Abstract
Purpose
The ultrasonographic and hemodynamic features of patients with carotid near-occlusion (CNO) are still not well known. Our aim was to describe the ultrasonographic and hemodynamic characteristics of a cohort of patients with CNO.
Methods
A prospective, observational, nationwide, and multicenter study was conducted from January/2010 to May/2016. Patients with digital subtraction angiography (DSA)–confirmed CNO were included. We collected information on clinical and demographic characteristics, carotid and transcranial ultrasonography and DSA findings, presence of full-collapse, collateral circulation, and cerebrovascular reactivity (CVR).
Results
One hundred thirty-five patients were analyzed. Ultrasonographic and DSA diagnosis of CNO were concordant in only 44%. This disagreement was related to the presence/absence of full-collapse: 45% of patients with CNO with full-collapse were classified as a complete carotid occlusion, and 40% with a CNO without full-collapse were interpreted as severe stenosis (p < 0.001). Mean velocities (mV) and pulsatility indexes (PIs) were significantly lower in the ipsilateral middle cerebral artery compared with the contralateral (43 cm/s vs 58 cm/s, p < 0.001; 0.80 vs 1.00, p < 0.001). Collateral circulation was identified in 92% of patients, with the anterior communicating artery (73%) being the most frequent. CVR was decreased or exhausted in 66% of cases and was more frequent in patients with a poor or absent collateral network compared with patients with ≥ 2 collateral arteries (82% vs 56%, p = 0.051).
Conclusion
The accuracy of carotid ultrasonography in the diagnosis of CNO seems to be limited, with significant discrepancies with DSA. Decreased ipsilateral mV, PI, and CVR suggest a hemodynamic compromise in patients with CNO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Carotid near-occlusion (CNO) is a tight atherosclerotic stenosis of the carotid artery resulting in a decrease in the diameter of the artery beyond the stenosis. CNO can be classified as with or without full-collapse, which is defined as a “threadlike” lumen distal to the stenosis [1]. CNO must be differentiated from “conventional” severe (≥ 50%) carotid stenosis, in which the distal reduction of the artery lumen is not observed. This distinction is relevant because the risk of stroke seems to be lower in CNO and the benefit of revascularization has not been demonstrated in this type of stenosis [2,3,4].
The diagnosis of CNO is based on digital subtraction angiography (DSA) or computed tomography angiography (CTA) [2, 5]. Carotid ultrasound has a low sensitivity and does not seem to be the adequate technique to identify CNO, as it may be misdiagnosed as complete carotid occlusions or conventional stenosis [6]. However, some ultrasonographic findings have been described, including filiform distal flow, very reduced velocities and altered Doppler pattern with a flattened, pseudovenous flow and low pulsatility. These features are highly specific for CNO with full-collapse [7,8,9]. Conversely, high peak systolic velocity has been frequently observed in CNO without full-collapse [6].
Two main mechanisms of stroke in CNO have been proposed: an embolic mechanism, in which the reduction of blood flow in the collapsed carotid artery favors blood stasis together with the production of thrombi and its embolization, and a hemodynamic mechanism in which collateral circulation and cerebrovascular reactivity may play an important role [10, 11].
Transcranial Doppler (TCD) findings in patients with CNO are not yet known, and the available information on the hemodynamic and ultrasonographic features of these patients is limited. Therefore, the objective of this study is to describe the ultrasonographic and hemodynamic characteristics of a cohort of patients with symptomatic CNO.
Methods
Study design and patient selection
The CAOS study is a prospective, observational, nationwide, multicenter registry study conducted in seventeen Spanish university hospitals from January 2010 to May 2016. The study methodology has been described previously [12]. The study population comprised adult patients with angiography-confirmed diagnosis of atherosclerotic internal carotid artery (ICA) near-occlusion and ipsilateral ischemic stroke, transient ischemic attacks (TIA), or retinal ischemia in the previous 6 months.
The study was supported by the “Proyecto Ictus” initiative of the Spanish Cerebrovascular Diseases Study Group, and approved by the ethics committees of Gregorio Marañón University Hospital and the other participating institutions. Written informed consent was obtained from all study participants.
