Abstract
Purpose
Meningiomas are mainly benign tumors, though a considerable proportion shows aggressive behaviors histologically consistent with atypia/anaplasia. Histopathological grading is usually assessed through invasive procedures, which is not always feasible due to the inaccessibility of the lesion or to treatment contraindications. Therefore, we propose a multi-parametric MRI assessment as a predictor of meningioma histopathological grading.
Methods
Seventy-three patients with 74 histologically proven and previously treated meningiomas were retrospectively enrolled (42 WHO I, 24 WHO II, 8 WHO III) and studied with MRI including T2 TSE, FLAIR, Gradient Echo, DWI, and pre- and post-contrast T1 sequences. Lesion masks were segmented on post-contrast T1 sequences and rigidly registered to ADC maps to extract quantitative parameters from conventional DWI and intravoxel incoherent motion model assessing tumor perfusion. Two expert neuroradiologists assessed morphological features of meningiomas with semi-quantitative scores.
Results
Univariate analysis showed different distributions (p < 0.05) of quantitative diffusion parameters (Wilcoxon rank-sum test) and morphological features (Pearson’s chi-square; Fisher’s exact test) among meningiomas grouped in low-grade (WHO I) and higher grade forms (WHO II/III); the only exception consisted of the tumor-brain interface. A multivariate logistic regression, combining all parameters showing statistical significance in the univariate analysis, allowed discrimination between the groups of meningiomas with high sensitivity (0.968) and specificity (0.925). Heterogeneous contrast enhancement and low ADC were the best independent predictors of atypia and anaplasia.
Conclusion
Our multi-parametric MRI assessment showed high sensitivity and specificity in predicting histological grading of meningiomas. Such an assessment may be clinically useful in characterizing lesions without histological diagnosis.
Key points • When surgery and biopsy are not feasible, parameters obtained from both conventional and diffusion-weighted MRI can predict atypia and anaplasia in meningiomas with high sensitivity and specificity. • Low ADC values and heterogeneous contrast enhancement are the best predictors of higher grade meningioma |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas are the most frequent central nervous system primary neoplasm in adults, accounting for 24–30% of intracranial primitive tumors [1, 2]. They develop from arachnoid meningothelial cells and usually appear as extra-axial masses, T1 isointense/slightly hypointense, and T2 hyperintense/isointense compared to gray matter (GM), with avid contrast enhancement (CE) [3].
Although meningiomas are overall considered benign tumors, a significant proportion of them displays more aggressive biological and clinical behaviors. Twenty to 30% of meningiomas are histologically classified by the WHO as atypical (WHO grade II) or anaplastic (WHO grade III). These high-grade meningiomas (HGM), which can either arise de novo or progress from low-grade meningiomas (LGM), show higher mortality due to the neurologic deterioration caused by their aggressive growth and the consequent compression of CNS structures [1, 3, 4].
The main treatment for meningiomas is surgery, usually performed when a lesion causes symptoms or grows significantly over time. Nonetheless, atypical and anaplastic meningiomas show higher recurrence rates, ranging from 29 to 52% and from 50 to 94%, respectively, compared to the much lower recurrence rate of LGMs, ranging from 7 to 20% [5, 6]. Adjuvant radiotherapy is commonly necessary in the course of HGM treatment.
While biopsy or excision is currently the only way to definitively confirm the grading of the tumor, these procedures are not free from risk [7, 8]. Several factors such as comorbidity, accessibility of the lesion, and age of the subjects might prevent the surgeons from performing invasive procedures, resulting in insufficient information to evaluate the possible need for radiotherapy and the adequate radiation dose.
Several authors have investigated the possibility of predicting the histological grade of meningiomas through MRI examinations. Specifically, most of the studies focused either on a qualitative assessment of morphological features or on a quantitative assessment of more advanced MRI techniques [9,10,11,12,13,14,15,16].
