Abstract
Purpose
Different studies showed correlations between gadolinium-based contrast agent (GBCA) administrations and dentate nucleus (DN) T1-weighted hyperintensity. The clinical impact of gadolinium retention, however, is still largely unknown. The aim of this study was to investigate relations between MRI and clinical disability in relapsing–remitting multiple sclerosis (RR-MS) patients.
Methods
In this retrospective study, clinical data were obtained from 74 RR-MS patients at baseline and after a mean follow-up time of 3.6 years, including the expanded disability status scale (EDSS) score and its change (ΔEDSS). Patients were considered showing clinical worsening if they score a ΔEDSS ≥ 1 (for baseline EDSS ≤ 5.5) or ΔEDSS ≥ 0.5 (for baseline EDSS > 5.5). From the MRI data, the presence of bilateral DN hyperintensity was recorded along with the calculation of longitudinal relaxation rate (R1) maps.
Results
Patients with DN hyperintensity showed similar ΔEDSS change compared to those without visible changes on T1-weighted images (p = 0.32). Similarly, no DN-R1 difference was found comparing stable patients with those showing a significant clinical worsening (p = 0.54). Finally, no significant effect of DN-R1 values explained the variance in ΔEDSS (p = 0.76), thus suggesting their independence from the clinical outcome.
Conclusions
MS patients with DN hyperintensity show similar EDSS changes compared to subjects without DN high-signal intensity. Furthermore, mean DN-R1 values of patients with significant clinical worsening were comparable to those of stable subjects and were unrelated to clinical disability. Taken together, these findings suggest that gadolinium retention in the brain of MS patients does not affect their clinical worsening, expressed by the EDSS change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gadolinium-based contrast agents (GBCAs) are a family of paramagnetic compounds consisting of gadolinium ion chelated with linear or macro-cyclic ligands to form a stable complex [1]. Largely used in diagnostic neuroradiology for their ability to shorten T1- and T2-relaxation times of tissues enhancing tissue characterization, GBCAs have been applied to a large range of central nervous system (CNS) diseases, especially in inflammatory or oncologic conditions requiring periodic follow-up [2].
Despite GBCA safety and the limited number of adverse reactions [3] and long-term complications [4], possible gadolinium retention in the brain of patients receiving multiple GBCAs doses has recently become a major public health concern [5], with possible implications for clinical practice [6].
The presence of spontaneous high-signal intensity on unenhanced T1-weighted images of the dentate nucleus (DN) and the globus pallidus (GP) in patients who had undergone multiple GBCA administration has been described by Kanda and colleagues in 2014, showing a direct correlation between increased relative signal intensity and the number of contrast-agent administrations [7]. Since this first report, several qualitative and quantitative MRI studies confirmed a T1 shortening, on unenhanced images, of the deep nuclei of subjects who had received multiple doses of GBCAs [8, 9], notwithstanding the presence of a conserved renal function, preserved hepatobiliary system, or intact blood–brain barrier [10,11,12]. Following these observations, both animal [13, 14] and human [11, 15, 16] ex vivo studies demonstrated that these regional signal changes correspond to gadolinium retention, occurring independently from the presence of renal dysfunction [15].
These findings exerted a great impact on the scientific community, as they have possible consistent implications for the daily clinical practice, with concerns about the potentially harmful effects of gadolinium deposits [10]. Although no clear relationship between GBCA administration and overall disability has been described neither in animal models nor in human [10], the possible clinical impact of gadolinium retention in the brain is still largely unknown. A recent study reported an association between increased DN signal intensity and lower verbal fluency in multiple sclerosis (MS) patients, although a causal relationship between the two could not be determined [17]. At the same time, other authors have proposed the existence of a “Gadolinium Deposition Disease” (GDD), a still controversial definition used to designate the onset of mild, non-specific, self-limiting, and, generally, self-reported systemic symptoms arising from hours to months after GBCA administration [18,19,20,21].
To expand the current knowledge about the clinical meaningfulness of GBCA retention, we have assessed the impact of repeated intravenous administration of GBCAs on the clinical evolution of patients with relapsing–remitting MS (RR-MS) by investigating the relationship between MRI signs suggestive of gadolinium retention and disability progression.