Diagnosis and imaging procedures
ICA near-occlusion was diagnosed in all patients using DSA which was considered the reference diagnostic technique. Based on the report by Fox et al. [2], diagnosis of ICA near-occlusion is confirmed when the angiographic findings fulfill at least 2 of the following 4 criteria: (1) delayed cranial arrival of ICA contrast compared with the external carotid artery; (2) intracranial collaterals seen as cross-filling of contralateral vessels or ipsilateral contrast dilution; (3) frankly reduced diameter of the ICA compared with the contralateral ICA; and (4) reduced ICA diameter compared with the ipsilateral external carotid artery.
Full-collapse was defined as a “threadlike” lumen distal to the stenosis [1]. Presence or absence of full-collapse was determined with DSA and/or CT angiography.
For the quantification of the degree of carotid stenosis with ultrasonography, we used the Society of Radiologists in Ultrasound Consensus Conference criteria. According to those criteria, ultrasonographic diagnosis of CNO can be established by demonstrating a markedly narrowed lumen at color or power Doppler mode rather than applying velocity parameters [13].
ICA findings in DSA and carotid ultrasonography were recorded as follows: normal; < 50% stenosis; 50–69% stenosis; 70–99% stenosis without near-occlusion criteria; near-occlusion; and total occlusion.
The mean velocity (mV) and pulsatility index (PI) values of both middle cerebral arteries (MCA) were recorded using transcranial ultrasonography.
Collateral circulation through anterior communicating artery (ACoA), ipsilateral posterior communicating artery (PCoA), or ipsilateral ophthalmic artery was assessed with DSA and/or transcranial ultrasonography.
Cerebrovascular reactivity (CVR) was assessed using the breath-holding index (BHI) according to the technique described by Markus et al. [14]. Previously published local reference BHI values were considered [15]. CVR values were classified as follows: normal CVR when the BHI was > 0.6 %/s, reduced when the BHI was between 0.3 and 0.6%/s, and exhausted CVR when the BHI was < 0.3%/s.
Clinical data
We prospectively recorded baseline demographics (age and gender), vascular risk factors (hypertension, diabetes, smoking, dyslipidemia, ischemic heart disease, atrial fibrillation, previous history of stroke or TIA, and peripheral arterial disease) and type of presenting event (ipsilateral ischemic stroke, ipsilateral TIA, ipsilateral retinal infarct, and ipsilateral amaurosis fugax).
Statistical analysis
The statistical analysis was performed using the IBM SPSS Statistics 22.0 software package for Windows. In the descriptive analysis, the results were expressed as proportions (%) for the qualitative variables and as mean ± standard deviation (SD) or median and interquartile range (IQR) for the quantitative variables. The normal distribution of the variables was verified with the Kolmogorov-Smirnov test. For the univariate comparative analysis, the Chi-square test was used for the dichotomous variables and the Student T or Mann-Whitney U tests for the continuous variables. p values ≤ 0.05 were considered statistically significant.
Results
A total of 141 patients with angiography-confirmed CNO were recruited in the CAOS registry. One hundred thirty-five subjects who underwent an ultrasonographic study could be included in this analysis. The baseline characteristics, distribution of risk factors, clinical presentation, and imaging findings are presented in Table 1.
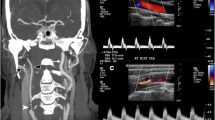
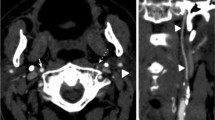
In 31 patients (23%) CNO was mistaken with a complete ICA occlusion, and a “conventional” ICA stenosis was diagnosed with ultrasonography in 44 cases (33%). Diagnosis of CNO was concordant with DSA in only 60 patients (44%). The presence or absence of full-collapse was related with the misdiagnosis of CNO with ultrasonography: 18 out of 40 patients (45%) with CNO with full-collapse were classified as a complete ICA occlusion, while 28 of 70 subjects (40%) with a CNO without full-collapse were interpreted as severe stenosis (p < 0.001) (Figs. 1 and 2).
Angiographic and ultrasonographic findings in patients with carotid near-occlusion with and without full-collapse. a–c Patient with a carotid near-occlusion of the left ICA without full-collapse. a DSA showing a reduced distal ICA diameter (white arrow) which is similar to ipsilateral external carotid artery (black arrow) and smaller than the contralateral ICA (b; white arrow). c Carotid ultrasonography showing increased peak systolic velocities, suggesting a conventional > 50% ICA stenosis. d–f Patient with a right carotid near-occlusion with full-collapse. d DSA showing a “threadlike” lumen distal to the ICA stenosis (white arrows), with a frankly reduced diameter compared with contralateral ICA (e; white arrow). f Carotid ultrasonography showing an absence of Doppler signal in the ICA, interpreted as a complete ICA occlusion. ICA, internal carotid artery; DSA, digital subtraction angiography
The analysis of the transcranial ultrasonography findings showed that both, mV and PIs, were significantly lower in the MCA ipsilateral to the CNO compared with the contralateral MCA (Table 2). No differences were observed in mV and PI values according to presence or absence of full-collapse, collateral status, or CVR results (Table 3).
