Abstract
Purpose
Computed tomography angiography (CTA) and magnetic resonance imaging/angiography (MRI/MRA) are used for the diagnosis of intracranial dural arteriovenous fistulas (DAVFs). The purpose of this study was to compare the diagnostic accuracy of CTA and magnetic resonance imaging/angiography (MRI/MRA) for detection of cortical venous reflux (CVR) in intracranial DAVFs.
Methods
The records of patients with angiography-confirmed intracranial DAVFs who also received CTA and MRI/MRA from January 2008 to July 2016 were reviewed. CTA and MRI/MRA were reviewed for signs of CVR, and the diagnostic accuracy of individual signs was evaluated by receiver operating curve (ROC) analysis.
Results
A total 108 patients were included in this study. CTA signs of CVR included abnormal dilatation, early enhancement, and the presence of a medullary or pial vein. MRI/MRA signs of CVR included abnormal dilatation, early enhancement, flow-related enhancement, flow void, and medullary or pial venous collaterals. The sensitivity of individual CTA signs ranged from 62 to 96%, and specificities from 79 to 94%. The sensitivities of individual MRI/MRA signs ranged from 58 to 83%, and specificities from 77 to 93%. The area under ROC curve (AUC) of CTA and MRI/MRA were 0.91 and 0.87, respectively (P = 0.04 in direct comparison). In subgroup analysis, CTA had better diagnostic accuracy for higher grade disease (P = 0.05) and non-aggressive manifestation (P = 0.04).
Conclusions
Both CTA and MRI/MRA have good diagnostic accuracy for detection of CVR in patients with intracranial DAVFs. There is modest evidence that CTA is better than MRI/MRA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intracranial dural arteriovenous fistulas (DAVFs) have variable clinical manifestation and natural history. A definite diagnosis is usually established by catheter angiography. Although there are many different classification systems, the classification schemes by Cognard [1] and Borden [2] are the most widely used. Both systems indicate that cortical venous reflux (CVR) is highly associated with an aggressive clinical course [3,4,5]. Although catheter angiography is considered the gold standard for diagnosis of CVR, it is invasive and radiation exposure is a concern. Less-invasive computed tomography angiography (CTA) and magnetic resonance imaging/angiography (MRI/MRA) are alternative imaging techniques [6, 7]. A prior meta-analysis suggested using MRI/MRA in symptomatic cases as a rule-in test because of its better accuracy [8].
Nevertheless, the role of diagnostic imaging in cases of DAVF is more than just detection. Imaging data should be used for risk classification, prognostication, and treatment planning. CVR is associated with more aggressive disease, and is used for the treatment planning of patients with DAVFs [9]. Modality choice based on diagnostic accuracy is important for practitioners in the initial clinical work-up. Previous reports have shown that CTA and MRI/MRA can both detect CVR with similar accuracy [5, 6, 9,10,11]. However, large-scale studies comparing their diagnostic accuracy have not been performed.
Thus, the purpose of this study was to compare the diagnostic accuracy of CTA and MRI/MRA for the detection of CVR in patients with an intracranial DAVF.
Methods
Patients
This retrospective study was approved by our Institutional Review Board, and because of the retrospective nature patients informed consent was waived. The records of patients treated at our neuroangiography were searched for patients with a DAVF treated from January 1, 2008, to July, 1, 2016. Patients who were diagnosed with a DAVF by catheter angiography who also received MRI/MRA and CTA within 2 months were included in the study. The quality of the CTA and MRI/MRA examinations were checked by a diagnostic neuroradiologist. Patients were excluded from the study if (1) any of the imaging studies were insufficient or of poor quality, (2) a direct type carotid-cavernous fistula was present, (3) a spinal dural arteriovenous fistula was present, and (4) the intracranial DAVF had been previously treated.
Patient clinical data
Patient clinical data were reviewed in the electronic medical record database. The clinical presentation was considered non-aggressive if the patients had no subjective complaints, eye symptoms, or pulsatile tinnitus. If focal neurological deficits, dementia or rapid functional decline, seizures, consciousness disturbance, or intracranial hemorrhage were present, the clinical presentation was considered aggressive. The Cognard classification was used to classify DAVFs [1]. Less advanced DAVFs without CVR were defined as Cognard type IIA+B or IIB, and advanced DAVFs with CVR were defined as Cognard type III, IV, or V. If with multiple DAVFs, then the location was defined according to the most advanced lesion.