Material and methods
Participants
This retrospective analysis was conducted on the same group of 74 RR-MS patients enrolled in a previous cross-sectional study [22]. For each patient included in the analysis, longitudinal clinical data were obtained within one week from the MRI acquisition and after a mean follow-up time of 3.6 (± 0.6) years. All patients fulfilled the 2010 revision of the McDonald’s criteria for MS diagnosis [23]. Seventy three out of 74 patients were in treatment with a disease-modifying therapy (DMT) at baseline (fingolimod n = 17; natalizumab n = 28; interferon beta-1a n = 20; interferon beta-1b n = 7; glatiramer acetate n = 1), with 35 patients under a different agent at the follow-up examination (fingolimod n = 23; natalizumab n = 19; interferon beta-1a n = 9; dimethyl fumarate n = 7; interferon beta-1b n = 5; alemtuzumab n = 4; rituximab n = 1; glatiramer acetate n = 1).
Global clinical disability was assessed at baseline and follow-up with the expanded disability status scale (EDSS) score, and disease duration (DD) was estimated as the interval period between the first occurrence of symptoms referable to the disease onset and the baseline assessment. Despite its known limitations [24], EDSS was selected as the outcome measure not only because it is routinely administered in our MS center and was the clinical measure more consistently available for retrospective collection across subjects and time points but also because, to date, it is still the most widely used measure of clinical disability progression in MS trials [25]. For each subject, the change in the EDSS score (ΔEDSS, calculated as Follow-up EDSS − Baseline EDSS) was reported. According to the criteria first described by Weinshenker and colleagues [26] and commonly applied in research setting and clinical trials [27, 28], patients were considered having a clinical worsening when scoring a ΔEDSS ≥ 1 (if baseline EDSS was 5.5 or lower), while for subjects with a baseline EDSS above 5.5, a ΔEDSS ≥ 0.5 was used to define progression. Finally, the number of new relapses (NR) and the change to a secondary progressive (SP-MS) type of the disease were recorded at the follow-up examination, as additional indices of clinical status.
Standard protocol approvals, registrations, and patient consents
This study was formerly approved by the local Ethics Committee. Written informed consent was obtained from all patients prior to enrollment.
MRI data acquisition
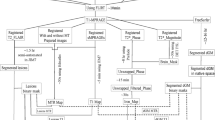
All MRI data were acquired on the same 3T scanner (Trio, Siemens Medical Systems, Erlangen, Germany) after one year (12.3 ± 0.9 months) from the last contrast-enhanced MRI scan. The following sequences were included in the acquisition protocol: a 3D Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) T1-weighted sequence (TR = 2500 ms; TE = 2.8 ms; TI = 900 ms; FA = 9°; voxel size = 1 × 1 × 1 mm3; 176 sagittal slices) acquired before contrast administration, a 3D Fluid-Attenuated Inversion Recovery sequence (FLAIR; TR = 6000 ms; TE = 396 ms; TI = 2200 ms; flip angle = 120°; voxel size = 1 × 1 × 1 mm3, 160 sagittal slices) used for the estimation of lesions load (LL) volume, and two unenhanced 3D double-echo spoiled GRE sequences (TR = 28 ms; TE1 = 7.63 ms; TE2 = 22.14 ms; FA1 = 3°; FA2 = 20°; GRAPPA factor = 2; bandwidth = 190 Hz/pixel; voxel size = 0.65 × 0.65 × 1.3 mm3; 128 axial slices).
MRI data processing and analysis
Hyperintense lesions on FLAIR images were identified and segmented for all patients included in the study. This analysis was performed in consensus by three observers (CP, MDS, EV) with more than 4 years of experience in brain imaging using a semiautomatic approach (Jim 7, Xinapse Systems, Northants, UK), and LL volume was obtained for each subject.
Corresponding lesion masks were then coregistered using an affine registration to the T1-weighted volume for an in-painting procedure, carried out using FSL v. 5.0.10 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl), to correct for the impact of white matter lesions during the segmentation procedure [29]. Using SIENAX [30], gray matter volume (GMV) (normalized by multiplying for the V-scaling factor) was obtained for each subject, as an index of cortical atrophy.
