Abstract
Purpose
Despite considerable published information about the clinical–radiological correlation of medullary infarcts, no study has determined whether topographic evaluations are performed accurately among researchers. Our purpose in this study was twofold: to evaluate the topographic pattern of medullary infarcts on diffusion-weighted imaging by their radiological aspect, and to assess interobserver agreement on the topographic pattern.
Methods
We retrospectively reviewed our imaging and clinical database for patients admitted to our radiology department between January 2014 and September 2019. Two radiologists evaluated the imaging studies independently. Consensus data were used in the analysis.
Results
The retrospective review yielded 92 patients with medullary infarction. The affected vascular territories were lateral (n = 58), anteromedial (n = 28), posterior (n = 3), and anterolateral (n = 1). Two patients had hemimedullary infarction. The rostrocaudal levels of the medullary infarct were superior (n = 34), middle (n = 31), inferior (n = 4), superior-middle (n = 13), and middle-inferior (n = 10). The medullary infarcts were divided into two types: lateral (n = 62) and medial (n = 28). The affected vascular territories differed with rostrocaudal topography of medullary infarct (p = 0.003). Excellent interobserver agreement was found for type of medullary infarct, compared with moderate for vascular territory and fair for rostrocaudal topography. The anterolateral and posterior territories were the most often misdiagnosed, while the level with the most disagreements in rostrocaudal topography was middle.
Conclusion
The accurate topographic evaluation of a medullary infarct can be an important basis for investigating stroke etiology. However, correct topographic evaluation may not always be available and smaller territories such as anterolateral and posterior should be assessed carefully.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medullary infarction constitutes 7% of all ischemic brainstem strokes [1]. Lateral medullary infarction (LMI) is approximately five times more frequent than medial medullary infarction (MMI) [2]. Although dorsal medullary infarcts may accompany cerebellar strokes, isolated dorsal medullary infarcts are considered to be a type of LMI [1].
The vascular zone of the medulla is divided into four territories: large anteromedial, small anterolateral, large lateral, and small dorsal. The anterolateral and posterior zones are smaller than the others. The anteromedial territory is supplied by the anterior spinal artery (ASA) and vertebral artery (VA) cranially, and the ASA inferiorly. The anterolateral territory is supplied by the ASA and posterior inferior cerebellar artery (PICA) inferiorly, and by the ASA and VA superiorly. The lateral territory feeds from the PICA, basilar artery (BA), VA, and anterior inferior cerebellar artery, from caudal to cranial. The posterior territory is supplied by the posterior spinal artery (PSA) inferiorly, and the PICA superiorly [1, 3,4,5].
Each vascular zone includes different cranial nerve nuclei and fascicles. Infarcts in different vascular zones may cause various symptoms [1], so the clinical picture of medullary infarcts is diverse [1, 5,6,7]. Among syndromes, Dejerine’s and Wallenberg’s are the most commonly known [1, 4, 6, 8,9,10]. Making an accurate diagnosis of the affected vascular zone and level of the medullary infarct can be important for building a clinical picture and determining the etiology of a stroke.
Most previous studies of medullary infarcts using diffusion-weighted imaging (DWI) were conducted by clinicians and focused on clinical–radiological correlations. However, the effect of differences in topographic assessments among researches has not been examined. This study aimed to evaluate the topography of medullary infarct on DWI by the radiological assessing and to investigate the interobserver agreement on the topographic pattern.
Materials and methods
Ethics, study design, and patients
The study protocol was reviewed and approved by our institutional ethics committee.
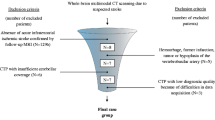
We retrospectively reviewed our imaging and clinical database for the 333 consecutive brainstem infarction patients admitted to our radiology department between January 2014 and September 2019 on a PACS imaging workstation. We evaluated any case with a diffusion restriction on DWI that developed due to either infarction or cytotoxic edema that can recover with blood flow restoration. Patients with pons or mesencephalic infarcts were excluded. After that, remaining 92 patients who had medullary infarct were included. We divided this population into two groups: LMI (n = 62) and MMI (n = 28). Two patients had both LMI and MMI together (i.e., hemimedullary infarction).