Collateral circulation could be analyzed in 127 patients (94%) and was more frequent through the ACoA (100 patients, 74%), followed by the ipsilateral PCoA (53 patients, 40%) and the ipsilateral ophthalmic artery (35 patients, 26%). In 63 patients (50%), collateral circulation was found through 2 or more of the arteries described. Collateral circulation was absent in only 8 patients (6%). No differences were found in the collateral status according to presence or absence of full-collapse (Table 3).
The CVR study was carried out in 60 patients but could be analyzed in only 50. CVR values ipsilateral to the CNO were normal in 17 subjects (34%), reduced in 22 (44%), and exhausted in 11 (22%). CVR contralateral to the CNO was normal in 45 patients (90%) and reduced or exhausted in 5 (10%). The presence or absence of full-collapse did not affect the CVR results (Table 3). CVR tended to be more frequently reduced in patients with a poor or absent collateral network (0 or 1 collateral) compared with patients with 2 or more collaterals (reduced or exhausted ipsilateral CVR in 82% and 56% respectively, p = 0.051) (Table 3).
Discussion
The main finding of this study was that the mean velocities and pulsatility indexes of the MCA ipsilateral to the CNO were significantly reduced compared with those observed in the contralateral MCA, reflecting a hemodynamic impairment in these patients. Additionally, the vast majority (more than 90%) of patients in our series presented collateral circulation through the ACoA, PCoA, and/or ophthalmic artery. The presence of compensatory collateral circulation in patients with CNO has been previously described, reaching between 64 and 96% of cases [16, 17]. Some authors suggest that the presence of collateral circulation in CNO is an almost constant finding; in fact, it constitutes one of the 4 angiographic diagnostic criteria [10]. An efficient collateral circulation in patients with conventional carotid stenosis has been shown to reduce the risk of stroke or TIA [16] and could also explain the observed low stroke rate in patients with CNO in the North American Symptomatic Endarterectomy Trial (NASCET), the European Carotid Surgery Trial (ECST), and in the CAOS study [2,3,4, 12]. However, recent studies have reported higher risk of recurrent stroke associated with CNO [11, 18]. Unfortunately, these studies did not provide information on collateral status and therefore its potential protective effect in patients with CNO cannot be fully established.
Two-thirds of our patients had a reduced or exhausted CVR. A similar proportion of impaired CVR has been described for conventional (> 50%) carotid stenosis [14, 19]. Reduced or exhausted CVR has been previously described in CNO patients [20,21,22], and Oka et al. observed that CVR was significantly lower in CNO than in patients with conventional carotid stenosis or healthy subjects [23]. In our series, the CVR seemed to be more frequently impaired in patients without collaterals or poor collateral circulation (< 2 arteries); however, our results did not reach statistical significance (p = 0.051) and should be considered cautiously.
The pathophysiology of stroke in CNO patients remains unclear. An embolic mechanism has been suggested, in which the reduction of blood flow in the collapsed carotid artery favors blood stasis and increases the risk of thrombi formation and their subsequent embolization to distal arterial territories [10]. However, some authors consider this mechanism unlikely because the low intraluminal pressure across the stenosis may not allow the dislodgement of emboli from the surface of the plaque [24]. Furthermore, a study using transcranial Doppler monitoring of microemboli in the MCA distal to a carotid stenosis, showed a very significant reduction in the number of the detected microemboli when the degree of stenosis exceeded 90% and specially in cases with near-occlusion [25]. On the other hand, the hemodynamic mechanism suggests that in certain situations such as prolonged arterial hypotension and syncope, a stroke could occur due to hypoperfusion [10]. It seems reasonable to consider that the subgroup of patients with CNO and a worse cerebral hemodynamic situation (poor collaterals and/or impaired CVR) could have a higher risk of suffering a stroke, although this has not been demonstrated so far.