The purpose of this study is to test a multi-parametric 3T MRI assessment that includes both morphological features and diffusion quantitative metrics as a possible predictor of meningioma histopathological grading. Detecting atypia and anaplasia in meningiomas with high sensitivity and specificity might allow prognostic stratification even in cases where invasive approaches are not feasible, guiding clinicians in choosing between different radiotherapy strategies.
Material and methods
Subjects
A total of 113 subjects with meningioma referred to our center between April 2016 and April 2019 to be evaluated for potential hadron-therapy treatment. A baseline brain 3T MRI was performed in all subjects prior to the start of the treatment. Forty subjects were excluded due to lack of histopathological report (n = 29), poor quality of the MRI study (n = 9), or difficulty in identifying the residual lesion after surgical excision (n = 2). A total of 74 meningiomas, distributed among 73 subjects, were then included in the current retrospective study. All participants signed a detailed written informed consent approved by the local ethics committee.
The average age of the subjects was 60.1 ± 14.7 years (range from 16 to 91). Forty subjects (55%) were female and 33 (45%) were male. Out of 74 meningiomas, 31 (9 LGMs and 22 HGMs) had already been treated with conventional radiotherapy, which was performed at least 10 months before baseline. The average time from treatment was 47 ± 40 months (median 36, range from 10 to 180). Out of 73 subjects, 7 underwent biopsy and 66 underwent partial excision. The average time from surgical procedures was 32 ± 44 months (median 17, range from 2 to 252). Out of 74 meningiomas, 42 (57%) were histologically confirmed to be grade I, 24 (32%) grade II, and 8 (11%) grade III, according to the 2016 WHO classification of CNS tumors. Patients that underwent biopsy and excision were distributed equally among groups (P = 0.2, chi-square test).
MRI protocol and imaging processing for quantitative analysis
MRI studies were acquired at the same institution on the same 3T scanner (Magnetom Verio, Siemens Healthineers) with an identical protocol that included the following sequences:
-
Axial T1 turbo spin echo (TSE); voxel size 0.8 × 0.8 × 3.0 mm, phase encoding AP, TR = 740 ms, TE = 11 ms, flip angle (FA) 150°
-
Axial T2 TSE; voxel size 0.7 × 0.5 × 3 mm, phase encoding AP, TR = 5200 ms, TE = 100 ms, FA 150°
-
Axial T2 gradient echo (GE); voxel size 0.8 × 0.8 × 3mm, phase encoding AP, TR = 870 ms, TE = 12 ms, FA 20°
-
Axial fluid-attenuated inversion recovery (FLAIR); voxel size 0.9 × 0.9 × 4mm, TR = 6900 ms, TE = 94 ms, TI = 2300 ms, FA 150°
-
Echo-planar imaging two-dimensional (EP2D) DWI with B values equal to 0, 50, 100, 150, 200, 400, and 1000, from which ADC maps were derived; voxel size 1.9 × 1.9 × 4mm, phase encoding AP, TE = 30 ms, TR = 4000 ms, FA 90°
-
Axial T1 volumetric interpolated breath-hold examination (VIBE) fat saturated with 0.6-mm isotropic voxel after injection of a gadolinium-based contrast agent (gadoteridol 0.2 mL/kg); voxel size 0.6 × 0.6 × 0.6 mm, TR = 5.35 ms, TE = 2.27 ms, FA 11.5°
Meningiomas were manually segmented on fat-saturated T1 VIBE in each single slide using the open-source software ITK-SNAP (http://www.itksnap.org). Since all subjects underwent biopsy or surgery before baseline, we observed GE sequences in order to exclude peripheral foci of magnetic susceptibility from segmentations. The lesion volumes were assessed as integrals of the segmented voxels. T1 VIBE studies of each patient were rigidly registered to their respective ADC maps, and the computed transformation was applied to consequently warp the segmented lesion (http://www.plastimatch.org). The registration outputs were reviewed by 2 neuroradiologists with 10 and 8 years of experience in the field (L.M.F. and A.B. respectively). We finally extracted, within the segmented lesions, quantitative metrics such as median ADC values, pure diffusion (D), pseudo-diffusion (D*), and perfusion fraction (f), based on conventional DWI imaging and intravoxel incoherent motion (IVIM) model [17].