Imaging protocols
DWIs were obtained using two 1.5 T magnetic resonance imaging (MRI) units (GE Signa HDxt and Signa Explorer; GE, Milwaukee, WI, USA). DWIs were acquired in the axial plane with parameters field of view 25 mm, repetition time 5000 ms, echo time 100 ms, acquisition time 1, number of excitations 1, and b values of 0 and 1000 s/mm2, isotropically weighted. DWI yielded 20 contiguous slices that were 7 mm thick and axial-oblique. A visual evaluation was performed. Besides, computed tomography angiography (CTA) and magnetic resonance angiography (MRA) were evaluated to search for etiology.
Imaging analysis
Two radiologists with significant experience in radiology (SND with 11 years in neuroradiology and AHB with 17 years in radiology) evaluated all DWI independently on a PACS imaging workstation (Infinitt PACS; Infinitt Healthcare, Seoul, Republic of Korea). The radiologists were blinded to neurologic symptoms during the retrospective imaging review, and discrepancies were resolved by consensus.
Stroke etiology was investigated by medical record review. Stroke etiologies were classified into five groups based on the Trial of ORG 10172 in Acute Stroke Treatment criteria [P12:10]: (1) large artery atherosclerosis (embolus/thrombosis), (2) cardioembolism, (3) small-vessel occlusion, (4) stroke of other determined etiology, and (5) stroke of undetermined etiology. Large artery atherosclerosis was defined as more than 50% stenosis at the relevant distal or proximal VA, or proximal BA. When the patient had normal angiographic findings and no cardioembolism, small vessel occlusion was defined when the infarction corresponded to a penetrator artery on MRI. CTA and MRA were performed and the degree of stenosis calculated based on the North American Symptomatic Carotid Endarterectomy Trial method. Cardioembolic disease was evaluated by 12-lead electrocardiography, transthoracic echocardiography, and 24-h rhythm Holter monitoring.
Infarct topography
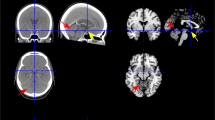
Infarcts were assigned four vascular zones based on previously published territory templates: anteromedial, anterolateral, lateral, and dorsal arterial (Fig. 1) [1, 3, 5]. The rostrocaudal topography of the infarct was assigned one of three levels: upper, middle, and lower (Fig. 2) [5]. While the upper medulla was identified by massive dorsolateral bulging of the restiform body, the middle medulla was identified by ventral-lateral bulging of the inferior olivary nucleus. The lower medulla has a relatively round shape without bulging.
Based on the affected vascular zone of the medulla, there were two types of medullary infarct: LMI and MMI. LMI was accepted when either lateral or anterolateral arterial zones were involved. Posterior infarcts were also considered a part of LMI when they were alone [5]. MMI was considered when the anteromedial arterial zone was included. However, when both MMI and LMI were concomitant, a hemimedullary infarct was identified.
Statistical analysis
NCSS 10 software (NCSS Statistical Software, Kaysville, UT, USA) was used for data analysis. Normality checks were performed by the Shapiro–Wilk test, and by drawing histograms, Q–Q plots, and box plots. Data were characterized by their mean, standard deviation, minimum, maximum, frequency, and percentage. The two categories of non-normally distributed variables were analyzed by the Mann–Whitney U test. Fisher’s exact probability test was used to determine the nominal variables. The significance level was taken as p < 0.05 and bidirectional. A kappa, κ, coefficient test was used to determine the strength of agreement between two observers’ judgments on the type, the affected vascular territory, and the rostrocaudal topography of medullary infarction. The strength of interobserver agreement above the level of chance was assessed according to the following ratings for κ: 0.00–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; and 0.81–1.00, excellent.
Results
General results
The retrospective review yielded 333 consecutive brainstem infarcts during the last 5 years. All patients had DWI. Among these, 92 patients had a medullary infarct and the prevalence of medullary infarct among brainstem infarction was 28%. Medullary infarcts located laterally in 62 (67.4%) patients, medially in 28 (30.4%), and hemimedullary in 2 (2.2%). The ratio of LMI to MMI was 2.2. The prevalence of LMI, MMI, and hemimedullary infarct was 18.6%, 8.4%, and 0.6%, respectively.
In the total cohort, 58 (63%) patients were male and 34 (37%) were female. The mean age was 61.5 (range, 21–90) years. The medullary infarction was located on the right side in 45 patients (48.9%) and on the left side in 47 (51.1%). Comparing patients with MMI or LMI, the age, sex, and side affected were similar for both, with p = 0.469, 0.496, and 0.07, respectively. The time from event to DWI ranged from 4 h to 7 days.