Imaging techniques
CTA was performed by one of two 64-slice CT scanners: a LightSpeed VCT (scanner A; GE Healthcare, Milwaukee, WI) and a Sensation 64 (scanner B; Siemens Medical Solutions, Forchheim, Germany). The scanning protocol used a previously reported hybrid algorithm [6]. In brief, patients were scanned from the aortic arch to the vertex (average, 32 cm) before and after administration of contrast medium. From aortic arch to vertex, the mean scanning time was 7.2 s for scanner A and 8 s for scanner B. Intravenous contrast medium was delivered via dual injectors. A bolus test was performed with 12 ml of contrast medium (Ultravist 370, Bayer Schering Pharm, Berlin Germany) and 16 ml of normal saline at rate of 4 ml/s to determine the peak enhancement, followed by 60 ml contrast medium and 35 ml normal saline at the same rate. Image acquisition was performed with 32 × 0.625 or 64 × 0.6 mm collimation, and reformatted into 1 mm axial, coronal, and sagittal views for review. 3D-reformatting was also available when necessary. The average dose-length products were 746.40 and 602.88 mGy, respectively.
MRI/MRA was performed with multiple scanners at our hospital, or at other hospitals, with 3T (N = 22) and 1.5T (N = 86) machines. All acquisition contained at least two planes. Contrast medium was used in 75 patients. The parameters in our institute were all recorded. T1-weighted images were as followed: repetition time (TR) of 400–900 msec, echo time (TE) of 9–21 msec, section thickness of 3–5 mm, and field of view (FOV) of 16–30 cm. T2-weighted images were as followed: repetition time (TR) of 2780–7067 msec, echo time (TE) of 80.64–103.3 msec, section thickness 2.5–5 mm, and field of view (FOV) of 16–24 cm. MRA was obtained using 3D time-of-flight (TOF, N = 92) and contrast-enhanced (N = 75) techniques. 3D-TOF parameters were as followed: TR of 6.69–40 msec, TE of 2.46–7.2 msec, flip angle of 15–25, FOV of 18–30 cm; section thickness of 0.5–2 mm. Contrast-enhanced MRA parameters were as followed: TR of 2.864–8.26 msec, TE of 1.052–4.2 msec, flip angle of 15–45, FOV of 20–30 cm; section thickness of 1–3.2 mm. MRA were evaluated in source images.
Catheter angiography
Experienced interventional neuroradiologists performed catheter angiography with a biplane angiography unit (Axioms, Artis; Siemen Medical Solution, Erlangen, Germany). Selective angiography of internal carotid artery, external carotid artery, and vertebral artery were routinely performed.
Image analysis
The CTA and MRI/MRA source images were read by board-certified neuroradiologists (YHL and YFW, with 4 and 7 years’ experience, respectively). No additional training in reading the images was given. Readers were unaware of the patients’ clinical characteristics and angiographic results when reading imaging studies, and the two modalities were not read in the same session. A standard high-quality monitor for diagnostic radiology (3 megapixels, 1536 × 2048 native resolution, monochrome) was used to view the images. The images were stored in the PACS system to simulate the usual diagnostic process. The images were viewed in a random sequence, and < 20 studies were reviewed in a single session.
Individual CTA and MRI/MRA imaging signs were recorded as positive or negative. The CTA signs of CVR included abnormal dilatation, early enhancement, and the presence of a medullary or pial vein [6]. The MRI/MRA signs of CVR included abnormal dilation, flow-related enhancement, clustered of flow void, early enhancement, and presence of a medullary or pial vein [11, 12]. Individual CTA and MRI/MRA signs with corresponding catheter angiography are shown in Fig. 1. The above signs comprised the grading system for CVR. If a discrepancy occurred between the two readers, the findings were discussed until a consensus was reached. The grading systems for CTA and MRI/MRA were used to consider the trade-off between sensitivity and specificity, as well as the correlation between different signs. Details of the grading systems are shown in Table 4.