The concordance of carotid ultrasonography with DSA for the detection of CNO was poor in our study. In more than half of cases, CNO was mistaken with a complete carotid occlusion or a conventional ICA stenosis. These ultrasonographic mimics have been previously described [1]. The published data about the use of ultrasonography in the diagnosis of CNO are limited. It has been described that the presence of a tight stenosis with reduced flow velocities constitutes a criteria with high specificity and moderate sensitivity for the ultrasonographic diagnosis of this condition [7, 8]. Furthermore, sensitivity of this criteria can be improved by adding a peak systolic velocity distal to the stenosis < 50 cm/s [26]. However, Khangure et al. recorded this finding in only 13% of their study population and failed to find other parameters with high sensitivity and specificity to differentiate CNO from a conventional carotid stenosis [6]. In our series, ultrasonographic mimics were related with the presence or absence of full-collapse: patients that were mistaken with a carotid occlusion presented more frequently a CNO with full-collapse, while those interpreted as a conventional ICA stenosis did not show a full-collapse. These findings may be related with the small low-flow signal that can be hardly detected with ultrasounds in full-collapse patients, and with the high flow velocities simulating a conventional high-grade stenosis in CNO without full-collapse as previously noted by Khangure et al. [1, 6]. There is no consensus on the real role of ultrasonography for the detection of CNO. Some authors consider that the diagnosis of CNO should be confirmed with another vascular imaging test such as DSA or CT angiography [1]. The poor results of ultrasonography in our study seem to confirm this approach.
Our study is subject to a series of limitations. First, the ultrasonographic diagnosis of CNO was based on the criteria of the Society of Radiologists in Ultrasound Consensus Conference and therefore no velocity parameters such as low flow velocity distal to the stenosis were applied. Second, the use of DSA as the diagnostic tool for patient inclusion may have generated selection bias, since older patients with comorbidities may have been excluded. Third, DSA, CT angiography, and ultrasonography were not centrally read, leading to inter-observer error. Finally, CVR study was performed in a small proportion of patients and this may have affected the accuracy and the statistical power of our results.
In conclusion, the accuracy of carotid ultrasonography in the diagnosis of CNO is limited, with significant discrepancies with DSA. CNO with full-collapse is often confused with complete carotid occlusions, while CNO without full-collapse is frequently interpreted as a conventional high-grade stenosis. Although the majority of patients with CNO present collateral circulation, the cerebral hemodynamics of these patients seems to be altered, as evidenced by the reduction of mV, PI, and CVR. The role of these findings in the pathophysiology of stroke in CNO needs to be clarified in further studies.
Data Availability
Data of the study are available upon reasonable request.
References
Johansson E, Fox A (2016) Carotid near-occlusion: a comprehensive review, Part 1—Definition, terminology, and diagnosis. Am J Neuroradiol. 37:2–10
Fox AJ, Eliasziw M, Rothwell P, Schmidt M, Warlow C, Barnett H (2005) Identification, prognosis, and management of patients with carotid artery near occlusion. Am J Neuroradiol. 26:2086–2094
Rothwell P, Eliasziw M, Gutnikov S, Fox A, Taylor D, Mayberg M, Warlow CP, Barnett HJM (2003) Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 361:107–116
Rothwell P, Gutnikov S, Warlow C, for the European Carotid Surgery Trial (2003) Reanalysis of the final results of the European Carotid Surgery Trial. Stroke. 34:514–523
Bartlett E, Walters T, Symons S, Fox A (2006) Diagnosing carotid stenosis near-occlusion by using CT angiography. Am J Neuroradiol. 27:632–637
Khangure S, Benhabib H, Machnowska M, Fox A, Grönlund C, Herod W, Maggisano R, Sjöberg A, Wester P, Hojjat SP, Hopyan J, Aviv RI, Johansson E (2018) Carotid near-occlusion frequently has high peak systolic velocity on Doppler ultrasound. Neuroradiology. 60(1):17–25
Hetzel A, Eckenweber B, Trummer B, Wernz M, Schumacher M, von Reutern G (1998) Colour-coded duplex sonography of preocclusive carotid stenoses. Eur J Ultrasound. 8:183–191