The stroke etiology was investigated for 74 (80.4%) patients. CTA was performed for 89.8% of patients, and MRA for 10.2%. The presumed cause of stroke in the total cohort was large artery disease in 38 patients (51.4%), small vessel disease in 21 (28.4%), cardioembolism in 9 (12.2%), other determined etiology in 2 (2.7%), and undetermined in 4 (5.4%). The two other determined etiologies were heterozygous methylenetetrahydrofolate reductase (MTHFR) mutation and heterozygous Factor V Leiden mutation. The etiology could not be investigated for the remaining 18 patients (19.6%) because one of them died within the first 24 h, and the others transferred to other referral centers. No significant difference in stroke etiology was observed between MMI and LMI (p = 0.73). The stroke etiologies for the vascular zone and rostrocaudal topography were also similar, with p = 0.92 and 0.851, respectively.
Tables 1 and 2 summarize the patient demographics for the total cohort and two groups, respectively.
Imaging-related results
Regarding the type of medullary infarct, the strength of interobserver agreement was excellent (κ = 0.829 ± 0.062, p < 0.001). Concerning the vascular territory of the medulla oblongata, the lateral territory was involved in 58 patients (63%), anteromedial in 28 (30.4%), posterior in 3 (3.3%), anterolateral in 1 (1.1%), and hemimedullary in 2 (2.2%). Lesions were most commonly identified in the lateral territory, followed by the anteromedial and posterior. Interobserver agreement on the affected territory was moderate (κ = 0.592 ± 0.066, p < 0.001). Disagreement was most commonly observed for small territory areas, such as the posterior and anterolateral.
Considering the rostrocaudal topography for the total cohort, the superior medulla was involved in 34 patients (37%), middle in 31 (33.7%), inferior in 4 patients (4.3%), superior-middle in 13 (14.1%), and middle-inferior in 10 (10.9%). Interobserver agreement on the rostrocaudal topography was fair (κ = 0.278 ± 0.052, p < 0.001). Disagreement was most commonly observed for the middle medulla. Table 3 summarizes the results from the imaging studies relating to vascular zone and rostrocaudal topography.
The involved vascular territories differed according to the rostrocaudal topography of the infarct (p = 0.003). Whereas anteromedial territory infarcts were located in the upper medulla (67.9%), lateral territory infarcts were more frequent in the middle medulla (37.9%). The distribution difference in rostrocaudal topography between MMI and LMI was significant (p = 0.005). Table 4 shows the distribution of infarctions with vascular zone and rostrocaudal topography.
Discussion
In the present study, we evaluated the topographic pattern of medullary infarctions based on the relevant vascular zone and rostrocaudal location by DWI. We also assessed the interobserver agreement of that topographic evaluation. We obtained several interesting findings. First, the strength of interobserver agreement was not the same among the vascular zone, rostrocaudal topography, and type of medullary infarction. Agreement on the mapping of the vascular zone was moderate, and agreement on the rostrocaudal topography infarction was fair. However, agreement on the type of medullary infarct was excellent. Second, the distributions in rostrocaudal topography of the infarct were significantly different among vascular territories and between MMI and LMI. Third, the ratio of LMI to MMI was low. Fourth, the etiology of stroke was similar between LMI and MMI. No significant differences in stroke etiology were observed for the vascular zone or rostrocaudal topography.
Most previous studies that were conducted by clinicians focused on the clinical–radiologic relationship. However, interobserver agreement on the topographic evaluation by radiologists as in the present study had not been assessed. To the best of our knowledge, this is the only study in which interobserver agreement has been evaluated. According to our results, there was a discrepancy in evaluating relatively small vascular territories, such as the anterolateral and posterior. However, it did not influence the typing of the medullary infarction because both of these vascular zones are considered LMI. The anteromedial territory includes the medial portion of the pyramidal tract, while the anterolateral territory includes the lateral portion of the pyramidal tract and the medial part of the inferior olive [5]. The border of the anterolateral and anteromedial territories was unclear because of the low resolution of the DWI.
