report of two cases of a rare cause of subarachnoid hemorrhage including unusual presentation and an emerging and effective treatment option. J Neurointerv Surg 5:e30–e30. https://doi.org/10.1136/neurintsurg-2012-010387
Sivakanthan S, Carlson A, van Loveren H, Agazzi S (2014) Surgical clipping of a basilar perforator artery aneurysm: a case of avoiding perforator sacrifice. J Neurol Surg Part A Cent Eur Neurosurg 76:79–82. https://doi.org/10.1055/s-0033-1356488
Da Ros V, Diana F, Sabuzi F et al (2020) Flow diverters for ruptured posterior circulation perforator aneurysms: multicenter experience and literature review. J Neurointerv Surg 12:688–694. https://doi.org/10.1136/neurintsurg-2019-015558
Sahu C, Ashpilaya A (2017) Case series on perforator aneurysm: endovascular stenting—a safe strategy. J Clin Interv Radiol ISVIR 01:179–183. https://doi.org/10.1055/s-0037-1606374
Lee JJ, Huang M, Guerrero J, Desai VR, Jenson A, Austerman R, Diaz O, Britz GW (2020) Operative treatment of a superior cerebellar artery perforator dissecting aneurysm. Oper Neurosurg 0:1–6. https://doi.org/10.1093/ons/opz407
Murata T, Nomura S, Yamamori E, Takahashi S, Takase K (2020) Thrombosis of a basilar perforator aneurysm associated with pontine infarction in a patient with systemic lupus erythematosus. Radiol Case Reports 15:757–760. https://doi.org/10.1016/j.radcr.2020.03.009
Shlobin NA, Cantrell DR, Ansari SA, Hurley MC, Shaibani A, Jahromi BS, Potts MB (2020) Conservative management and natural history of ruptured basilar perforator artery aneurysms: two cases and literature review. World Neurosurg 138:218–222. https://doi.org/10.1016/j.wneu.2020.03.042
Inoue Y, Kusaka N, Ikushima K, Edaki H, Shinji Y, Itami H, Otsuka S, Nishiura T, Ogihara K (2020) A case of simple stenting for ruptured basilar perforator aneurysm. J Stroke Cerebrovasc Dis 29:104855. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.104855
Enomoto N, Shinno K, Tamura T, Shikata E, Shono K, Takase K (2020) Ruptured basilar artery perforator aneurysm: a case report and review of the literature. NMC Case Rep J 7:93–100. https://doi.org/10.2176/nmccrj.cr.2019-0143
Giordan E, Rabinstein AA, Cloft HJ, Lanzino G (2018) Teaching neuroimages: rupture and spontaneous resolution of a P1 perforator pseudoaneurysm. Neurology 90:e1730–e1731. https://doi.org/10.1212/WNL.0000000000005485
Apok V, Tarnaris A, Brydon HL (2013) An unusual aneurysm of a basilar perforating artery presenting with a subarachnoid haemorrhage. Br J Neurosurg 27:105–107. https://doi.org/10.3109/02688697.2012.717977
Bhogal P, AlMatter M, Hellstern V, Pérez MA, Lehmberg J, Ganslandt O, Bäzner H, Henkes H (2019) Basilar artery perforator aneurysms: report of 9 cases and review of the literature. J Clin Neurosci 63:122–129. https://doi.org/10.1016/j.jocn.2019.01.026
Chalouhi N, Jabbour P, Starke RM, Zanaty M, Tjoumakaris S, Rosenwasser RH, Gonzalez LF (2014) Treatment of a basilar trunk perforator aneurysm with the pipeline embolization device. Neurosurgery 74:E697–E701. https://doi.org/10.1227/NEU.0000000000000308
Marinkovic SV, Gibo H (1993) The surgical anatomy of the perforating branches of the basilar artery. Neurosurgery 33:80–87. https://doi.org/10.1227/00006123-199307000-00012
Marinković S, Milisavljević M, Gibo H, Maliković A, Djulejić V (2004) Microsurgical anatomy of the perforating branches of the vertebral artery. Surg Neurol 61:190–197. https://doi.org/10.1016/S0090-3019(03)00577-9
Rinkel GJ, Wijdicks EF, Vermeulen M et al Nonaneurysmal perimesencephalic subarachnoid hemorrhage: CT and MR patterns that differ from aneurysmal rupture. AJNR Am J Neuroradiol 12:829–834
Brinjikji W, Kallmes DF, White JB, Lanzino G, Morris JM, Cloft HJ (2010) Inter- and intraobserver agreement in CT characterization of nonaneurysmal perimesencephalic subarachnoid hemorrhage. Am J Neuroradiol 31:1103–1105. https://doi.org/10.3174/ajnr.A1988
Hasan D, Vermeulen M, Wijdicks EFM et al (1989) Management problems in acute hydrocephalus after subarachnoid hemorrhage. Stroke 20:747–753. https://doi.org/10.1161/01.STR.20.6.747
Heros RC (1989) Acute hydrocephalus after subarachnoid hemorrhage. Stroke 20:715–717. https://doi.org/10.1161/01.STR.20.6.715
O’Kelly CJ, Kulkarni AV, Austin PC et al (2009) Shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage: incidence, predictors, and revision rates. J Neurosurg 111:1029–1035. https://doi.org/10.3171/2008.9.JNS08881
Cagnazzo F, Di Carlo DT, Petrella G, Perrini P (2020) Ventriculostomy-related hemorrhage in patients on antiplatelet therapy for endovascular treatment of acutely ruptured intracranial aneurysms. A meta-analysis. Neurosurg Rev 43:397–406. https://doi.org/10.1007/s10143-018-0999-0
Chalouhi N, Jabbour P, Singhal S, Drueding R, Starke RM, Dalyai RT, Tjoumakaris S, Gonzalez LF, Dumont AS, Rosenwasser R, Randazzo CG (2013) Stent-assisted coiling of intracranial aneurysms. Stroke 44:1348–1353. https://doi.org/10.1161/STROKEAHA.111.000641
Phillips TJ, Wenderoth JD, Phatouros CC, Rice H, Singh TP, Devilliers L, Wycoco V, Meckel S, McAuliffe W (2012) Safety of the pipeline embolization device in treatment of posterior circulation aneurysms. Am J Neuroradiol 33:1225–1231. https://doi.org/10.3174/ajnr.A3166
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Ethics approval was obtained from the Ethics Committee of our Institution.
Consent to participate
Informed consent was not required for this retrospective study and review.
Consent for publication
Not required because individual patient data are not presented.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 53 kb)
Rights and permissions
About this article
Cite this article
Gardijan, D., Herega, T., Premužić, V. et al. Comparison between stenting and conservative management of posterior circulation perforator aneurysms: Systematic review and case series. Neuroradiology 63, 639–651 (2021). https://doi.org/10.1007/s00234-020-02618-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02618-2