Semi-quantitative assessment of morphological features
The two neuroradiologists (L.M.F. and A.B.) independently reviewed the MRI studies and assessed several morphological parameters through semi-quantitative scales validated in literature [7, 11, 12, 16,17,18,19,20] while blinded to the histological grading. No significant differences were observed between the two experts’ assessments (Wilcoxon rank-sum test, p < 0.05). Each parameter was specifically assessed and labeled as follows:
-
a)
Location: 1 for skull base, 2 for convexity, 3 for falx cerebri, 4 for posterior fossa.
-
b)
T1 signal intensity (compared to GM): 1 for hypointense, 2 for isointense, 3 for hyperintense, 4 for mixed.
-
c)
T2 signal intensity (compared to GM): 1 for hypointense, 2 for isointense, 3 for hyperintense, 4 for mixed.
-
d)
Shape: 1 for rounded, 2 for lobulated, 3 for irregular. The irregular subtype included both meningiomas with infiltrating margins and extensions through the skull base foramina as well as meningiomas not adequately classifiable as either rounded or lobulated.
-
e)
Peritumoral edema (PTE): assessed on axial T2 TSE as hyperintense areas of the brain parenchyma contiguous to the lesion. 0 for none, 1 for mild (< 10 mm from the lesion edge) not associated to mass effect, 2 for moderate (> 10 mm from the lesion edge) associated with mass effect [18].
-
f)
CE: assessed after gadolinium injection. 0 if heterogeneous, 1 if homogeneous.
-
g)
Capsular enhancement (CapE): assessed after gadolinium administration and defined as contrast enhancement of the peripheral portions of the tumor. 0 if absent, 1 if present.
-
h)
Tumor-brain interface (TBI): 0 if unclear boundaries, 1 if clear boundaries.
Statistical analysis
The purpose of this study is to test a multi-parametric 3T MRI assessment as a predictor of histopathological grading of meningiomas. Specifically, we looked for differences in each established MRI parameter between the two subgroups of lesions: LGMs, classified as grade I by the WHO, and HGMs, classified as either grade II or grade III by the WHO. We chose this stratification in order to get subgroups of lesions with similar and comparable sample sizes, specifically, 42 LGMs and 32 HGMs. Sample size for anaplastic meningiomas (n = 8) was too small to get any statistically significant results.
We used Matlab® 2019 to perform all the following analyses:
-
Univariate analysis of morphological parameters using Pearson’s chi-square test for variables expressed by multiple categories, and Fisher’s exact test for categorical variables (alpha = 5%).
-
Univariate analysis of diffusion parameters using Wilcoxon rank-sum test (alpha = 5%).
-
Multivariate logistic regression considering all morphological and quantitative diffusion parameters that were found to be statistically significant (p < 0.05) in the univariate analyses. This analysis was described by sensitivity, specificity, and AUC computed from ROC curves.
Results
Univariate analysis of morphological parameters
As reported in Table 1, univariate analysis showed statistically different distributions among LGMs and HGMs of all morphological parameters except for TBI. Specifically, HGMs, compared to LGMs, were more frequently localized at the convexity and falx cerebri and showed more often hypointense or mixed T1 signal and hyperintense or mixed T2 signal. HGMs, compared to LGMs, were more frequently lobulated in shape and showed heterogeneous CE, CapE, and mild edema of the contiguous brain more often.
Univariate quantitative analysis of diffusion parameters
As showed in Table 2, the two groups of meningiomas showed statistically significant differences in all diffusion parameters considered. Specifically, HGM showed lower median ADC, D, D*, and f compared to LGM.