Interobserver agreement on the rostrocaudal topographic evaluation was worse than on the others, and disagreement was observed most often for the middle medulla. This may be because the medulla was not always imaged at the same level in the axial plane in all patients. Thus, the borders of the levels might have overlapped so that the level of the medulla could not be decided exactly in every patient.
Another aspect to consider is the distribution of rostrocaudal topography among vascular territories and types of medullary infarction. Consistent with the literature, the distributions of rostrocaudal topography among vascular territories and between MMI and LMI were significantly different [11, 12]. Anteromedial territory infarctions were located distinctly in the upper medulla, as in previous studies [11,12,13,14]. In the upper medulla, anteromedial arteries usually emerge from the VA and ASA. In the lower medulla, anteromedial arteries arise from the ASA. After the coupled ASA supply a part of the upper medulla with the VA, they fuse to form a single artery. After that, it feeds the middle and lower medulla. Occlusion of the ASA before the union might not produce infarction in the middle and lower medulla. The ASA also has anastomosis with the PSA. This could explain the predisposition towards anteromedial zone infarction in the upper medulla [11, 12]. Consistent with previous studies, the lateral territory was involved most often in the middle medulla [11, 12, 14, 15]. However, the involvement of more than one level of the medulla occurred mostly for lateral territory infarcts, rather than for other territories. The tendency towards LMI in the middle and middle-lower medulla could be explained by the anatomical course of the VAs. A pair of VAs lies adjacent to the lateral surface of the caudal medulla and ascends anteroinferiorly to merge with the BA [15]. On the other hand, the various rostrocaudal levels of LMI could be explained by the variability of the PICA origin from the VA, variable length of the ascending loop of the PICA, different degrees of collateralization from the VA or anterior inferior cerebellar artery, and different levels of occlusion of penetrating branches from the PICA.
In previous studies [11, 14], the posterior territory was frequently involved in the middle and lower medulla. However, in the present study, the posterior territory was involved only in the middle and middle-superior medulla; this apparent discrepancy needs to be explained. The posterior territory of the caudal medulla is supplied by the PSA, which is a relatively distal branch of the PICA. This area may often be spared because atherosclerotic disease is more severe in the proximal part of the PICA [16] and the most common presumed etiology was large vessel disease in the present study. On the other hand, while the small number of posterior vascular zone infarctions could cause this disagreement with previous studies [11, 14], as mentioned above, interobserver agreement was not good in small territories, such as the posterior. So, the posterior territory might need to be evaluated more carefully.
The ratio of LMI to MMI was 2.2, which is lower than in previous reports [2, 12, 14, 17]. As reported previously, MMI may be underdiagnosed when DWI is not repeated [8, 10].
Concerning stroke etiology, large vessel disease was the most frequent etiology in the present study. Small vessel disease was seen more commonly than cardioembolism, as in previous studies [11, 12, 15]. The distributions of stroke etiology among vascular territories and rostrocaudal topography were similar. However, Kim et al. reported that the distribution of stroke etiology in rostrocaudal topography was similar, but a significant difference was present between the infarct mechanism and vascular zone [11]. Kim concluded that the most common etiology in LMI was large vessel disease [15]. There are several studies reporting different results regarding the etiology of MMI. Akimoto et al. reported that large vessel disease was the most common vascular pathology in MMI [8]. However, small vessel disease was reported as the most common mechanism of MMI in different studies [6, 11, 13]. In the present study, no significant difference in stroke etiology was present between MMI and LMI, consistent with a previous study [12].
Several limitations to this study need to be acknowledged. First, the study was retrospective and suffers from the limitations of all such studies. Second, it was a single-center study. Third, only a small number of infarctions were present for certain vascular territories. Fourth, the etiology of stroke could not be investigated in all patients. Fifth, arterial lesions were determined via CTA and MRA, not digital subtraction angiography.
Conclusion
There could be some discordance in mapping territories, especially smaller territories such as the anterolateral-posterior, and in evaluating the rostrocaudal topography of an infarction. The relevant arteries are different for each vascular zone at different levels of the medulla. Accurate topographic evaluation is important to avoid clinical–radiological discrepancies. Exact mapping of a medullary infarct can provide a guide to which artery is most likely to be affected, and this may play an important role in investigating etiology.