Qualitative and quantitative evaluation of DN hyperintensity were respectively used as clinical and advanced MRI indices of GBCA retention.
Qualitative evaluation was performed on volumetric T1w images, visually inspected independently by three different readers (CP, MDS, EV). In case of dubious interpretation of MRI findings, a fourth expert reader (AB) with more than 25 years of experience in neuroradiology was asked to review the images, to reach a final decision. For each patient, readers were asked to evaluate whether a clear and defined bilateral hyperintensity affecting DN was present. DN hyperintensity was considered present when reported by at least 2 out of 3 of the readers.
Quantitative evaluation was achieved through calculation of longitudinal relaxation rate (R1) maps according to [31, 32]. For each patient, the R1 values of the DN were obtained by hand-drawing two irregular bilateral regions of interest (ROIs) on the axial slice of the GRE images where the best representation of the DN was available, and mean values were normalized with those obtained by placing two circular ROIs on the brainstem. A complete and detailed description of R1 image analysis is available in [22].
Statistical analysis
The Fleiss’ kappa coefficient was calculated to assess the agreement between readers in evaluating the presence of DN hyperintensity, with the following classification: ≤ 0.20 = poor agreement; 0.20–0.40 = fair agreement; 0.40–0.60 = moderate agreement; 0.60–0.80 = good agreement; ≥ 0.80 = excellent agreement.
An independent two-sample t test was used to evaluate possible differences in terms of age, DD, and number of contrast-enhanced MRI between MS patients with and without a significant clinical worsening at the follow-up examination, while differences in terms of sex and EDSS values were probed via chi-squared and Mann–Whitney tests, respectively.
To verify the reliability of visually detected DN hyperintensity as an actual index of objective relaxometric modifications (and, thus, of GBCA retention), an independent two-sample t test was used with the aim of demonstrating differences in terms of DN R1 values between MS patients with and without DN hyperintensity.
Furthermore, possible differences in terms of ΔEDSS between patients with and without DN hyperintensity, as well as the comparison of DN R1 values between stable patients and those showing a clinical worsening were probed via the general linear model, removing the effects of potential confounding factors, such as age, sex, MS type, GMV, LL, DMT, number of NR, and DD.
Similarly, the possible relationship between R1 values and ΔEDSS was tested via hierarchical multiple linear regression analysis, including age, sex, MS type, DMT, number of NR, and DD in the first block and MRI variables in the second.
Analyses were carried out using the Statistical Package for Social Science (SPSS) package (SPSS Inc., version 17.0, Chicago, IL), with a p = 0.05 set to indicate a statistically significant difference in the between-group comparison and regression analyses.
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
Of the 74 patients enrolled in the baseline evaluation, 4 subjects did not return for the follow-up clinical examination and were, therefore, dropped-out from the subsequent analyses, thus leaving a total number of 70 subjects included in this study. Among these, 6 patients (8.6%) converted to a progressive phenotype and 12 patients (17.1%) showed a significant clinical worsening at the follow-up examination, with a mean ΔEDSS of 1.3 (± 0.78, ranging from 0.5 to 3.5 points). A complete list of demographical and clinical data of the studied population is available in Tables 1 and 2.
A good agreement between readers was achieved when evaluating the presence of DN hyperintensity (κ = 0.64), with consensus reached in 74.3% of the cases (52/70 subjects). DN hyperintensity was visible in 27 out of 70 (37.0%) of the examined MS patients. An example of a positive DN involvement is shown in Fig. 1.
As reported in the baseline work in which quantitative MRI data were evaluated, the normalized DN R1 values proved to be significantly correlated with the number of GBCA administrations (p < 0.001) [22]. This correlation remained significant after correction for MS-related variables and was mainly linked to linear GBCAs rather than macro-cyclic chelates [22].
When investigating the correspondence between qualitative and quantitative DN alterations, significantly higher DN R1 values were found in MS patients showing DN hyperintensity on unenhanced T1-weighted images compared to those without visible MRI changes (1.15 ± 0.01 vs 1.11 ± 0.01 s−1, p = 0.03).