Multivariate analysis
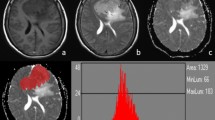
A multivariate logistic regression analysis was performed using all morphological and quantitative parameters that reached statistical significance in the univariate analysis. As showed in Fig. 1, this model allowed discrimination between LGMs and HGMs with an AUC of 0.978, resulting in sensitivity and specificity of 0.968 and 0.925 respectively. Specifically, the model indicated heterogeneous CE and low ADC median values as the best independent predictors of HGM (see Fig. 2).
a ROC curve and the corresponding AUC of the multivariate logistic regression considering all morphological and quantitative diffusion parameters that showed statistical significance in the univariate analysis. AUC is equal to 0.978, resulting in sensitivity and specificity of 0.968 and 0.925 respectively. R2 is equal to 0.78. b Model output probabilities showing different distributions of model output between the two groups of meningioma. The optimal threshold for the model is equal to 0.275
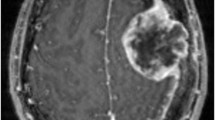
Panels a and b respectively show ADC map and contrast-enhanced VIBE of a higher grade meningioma (HGM) histologically classified as WHO grade III: the mass shows low values in the ADC map (lower than the contiguous brain parenchyma; ADC median value = 658 × 10−6 mm2/s). CE is heterogeneous. Panels c and d respectively show ADC map and contrast-enhanced VIBE of a low-grade meningioma (LGM) histologically classified as WHO grade I: the mass shows moderately low values in the ADC map (higher than the contiguous brain parenchyma; ADC median values = 930 × 10−6 mm2/s). CE is homogeneous
Discussion
The purpose of this study is to test a multi-parametric 3T MRI assessment as a predictor of meningioma histopathological grading. We built a systematic model to distinguish LGMs (WHO grade I) from HGMs (WHO grade II and III) using morphological parameters as well as quantitative diffusion assessment. We examined 74 meningiomas assessing, with semi-quantitative scores, tumor location, T1 and T2 signal intensity, shape, PTE, CE, CapE, and TBI. ADC median values derived from conventional DWI and D, D*, and f derived from the IVIM model were quantitatively assessed.
Univariate analysis showed statistically significant different distributions of MRI features among LGMs and HGMs for each quantitative and morphological parameter analyzed, with the only exception of TBI. All results have been successively integrated in a multivariate logistic regression analysis, which allowed discrimination between LGMs and HGMs with sensitivity and specificity of 0.968 and 0.925 respectively (AUC 0.978; R2 = 0.78) (see Fig. 1). Specifically, the model indicated heterogeneous CE and low ADC median values to be the best independent predictors of atypia and anaplasia in meningiomas (see Fig. 2).
The results showing the association between a homogeneous CE pattern and LGMs as well as the association between a heterogeneous pattern and HGMs are in line with literature. A heterogeneous CE pattern is thought to be connected with a greater intra-tumoral heterogeneity, which can be detected in atypical and anaplastic meningiomas, characterized by a faster growth rate and consequently a possibly larger necrotic component [9, 13, 15, 19].
The finding suggesting that LGMs have higher ADC median values compared to the atypical and anaplastic form is also supported by literature. In particular, since the diffusivity of water molecules in biological tissues depends on the relationship between extra-cellular and intra-cellular spaces, it is much more likely that HGMs, characterized by a greater mitotic rate, smaller cells, and higher nuclear-cytoplasmic ratio, show lower ADC values compared to LGMs [13, 16, 20, 21].
Most of the other differences in morphological parameters between the two subgroups detected in our study were also supported by previous studies. Specifically, PTE is known to be more frequent in HGM than in LGM. PTE in meningiomas is caused by a disruption of physiological barriers, such as arachnoid cell layer and CSF, separating the tumor and the brain. This can be due to vascular and non-vascular causes, the former being seen as a result of ischemia in the contiguous parenchyma compressed by large masses, while the latter is usually due to direct infiltration by tumoral cells [13, 14, 22, 23]. Other studies, however, highlighted that PTE may be caused by a higher expression of aquaporin-4, a membrane protein crucial for water transport and fluid balance, rather than being a direct expression of the tumor grade [24, 25]. These latter studies focused mainly on LGMs; further studies are therefore needed to confirm these suggestions.