References
Burger KM, Tuhrim S, Naidich TP (2005) Brainstem vascular stroke anatomy. Neuroimaging Clin N Am 15:297–324, x. https://doi.org/10.1016/j.nic.2005.05.005
Fung SH, Roccatagliata L, Gonzalez RG, Schaefer PW (2011) MR diffusion imaging in ischemic stroke. Neuroimaging Clin N Am 21:345–377, xi. https://doi.org/10.1016/j.nic.2011.03.001
Tatu L, Moulin T, Bogousslavsky J, Duvernoy H (1998) Arterial territories of the human brain cerebral hemispheres. Neurology 50:1699–1708. https://doi.org/10.1212/WNL.50.6.1699
De Mendivil AO, Alcalá-Galiano A, Ochoa M et al (2013) Brainstem stroke: anatomy, clinical and radiological findings. Semin Ultrasound CT MRI 34:131–141. https://doi.org/10.1053/j.sult.2013.01.004
Bassetti C, Bogousslavsky J, Mattle H, Bernasconi A (1997) Medial medullary stroke: report of seven patients and review of the literature. Neurology 48:882–890
Shono Y, Koga M, Toyoda K, Matsuoka H, Yokota C, Uehara T, Yamamoto H, Minematsu K (2010) Medial medullary infarction identified by diffusion-weighted magnetic resonance imaging. Cerebrovasc Dis 30:519–524. https://doi.org/10.1159/000319887
Kim JS, Lee JH, Suh DC, Lee MC (1994) Spectrum of lateral medullary syndrome correlation between clinical findings and magnetic resonance imaging in 33 subjects. Stroke 25:1405–1410. https://doi.org/10.1161/01.STR.25.7.1405
Akimoto T, Ogawa K, Morita A, Suzuki Y, Kamei S (2017) Clinical study of 27 patients with medial medullary infarction. J Stroke Cerebrovasc Dis 26:2223–2231. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.05.004
Kitis O, Calli C, Yunten N, Kocaman A, Sirin H (2004) Wallenberg’s lateral medullary syndrome: diffusion-weighted imaging findings. Acta Radiol 45:78–84. https://doi.org/10.1080/02841850410000692
Kumral E, Afsar N, KIrbas D et al (2002) Spectrum of medial medullary infarction: clinical and magnetic resonance imaging findings. J Neurol 249:85–93. https://doi.org/10.1007/PL00007852
Kim K, Lee HS, Jung YH, Kim YD, Nam HS, Nam CM, Kim SM, Heo JH (2012) Mechanism of medullary infarction based on arterial territory involvement. J Clin Neurol 8:116–122. https://doi.org/10.3988/jcn.2012.8.2.116
Hong Y-H, Zhou L-X, Yao M, Zhu YC, Cui LY, Ni J, Peng B (2018) Lesion topography and its correlation with etiology in medullary infarction: analysis from a multi-center stroke study in China. Front Neurol 9:813. https://doi.org/10.3389/fneur.2018.00813
Kim JS, Han YS (2009) Medial medullary infarction: clinical, imaging, and outcome study in 86 consecutive patients. Stroke 40:3221–3225. https://doi.org/10.1161/STROKEAHA.109.559864
Kameda W, Kawanami T, Kurita K, Daimon M, Kayama T, Hosoya T, Kato T, Study Group of the Association of Cerebrovascular Disease in Tohoku (2004) Lateral and medial medullary infarction: a comparative analysis of 214 patients. Stroke 35:694–699. https://doi.org/10.1161/01.STR.0000117570.41153.35
Kim JS (2003) Pure lateral medullary infarction: clinical-radiological correlation of 130 acute, consecutive patients. Brain 126:1864–1872. https://doi.org/10.1093/brain/awg169
Kim JS, Lee JH, Choi CG (1998) Patterns of lateral medullary infarction: vascular lesion-magnetic resonance imaging correlation of 34 cases. Stroke 29:645–652. https://doi.org/10.1161/01.STR.29.3.645
Fukuoka T, Takeda H, Dembo T et al (2012) Clinical review of 37 patients with medullary infarction. J Stroke Cerebrovasc Dis 21:594–599. https://doi.org/10.1016/j.jstrokecerebrovasdis.2011.01.008
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dogan, S.N., Bayrak, A.H. & Yazgu, R. Topographic evaluation of medullary infarcts from the radiologist’s point of view. Neuroradiology 62, 947–953 (2020). https://doi.org/10.1007/s00234-020-02398-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02398-9