When testing for possible differences in ΔEDSS between patients showing DN hyperintensity and patients without visible changes on unenhanced T1-weighted images, no significant difference emerged between the two groups (p = 0.32) (Fig. 2). Similarly, no difference was found in terms of DN R1 when subjects showing a significant clinical worsening were compared to stable patients (p = 0.54) (Fig. 3).
Finally, when possible relationships between DN R1 values and ΔEDSS were tested, the regression model explained 23.4% of the variance of ΔEDSS, without any significant effect of DN R1 values (p = 0.76). A scatter plot of the correlation between MRI data and clinical findings is reported in Fig. 4.
Discussion
In this study, we explored the possible impact of gadolinium retention in the brain on global motor disability in RR-MS patients, showing that qualitative and quantitative MRI features suggestive of GBCAs deposition are not associated with significant MS-related global disability progression.
Free gadolinium ion is known to show high toxicity, whereas chelated gadolinium has been historically considered relatively safe [33, 34]. Although animal models failed to demonstrate significant harm or behavioral changes in rats after repeated GBCA administrations [13, 14], some evidences of acute toxicity in vitro [35] or in animal models [36] have been reported. In human, besides some anecdotal reports of nephrotoxicity [37], neurotoxicity [38], and pancreatitis [39], very few data are available on the long-term effects of GBCA retention in the brain tissue.
In this work, we expanded the current knowledge about gadolinium deposition investigating, in a cohort of MS patients, the global motor disability evolution as function of the GBCA retention. We found no difference in terms of disability progression when comparing MS patients with and without DN hyperintensity on T1-weighted unenhanced images, suggesting a relative independence between the most recognizable indirect marker of gadolinium retention in the brain and the clinical course of the disease. Nevertheless, although in our analysis the readers reached a good agreement in evaluating this neuroradiological feature, visual assessment of DN T1-weighted hyperintensity shows the limits of any qualitative analysis, both in terms of reproducibility and reliability. These limitations can be overcome using quantitative measurements, which allow for an accurate and solid evaluation of tissue properties and provide information on microstructural changes not achievable using qualitative analyses [40]. Therefore, in the present study, the lack of association between gadolinium retention qualitatively estimated as DN hyperintensity and clinical progression has been confirmed by quantitative data, demonstrating no significant difference in terms of DN R1 values between patients with a stable disease and those who manifested a significant clinical worsening. Furthermore, when investigating the possible relationship between DN R1 values and disability progression rate, measured as ΔEDSS change between baseline and follow-up examination, no significant association was found. This result, in addition to the reported lack of effect of DD and EDSS on R1 values at the cross-sectional analysis [22], further supports the independence between DN relaxometry changes induced by gadolinium deposition and clinical status.
Our results mitigate against the presence of conditions, such as the GDD, in which unspecific symptoms, such as tightness, pain, and persistent headache with clouded mentation (“brain fog”), are reported usually within 1 month after GBCA administration [20, 21]. In contrast with these results, which were obtained collecting data via an online survey in which MS patients self-reported their symptoms without a control group, our findings suggest that clinical worsening, although evaluated using a rather rough but widely used scale, such as the EDSS [25], is independent from objective and quantitative MR measures, such as R1.
A study recently published has proposed an association between MRI signs of gadolinium retention and cognitive disability in MS [17]. In this light, our results substantiate these evidences, proving additional information about the possible clinical reflections of GBCA deposition in MS patients. Indeed, the reported relation between increased DN signal intensity and poorer performances at verbal fluency proved to be independent from EDSS scores, a result in line with the one we obtained, further advocating against a possible role of GBCA retention in global motor disability evolution. However, it has to be noted that EDSS is unable to capture subtle functional changes linked to the physiological roles of the DN (e.g., planning, initiation, and control of voluntary movements) [41]. For this reason, future prospective studies are warranted with the inclusion of a more focused neurological examination, to unravel the possible association between gadolinium retention in the brain and subtle neurological and cognitive deficits. Furthermore, the lack of follow-up MRI data has limited our ability to evaluate the relationship between changes in DN R1 and clinical worsening. In this regard, assuming a linear relation between gadolinium dose and DN R1, we have explored the correlation between number of gadolinium administrations over the follow-up period and delta EDSS in subjects showing DN hyperintensity on the baseline scan by means of a Spearman’s rank analysis (data not shown). The lack of correlation between the two metrics seems to further support the independence of clinical worsening from repeated gadolinium administrations.