Irregular shape and falx cerebri/convexity location are also known to be more frequent in HGMs [14, 20]. Regarding the location, in particular, the majority of literature agrees with the higher prevalence of LGM at the skull base compared to falx cerebri or convexity [13, 14, 22, 26, 27]. This might be connected to different genetic and molecular mechanisms that contribute to the tumor development. Specific mutations may be associated with LGMs located in the skull base, similarly to how NF-2 mutation is associated with HGM located in the cranium convexity [26, 28].
Regarding signal intensity in T1- and T2-weighted sequences, our data show that HGMs were characterized by higher T2 and lower T1 signal overall compared to LGMs. The finding of higher T2 signal in atypical and anaplastic meningioma is already reported in literature and may be connected with the presence of cystic and necrotic areas, which are far more common in tumors with faster growth rates [20, 27]. Our study also shows an association between low T1 signal intensity and atypia/anaplasia; this finding may also be explained by the possible presence of cystic areas and overall consistency of the tumor, though we have not found previous studies in literature that confirm this specific association. However, Hower et al. (2011) reported that tumors characterized by a softer consistency appear T1 hypointense more often than the ones characterized by a firm consistency [29].
Results regarding other morphological parameters showed more contrasting findings compared to previous studies. For example, our results that show an association between CapE and HGMs is in contrast with a recent study that indicates CapE to be more frequent within LGMs [9]. This latter observation is thought to be connected with a chronic reaction that produces a more extensive external fibrous layer in tumors characterized by a slower growth rate. However, several other studies did not detect any difference in the presence of CapE between the three subgroups of meningiomas [22, 23], and at least two of these found CapE to be more frequent in HGM [13, 30], which is consistent with our findings.
The presence of a clear or unclear TBI in our study was not associated with LGM or HGM in either univariate or multivariate analysis. This is in contrast with a previous study by Lin et al. (2014) which suggests that a peritumoral ring with hypointense T1 signal and hyperintense T2 signal might indicate the presence of a physiological barrier between the brain and tumor [13]. Although a less clear TBI may be read as a disruption of this physiological barrier, this imaging finding was not associated with invasive brain growth in a microscopic histological analysis performed on a cohort of more than 600 subjects with meningiomas [27].
In this current study, we assessed tumor perfusion through the use of IVIM. Among IVIM parameters, the D coefficient was shown to be higher in LGM compared to HGM. This finding, already reported in literature [11], is likely to be connected with the fact that the D coefficient is usually lower in tissues with higher cellularity. The D* coefficient, which reflects molecular motion in the context of microcirculation, was also shown to be higher in LGMs compared to HGMs. This last result is in contradiction with another study which observed higher D* values in atypical meningiomas compared to WHO grade I meningioma [11]. Curiously, however, the same work confirms our own data regarding f values, which describe the volume fraction of intravoxel incoherent signals coming from the vascular component, which were shown to be higher in LGM compared to atypical meningiomas [11]. Additionally, a more recent study that focused mainly on differences between LGMs and atypical meningiomas showed no D* differences between the two subgroups [12].
Regarding the use of other MRI techniques to potentially assess meningioma perfusion, susceptibility contrast-enhanced (DSC) showed limited utility. The PTE of anaplastic meningiomas showed a significantly elevated relative cerebral blood volume compared to the PTE of benign meningiomas [31], whereas no significant difference in perfusion was detectable between different grades of meningiomas [31, 32]. Dynamic contrast-enhanced (DCE) perfusion is technically more complex than DSC, as the relationship between T1 signal intensity and contrast agent concentration is non-linear; its oncological applications are mainly focused outside of the brain [33]. Interestingly, the assessment of tumor blood flow with the arterial spin labelling technique found that WHO grade I meningiomas presented homogeneous hyperperfusion patterns, whereas atypical and anaplastic forms showed respectively heterogeneous hyperperfusion and no substantial hyperperfusion pattern [34]. This finding, already reported in literature [13], suggests that the correlation between tumor grade and vascularization in meningiomas may not be positive [34], consistently with our results.