Ashraf Mansour M, Mattos M, Hood D, Hodgson K, Barkmeier L, Ramsey D et al (1995) Detection of total occlusion, string sign, and preocclusive stenosis of the internal carotid artery by color-flow duplex scanning. Am J Surg. 170:154–158
El-Saden S, Grant E, Hathout G, Zimmerman P, Cohen S, Baker J (2001) Imaging of the internal carotid artery: the dilemma of total versus near total occlusion. Radiology. 221:301–308
Johansson E, Fox A (2016) Carotid near-occlusion: a comprehensive review, Part 2—Prognosis and treatment, pathophysiology, confusions, and areas for improvement. Am J Neuroradiol. 37:200–204
Johansson E, Öhman K, Wester P (2015) Symptomatic carotid near-occlusion with full collapse might cause a very high risk of stroke. J Intern Med. 277:615–623
García-Pastor A, Gil-Núñez A, Ramírez-Moreno J, González-Nafría N, Tejada J, Moniche F et al (2019) The risk of recurrent stroke at 24 months in patients with symptomatic carotid near-occlusion: results from CAOS, a multicentre registry study. Eur J Neurol. 26:1391–1398
Grant E, Benson C, Moneta G, Alexandrov A, Baker J, Bluth E et al (2003) Carotid artery stenosis: gray-scale and Doppler US diagnosis--Society of Radiologists in Ultrasound Consensus Conference. Radiology. 229:340–346
Markus H, Harrison M (1992) Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath-holding as the vasodilatory stimulus. Stroke. 23:668–673
Jiménez-Caballero P, Segura T (2006) Valores de normalidad de la reactividad vasomotora cerebral mediante el test de apnea voluntaria. Rev Neurol. 43:598–602
Henderson R, Eliasziw M, Fox A, Rothwell P, Barnett H (2000) Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. Stroke. 31(1):128–132
Morgenstern L, Fox A, Sharpe B, Eliasziw M, Barnett H, Grotta J (1997) The risks and benefits of carotid endarterectomy in patients with near occlusion of the carotid artery. Neurology. 48:911–915
Gu T, Aviv R, Fox A, Johansson E (2020) Symptomatic carotid near-occlusion causes a high risk of recurrent ipsilateral ischemic stroke. J Neurol. 267:522–530
Silvestrini M, Troisi E, Matteis M, Cupini L, Caltagirone C (1996) Transcranial Doppler assessment of cerebrovascular reactivity in symptomatic and asymptomatic severe carotid stenosis. Stroke. 27:1970–1973
Terada T, Tsuura M, Matsumoto H, Masuo O, Tsumoto T, Yamaga H, Itakura T (2006) Endovascular treatment for pseudo-occlusion of the internal carotid artery. Neurosurgery. 59:301–309
Choi B, Park J, Shin J, Lü P-H, Kim J, Kim S et al (2010) Outcome evaluation of carotid stenting in high-risk patients with symptomatic carotid near occlusion. Interv Neuroradiol. 16:309–316
González A, Gil-Peralta A, Mayol A, Gonzalez-Marcos J, Moniche F, Aguilar M et al (2011) Internal carotid artery stenting in patients with near occlusion: 30-day and long-term outcome. Am J Neuroradiol. 32:252–258
Oka F, Ishihara H, Kato S, Oku T, Yamane A, Kunitugu I, Suzuki M (2013) Cerebral hemodynamic benefits after carotid artery stenting in patients with near occlusion. J Vasc Surg. 58:1512–1517
Rothwell P, Warlow C (2000) Low risk of ischemic stroke in patients with reduced internal carotid artery lumen diameter distal to severe symptomatic carotid stenosis: cerebral protection due to low poststenotic flow? On behalf of the European Carotid Surgery Trialists’ Collaborative. Stroke. 31:622–630
Molloy J, Markus H (1999) Asymptomatic embolization predicts stroke and TIA risk in patients with carotid artery stenosis. Stroke. 30:1440–1443
Johansson E, Benhabib H, Herod W, Hopyan J, Machnowska M, Maggisano R et al (2018) Carotid near-occlusion can be identified with ultrasound by low flow velocity distal to the stenosis. Acta radiol. 60(3):396–404
Acknowledgements
The data shown in this manuscript has been previously presented as an E-Poster Viewing Abstract at the 5th European Stroke Organisation Conference held in Milan, Italy in May 2019.
Funding
This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study obtained ethics approval from “Comité de Ética e investigación Clínica Hospital General Universitario Gregorio Marañón”, with ID number of the approval: 122/09.
Consent to participate
Participants gave informed consent before taking part in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palacios-Mendoza, M.A., García-Pastor, A., Gil-Núñez, A. et al. Ultrasonographic and hemodynamic characteristics of patients with symptomatic carotid near-occlusion: results from a multicenter registry study. Neuroradiology 63, 705–711 (2021). https://doi.org/10.1007/s00234-020-02567-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02567-w