Another limitation of the present study is that the specific molecule administered in our population was not always reported in scans previously performed in different centers [22], thus not allowing to investigate a possible different behavior between linear and macro-cyclic agents in determining significant clinical changes. However, it is known that linear agents are more prone to release gadolinium ions compared to macro-cyclic ones [13] and that they induce significant R1 changes [22]. As we assume a potential relation between clinical changes and gadolinium retention, it is unlikely that macro-cyclic GBCAs, being less prone to deposition, could impact more profoundly clinical scores compared to those that accumulate but (at least in our studied patient group) do not induce significant clinical changes. Nevertheless, this speculation needs to be tested in future longitudinal studies in which the class of GBCAs is known for all subjects.
Finally, our mean follow-up time was 3.5 years, thus limiting our observation to a relatively short period, not allowing to conclude whether gadolinium retention in the brain causes more delayed toxicity.
Nevertheless, our results indicate that MS patients showing DN hyperintensity on unenhanced T1-weighted images, a neuroradiological finding with a significant correlation with the number of previous GBCA administration, have similar EDSS changes over a period of 3.6 years compared to those without DN high-signal intensity. Furthermore, mean DN R1 values of patients with a significant clinical worsening were comparable to those obtained in more stable subjects, and were unrelated to global motor disability.
These findings, taken together, provide evidences that qualitative and quantitative signs of gadolinium retention in the brain of MS patients who underwent multiple GBCA-enhanced MRI examinations do not determine significant changes on global motor performances and, thus, do not affect the course of the disease.
References
Aime S, Caravan P (2009) Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging 30(6):1259–1267. https://doi.org/10.1002/jmri.21969
Kanal E, Maravilla K, Rowley HA (2014) Gadolinium contrast agents for CNS imaging: current concepts and clinical evidence. AJNR Am J Neuroradiol 35(12):2215–2226. https://doi.org/10.3174/ajnr.A3917
Jung JW, Kang HR, Kim MH, Lee W, Min KU, Han MH, Cho SH (2012) Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology 264(2):414–422. https://doi.org/10.1148/radiol.12112025
Grobner T (2006) Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 21(4):1104–1108. https://doi.org/10.1093/ndt/gfk062
McDonald RJ, Levine D, Weinreb J, Kanal E, Davenport MS, Ellis JH, Jacobs PM, Lenkinski RE, Maravilla KR, Prince MR, Rowley HA, Tweedle MF, Kressel HY (2018) Gadolinium retention: a research roadmap from the 2018 NIH/ACR/RSNA workshop on gadolinium chelates. Radiology 181151:517–534. https://doi.org/10.1148/radiol.2018181151
U.S. Food & Drug Administration (2017) 2017 Meeting Materials, Medical Imaging Drugs Advisory Committee. https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/ucm553470.htm
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270(3):834–841. https://doi.org/10.1148/radiol.13131669
Roberts DR, Holden KR (2016) Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain and Development 38(3):331–336. https://doi.org/10.1016/j.braindev.2015.08.009
Tedeschi E, Caranci F, Giordano F, Angelini V, Cocozza S, Brunetti A (2017) Gadolinium retention in the body: what we know and what we can do. Radiol Med 122(8):589–600. https://doi.org/10.1007/s11547-017-0757-3
Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB (2017) Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 16(7):564–570. https://doi.org/10.1016/S1474-4422(17)30158-8
McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275(3):772–782. https://doi.org/10.1148/radiol.15150025
Ramalho J, Castillo M, AlObaidy M, Nunes RH, Ramalho M, Dale BM, Semelka RC (2015) High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology 276(3):836–844. https://doi.org/10.1148/radiol.2015150872
Robert P, Fingerhut S, Factor C, Vives V, Letien J, Sperling M, Rasschaert M, Santus R, Ballet S, Idee JM, Corot C, Karst U (2018) One-year retention of gadolinium in the brain: comparison of gadodiamide and gadoterate meglumine in a rodent model. Radiology 172746:424–433. https://doi.org/10.1148/radiol.2018172746
Smith AP, Marino M, Roberts J, Crowder JM, Castle J, Lowery L, Morton C, Hibberd MG, Evans PM (2017) Clearance of gadolinium from the brain with no pathologic effect after repeated administration of gadodiamide in healthy rats: an analytical and histologic study. Radiology 282(3):743–751. https://doi.org/10.1148/radiol.2016160905
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, Haruyama T, Kitajima K, Furui S (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276(1):228–232. https://doi.org/10.1148/radiol.2015142690
Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, Maravilla KR (2016) Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Investig Radiol 51(7):447–453. https://doi.org/10.1097/RLI.0000000000000252
Forslin Y, Shams S, Hashim F, Aspelin P, Bergendal G, Martola J, Fredrikson S, Kristoffersen-Wiberg M, Granberg T (2017) Retention of gadolinium-based contrast agents in multiple sclerosis: retrospective analysis of an 18-year longitudinal study. AJNR Am J Neuroradiol 38(7):1311–1316. https://doi.org/10.3174/ajnr.A5211
Burke LM, Ramalho M, AlObaidy M, Chang E, Jay M, Semelka RC (2016) Self-reported gadolinium toxicity: a survey of patients with chronic symptoms. Magn Reson Imaging 34(8):1078–1080. https://doi.org/10.1016/j.mri.2016.05.005
Semelka RC, Commander CW, Jay M, Burke LM, Ramalho M (2016) Presumed gadolinium toxicity in subjects with normal renal function: a report of 4 cases. Investig Radiol 51(10):661–665. https://doi.org/10.1097/RLI.0000000000000318
Semelka RC, Ramalho J, Vakharia A, AlObaidy M, Burke LM, Jay M, Ramalho M (2016) Gadolinium deposition disease: initial description of a disease that has been around for a while. Magn Reson Imaging 34(10):1383–1390. https://doi.org/10.1016/j.mri.2016.07.016
Semelka RC, Ramalho M, AlObaidy M, Ramalho J (2016) Gadolinium in humans: a family of disorders. AJR Am J Roentgenol 207(2):229–233. https://doi.org/10.2214/AJR.15.15842
Tedeschi E, Palma G, Canna A, Cocozza S, Russo C, Borrelli P, Lanzillo R, Angelini V, Postiglione E, Morra VB, Salvatore M, Brunetti A, Quarantelli M (2016) In vivo dentate nucleus MRI relaxometry correlates with previous administration of gadolinium-based contrast agents. Eur Radiol 26(12):4577–4584. https://doi.org/10.1007/s00330-016-4245-2
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302. https://doi.org/10.1002/ana.22366
Meyer-Moock S, Feng YS, Maeurer M, Dippel FW, Kohlmann T (2014) Systematic literature review and validity evaluation of the expanded disability status scale (EDSS) and the multiple sclerosis functional composite (MSFC) in patients with multiple sclerosis. BMC Neurol 14:58. https://doi.org/10.1186/1471-2377-14-58
Uitdehaag BMJ (2018) Disability outcome measures in phase III clinical trials in multiple sclerosis. CNS Drugs. https://doi.org/10.1007/s40263-018-0530-8
Weinshenker BG (1996) Epidemiology of multiple sclerosis. Neurol Clin 14(2):291–308
Cohen JA, Khatri B, Barkhof F, Comi G, Hartung HP, Montalban X, Pelletier J, Stites T, Ritter S, von Rosenstiel P, Tomic D, Kappos L (2016) Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: results from the extension of the randomised TRANSFORMS study. J Neurol Neurosurg Psychiatry 87(5):468–475. https://doi.org/10.1136/jnnp-2015-310597