The current study has several limitations. In contrast to most of the recent studies, our work focused on previously treated meningiomas. Biopsy or partial surgical excision was performed in all the subjects before baseline. Additionally, 31 subjects underwent conventional radiotherapy, which was completed at least 10 months before baseline. Radiotherapy might induce changes within tissues such as intralesional scarring, which might affect both CE and quantitative diffusion metrics. Nonetheless, it should be noted that the parts of tumors that were segmented and analyzed tended to increase in size following the abovementioned therapies as a result of ineffective treatments. While the presence of hemosiderin caused by previous treatments may have affected ADC values, we believe that the use of GE sequences as guides in avoiding areas of lower signal minimized the issue. Additionally, no different distribution of surgical procedures was detectable among groups.
While focusing on previously treated tumors may be seen as a limit in the imaging-based assessment of the histological grade of meningiomas, we believe it also represents one of the major strengths of this study. Our finding that ADC values and CE heterogeneity can still discern HGMs from LGMs with high sensitivity and specificity, even after treatments and even in recurring forms, can be easily generalized to a wider range of clinical situations compared to studies focusing exclusively on treatment-naïve meningiomas.
Finally, recent evidences concerning the potential development of histological heterogeneity within meningiomas make our model even more clinically relevant. A multi-site biopsy approach showed that genetic anomalies indicative of atypia can be heterogeneously detectable in distinct areas within the same tumor [35]. A multi-parametric MRI assessment might therefore be helpful in several clinical scenarios.
A pre-surgical MRI might guide surgeons in performing biopsies specifically in regions showing low ADC values and heterogeneous CE in order to histologically evaluate the specific area most likely to show histological features compatible with a higher grade. Similarly, a pre-radiotherapy assessment might be used to plan treatments focusing specifically on areas that show MRI features consistent with atypia and anaplasia which might be demand higher radiation doses.
Conclusion
In this study, we showed that a multi-parametric 3T MRI assessment including conventional morphological features as well as diffusion quantitative metrics can suggest atypia and anaplasia in meningiomas with a high sensitivity and specificity. Specifically, low values of ADC and heterogeneous CE are the best predictors of HGMs. Such an assessment represents a useful clinical tool capable of:
-
a)
Characterizing lesions localized in surgically inaccessible areas, as well as lesions in patients unsuitable for invasive procedures, compensating for the lack of information regarding the possible need for radiotherapy.
-
b)
Tailoring biopsies, as well as radiotherapy treatments, on a case-by-case basis.
Abbreviations
- CE:
-
Contrast enhancement
- HGM:
-
High-grade meningioma
- LGM:
-
Low-grade meningioma
- IVIM:
-
Intravoxel incoherent motion
- VIBE:
-
Volumetric interpolated breath-hold examination
- PTE:
-
Peritumoral edema
- CapE:
-
Capsular enhancement
- TBI:
-
Tumor-brain interface
- AUC:
-
Area under the curve
References
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820
Perry A, Louis DN, Scheithauer BW, Budka H, von Deimling A. Meningiomas. In: David N Louis, et al, editors. (2000) WHO classification of tumors of the central nervous system. Lyon: IARC p. 164–172
Buetow MP, Buetow PC, Smirniotopoulos JG (1991) Typical, atypical, and misleading features in meningioma. Radiographics. 11(6):1087–1106
Moliterno J, Cope WP, Vartanian ED, Reiner AS, Kellen R, Ogilvie SQ, Huse JT, Gutin PH (2015) Survival in patients treated for anaplastic meningioma. J Neurosurg 123(1):23–30
Wen PY, Quant E, Drappatz J, Beroukhim R, Norden AD (2010) Medical therapies for meningiomas. J Neuro-Oncol 99(3):365–378
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol 114(2):97–109
Chen CC, Hsu PW, Erich Wu TW et al (2009) Stereotactic brain biopsy: single center retrospective analysis of complications. Clin Neurol Neurosurg 111(10):835–839
Schipmann S, Brix T, Varghese J, Warneke N, Schwake M, Brokinkel B, Ewelt C, Dugas M, Stummer W (2019) Adverse events in brain tumor surgery: incidence, type, and impact on current quality metrics. Acta Neurochir 161(2):287–306
Kawahara Y, Nakada M, Hayashi Y, Kai Y, Hayashi Y, Uchiyama N, Nakamura H, Kuratsu JI, Hamada JI (2012) Prediction of high-grade meningioma by preoperative MRI assessment. J Neuro-Oncol 108(1):147–152
Aslan K, Gunbey HP, Tomak L, Incesu L (2018) The diagnostic value of using combined MR diffusion tensor imaging parameters to differentiate between low- and high-grade meningioma. Br J Radiol 91(1088):20180088
Yiping L, Kawai S, Jianbo W, Li L, Daoying G, Bo Y (2017) Evaluation parameters between intra-voxel incoherent motion and diffusion-weighted imaging in grading and differentiating histological subtypes of meningioma: a prospective pilot study. J Neurol Sci 372:60–69
Bohara M, Nakajo M, Kamimura K, Yoneyama T, Fukukura Y, Kiyao Y, Yonezawa H, Higa N, Kirishima M, Yoshiura T (2020) Histological grade of meningioma: prediction by Intravoxel incoherent motion histogram parameters. Acad Radiol 27(3):342–353
Lin BJ, Chou KN, Kao HW, Lin C, Tsai WC, Feng SW, Lee MS, Hueng DY (2014) Correlation between magnetic resonance imaging grading and pathological grading in meningioma. J Neurosurg 121(5):1201–1208
Zhang S, Chiang GC, Knapp JM et al (2019) Grading meningiomas utilizing multiparametric MRI with inclusion of susceptibility weighted imaging and quantitative susceptibility mapping. J Neuroradiol S0150-9861(19):30162–30162
Abdel-Kerim A, Shehata M, El Sabaa B, Fadel S, Heikal A, Mazloum Y (2018) Differentiation between benign and atypical cranial meningiomas. Can ADC measurement help? MRI findings with hystopathologial correlation. Egypt J Radiol Nucl Med 49(1):172–175
Surov A, Ginat DT, Sanverdi E, Lim CCT, Hakyemez B, Yogi A, Cabada T, Wienke A (2016) Use of diffusion weighted imaging in differentiating between maligant and benign meningiomas. A Multicenter Analysis. World Neurosurg 88:598–602
Le bihan D (2019) What can we see with IVIM MRI? Neuroimage 187:56–67
Tini P, Nardone V, Pastina P, Battaglia G, Vinciguerra C, Carfagno T, Rubino G, Carbone SF, Sebaste L, Cerase A, Federico A, Pirtoli L (2017) Perilesional edema in brain metastasis from non-small cell lung cancer (NSCLC) as predictor of response to radiosurgery (SRS). Neurol Sci 38(6):975–982
Coroller TP, Bi WL, Huynh E, Abedalthagafi M, Aizer AA, Greenwald NF, Parmar C, Narayan V, Wu WW, Miranda de Moura S, Gupta S, Beroukhim R, Wen PY, al-Mefty O, Dunn IF, Santagata S, Alexander BM, Huang RY, Aerts HJWL (2017) Radiographic prediction of meningioma grade by semantic and radiomic features. PLoS One 12(11):e0187908
Watanabe Y, Yamasaki F, Kajiwara Y, Takayasu T, Nosaka R, Akiyama Y, Sugiyama K, Kurisu K (2013) Preoperative histological grading of meningiomas using apparent diffusion coefficient at 3T MRI. Eur J Radiol 82(4):658–663
Surov A, Hamerla G, Meyer HJ, Winter K, Schob S, Fiedler E (2018) Whole lesion histogram analysis of meningiomas derived from ADC values. Correlation with several cellularity parameters, proliferation index KI 67, nucleic content, and membrane permeability. Magn Reson Imaging 51(March):158–162