Kalincik T, Cutter G, Spelman T, Jokubaitis V, Havrdova E, Horakova D, Trojano M, Izquierdo G, Girard M, Duquette P, Prat A, Lugaresi A, Grand'Maison F, Grammond P, Hupperts R, Oreja-Guevara C, Boz C, Pucci E, Bergamaschi R, Lechner-Scott J, Alroughani R, Van Pesch V, Iuliano G, Fernandez-Bolanos R, Ramo C, Terzi M, Slee M, Spitaleri D, Verheul F, Cristiano E, Sanchez-Menoyo JL, Fiol M, Gray O, Cabrera-Gomez JA, Barnett M, Butzkueven H (2015) Defining reliable disability outcomes in multiple sclerosis. Brain 138(Pt 11):3287–3298. https://doi.org/10.1093/brain/awv258
Battaglini M, Jenkinson M, De Stefano N (2012) Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp 33(9):2062–2071. https://doi.org/10.1002/hbm.21344
Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N (2002) Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 17(1):479–489
Borrelli P, Palma G, Tedeschi E, Cocozza S, Comerci M, Alfano B, Haacke EM, Salvatore M (2015) Improving signal-to-noise ratio in susceptibility weighted imaging: a novel multicomponent non-local approach. PLoS One 10(6):e0126835. https://doi.org/10.1371/journal.pone.0126835
Palma G, Tedeschi E, Borrelli P, Cocozza S, Russo C, Liu S, Ye Y, Comerci M, Alfano B, Salvatore M, Haacke EM, Mancini M (2015) A novel multiparametric approach to 3D quantitative MRI of the brain. PLoS One 10(8):e0134963. https://doi.org/10.1371/journal.pone.0134963PONE-D-15-18675
Rogosnitzky M, Branch S (2016) Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals 29(3):365–376. https://doi.org/10.1007/s10534-016-9931-7
Sherry AD, Caravan P, Lenkinski RE (2009) Primer on gadolinium chemistry. J Magn Reson Imaging 30(6):1240–1248. https://doi.org/10.1002/jmri.21966
Heinrich MC, Kuhlmann MK, Kohlbacher S, Scheer M, Grgic A, Heckmann MB, Uder M (2007) Cytotoxicity of iodinated and gadolinium-based contrast agents in renal tubular cells at angiographic concentrations: in vitro study. Radiology 242(2):425–434. https://doi.org/10.1148/radiol.2422060245
Chen R, Ling D, Zhao L, Wang S, Liu Y, Bai R, Baik S, Zhao Y, Chen C, Hyeon T (2015) Parallel comparative studies on mouse toxicity of oxide nanoparticle- and gadolinium-based T1 MRI contrast agents. ACS Nano 9(12):12425–12435. https://doi.org/10.1021/acsnano.5b05783
Akgun H, Gonlusen G, Cartwright J Jr, Suki WN, Truong LD (2006) Are gadolinium-based contrast media nephrotoxic? A renal biopsy study. Arch Pathol Lab Med 130(9):1354–1357. https://doi.org/10.1043/1543-2165(2006)130[1354:AGCMNA]2.0.CO;2
Hui FK, Mullins M (2009) Persistence of gadolinium contrast enhancement in CSF: a possible harbinger of gadolinium neurotoxicity? AJNR Am J Neuroradiol 30(1):E1. https://doi.org/10.3174/ajnr.A1205
Blasco-Perrin H, Glaser B, Pienkowski M, Peron JM, Payen JL (2013) Gadolinium induced recurrent acute pancreatitis. Pancreatology 13(1):88–89
Monti S, Borrelli P, Tedeschi E, Cocozza S, Palma G (2017) RESUME: turning an SWI acquisition into a fast qMRI protocol. PLoS One 12(12):e0189933. https://doi.org/10.1371/journal.pone.0189933
Dimitrova A, de Greiff A, Schoch B, Gerwig M, Frings M, Gizewski ER, Timmann D (2006) Activation of cerebellar nuclei comparing finger, foot and tongue movements as revealed by fMRI. Brain Res Bull 71(1–3):233–241. https://doi.org/10.1016/j.brainresbull.2006.09.015
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
SC and CR receive speaking fees from Genzyme. MM has received research grants from ECTRIMS-MAGNIMS and Merck.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cocozza, S., Pontillo, G., Lanzillo, R. et al. MRI features suggestive of gadolinium retention do not correlate with Expanded Disability Status Scale worsening in Multiple Sclerosis. Neuroradiology 61, 155–162 (2019). https://doi.org/10.1007/s00234-018-02150-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-018-02150-4