Hale AT, Wang L, Strother MK, Chambless LB (2018) Differentiating meningioma grade by imaging features on magnetic resonance imaging. J Clin Neurosci 48:71–75
Palaniandy K, Haspani MSM, Zain NRM (2017) Prediction of histological grade and completeness of resection of intracranial meningiomas: role of peritumoural brain edema. Malays J Med Sci 24(3):33–43
Gawlitza M, Fiedler E, Schob S, Hoffmann KT, Surov A (2017) Peritumoral brain edema in meningiomas depends on Aquaporin-4 expression and not on tumor grade, tumor volume, cell count, or Ki-67 labeling index. Mol Imaging Biol 19(2):298–304
Ng WH, Hy JW, Tan WL, Liew D, Lim T, Ang BT, Ng I (2009) Aquaporin-4 expression is increased in edematous meningiomas. J Clin Neurosci 16(3):441–443
Cornelius JF, Slotty PJ, Steiger HJ, Hänggi D, Polivka M, George B (2013) Malignant potential of skull base versus non-skull base meningiomas: clinical series of 1,663 cases. Acta Neurochir 155(3):407–413
Adeli A, Hess K, Mawrin C, Streckert EMS, Stummer W, Paulus W, Kemmling A, Holling M, Heindel W, Schmidt R, Spille DC, Sporns PB, Brokinkel B (2018) Prediction of brain invasion in patients with meningiomas using preoperative magnetic resonance imaging. Oncotarget. 9(89):35974–35982
Kalamarides M, Stemmer-rachamimov AO, Niwa-kawakita M et al (2011) Identification of a progenitor cell of origin capable of generating diverse meningioma histological subtypes. Oncogene. 30(20):2333–2344
Hoover JM, Morris JM, Meyer FB (2011) Use of preoperative magnetic resonance imaging T1 and T2 sequences to determine intraoperative meningioma consistency. Surg Neurol Int 2:142
Li H, Zhao M, Jiao Y, Ge P, Li Z, Ma J, Wang S, Cao Y, Zhao J (2016) Prediction of high-grade pediatric meningiomas: magnetic resonance imaging features based on T1-weighted, T2-weighted, and contrast-enhanced T1-weighted images. World Neurosurg 91:89–95
Zhang H, Rödiger LA, Shen T, Miao J, Oudkerk M (2008) Perfusion MR imaging for differentiation of benign and malignant meningiomas. Neuroradiology. 50(6):525–530
Zampini MA, Buizza G, Paganelli C, Fontana G, D’Ippolito E, Valvo F, Preda L, Baroni G (2020) Perfusion and diffusion in meningioma tumors: a preliminary multiparametric analysis with dynamic susceptibility contrast and IntraVoxel incoherent motion MRI. Magn Reson Imaging 67:69–78
Essig M, Shiroishi MS, Nguyen TB, Saake M, Provenzale JM, Enterline D, Anzalone N, Dörfler A, Rovira À, Wintermark M, Law M (2013) Perfusion MRI: the five most frequently asked technical questions. AJR Am J Roentgenol 200(1):24–34
Qiao XJ, Kim HG, Wang DJJ, Salamon N, Linetsky M, Sepahdari A, Ellingson BM, Pope WB (2017) Application of arterial spin labeling perfusion MRI to differentiate benign from malignant intracranial meningiomas. Eur J Radiol 97:31–36
Juratli TA, Thiede C, Koerner MVA, Tummala SS, Daubner D, Shankar GM, Williams EA, Martinez-Lage M, Soucek S, Robel K, Penson T, Krause M, Appold S, Meinhardt M, Pinzer T, Miller JJ, Krex D, Ely HA, Silverman IM, Christiansen J, Schackert G, Wakimoto H, Kirsch M, Brastianos PK, Cahill DP (2017) Intratumoral heterogeneity and promoter mutations in progressive/higher-grade meningiomas. Oncotarget. 8(65):109228–109237
Acknowledgements
The authors would like to thank our patients and their families for participating in our research.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sacco, S., Ballati, F., Gaetani, C. et al. Multi-parametric qualitative and quantitative MRI assessment as predictor of histological grading in previously treated meningiomas. Neuroradiology 62, 1441–1449 (2020). https://doi.org/10.1007/s00234-020-02476-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02476-y