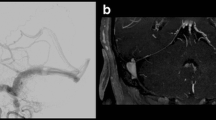
a–h A 31-year-old man with left transverse-sigmoid sinus DAVF was presented with seizure. a Axial CT angiography demonstrates dilated cortical venous pouch (white arrow). Early enhancement is revealed as compared with the adjacent dural sinuses. b Sagittal view of CTA shows dilated medullary veins in the occipital lobe (black arrows). c Reformatting CT angiography clearly depicts the venous pouch and its drainage (white arrow). d Axial T2-weighted image shows the dilated structure (white arrow) with dark signal, suggestive of flow void in the venous structure. e Axial time-of-fight image reveals the flow-related signal in the venous pouch (white arrow). f Coronal contrast-enhanced MR angiography demonstrates there is early, intense, enhancement in the venous structure as compared with adjacent dural sinus (white arrow). g Sagittal T1-weighted post-contrast image shows the dilated medullary vein the occipital lobe as well (black arrows). h Angiography confirms the Cognard type IV DAVF with direct drainage into ectatic cortical veins (black arrows). The image is corresponding to the reformatting CTA nicely
Catheter angiography results were examined for the presence and location of CVR by one of the authors (YHL) unaware of the patients’ clinical data and CTA and MRI/MRA results. The reading results were compared with those of the original report in the medical records.
Statistical analysis
Patient characteristics were summarized by descriptive statistics. Diagnostic accuracy indicators, including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated for individual imaging signs. The grading system was analyzed by receiver operating characteristic (ROC) curve analysis, with the area under the ROC curve (AUC) obtained by non-parametric method. Inter-observer agreement of the individual signs and grading system were evaluated by simple and weighted kappa values, respectively. Point and interval estimates were calculated. Direct comparisons between CTA and MRI/MRA ROCs were performed using a logistic regression model with contrast as CTA over MRI/MRA. The significance level was set at 0.05. We have reported the results according to the Standards for Reporting Diagnostic accuracy studies (STARD 2015) guidelines(see Supplementary material 1). Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Results
Patient characteristics
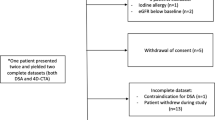
A total of 237 patients with angiography-proven intracranial DAVFs seen from January 1, 2008, to July, 1, 2016, were identified in the medical records. Among them, 108 (46%) who received both CTA and MRI/MRA were included in the analysis. Of all MRI/MRA studies, 31 (29%) did not contain contrast-enhanced MRA data, 12 (11%) did not contain time-of-flight MRA data, and 5 (5%) had poor quality of T2-weighted images. These studies were included for evaluating the individual imaging signs, but not in the ROC analysis. Thus, data of a total of 92 (85%) patients completely fulfilled the inclusion criteria and were used for the ROC analysis.
Of the patients, 70 (65%) presented with non-aggressive disease, and the others 38 (35%) presented with aggressive disease. Among them, 28 (26%) had DAVFs in the cavernous sinus, and 46 (43%) in the transverse to sigmoid sinuses. Other locations included the superior sagittal sinus, hypoglossal or anterior condylar region, temporal or sphenoid osseous-dural region, tentorium, olfactory groove, foramen magnum, and falx cerebelli. Using the Cognard classification, 47 (44%) patients were type I or IIA, 34 (31%) were type IIB or IIA+B, and the remaining 27 (25%) patients were higher types. Ninety-nine (92%) patients received endovascular embolization, 3 (3%) received surgery, and 6 (6%) received radiosurgery. Patient characteristics are summarized in Table 1.
Individual signs
For CTA, early cortical venous enhancement had the highest sensitivity of 98%, and the presence of a medullary vein or pial vein had the highest specificity of 94% for diagnosis of CVR. For MRI/MRA, cortical venous dilatation had the highest sensitivity of 83%, and the presence of a medullary vein or pial vein had the highest specificity of 93% for diagnosis of CVR.
For inter-observer agreement of the individual CTA signs, the kappa values ranged from 0.85 to 0.96, indicating substantial agreement. For individual MRI/MRA signs, the kappa values ranged from 0.74 to 0.96, indicating moderate to substantial agreement from. Details of the kappa analysis, and the PPV, NPV, and overall accuracy are show in Table 2.
ROC analysis
The ROC analysis included 92 patients, and the results are summarized in Table 3. The consensus results indicated the AUC of CTA (Fig. 2) was 0.91, and the AUC of MRI/MRA was 0.87 (P = 0.04). The weighted kappa value between the two readers was 0.88 for CTA, and 0.81 for MRI/MRA.
Receiver operating characteristic (ROC) curve of CTA and MRI/MRA of the CTA and MRI/MRA on the cortical venous reflux of intracranial DAVF. The curve were constructed with non-parametric method according to the grading system in Table 4
To consider the effect of MRI contrast medium, we perform ROC analysis for MRI/MRA after exclusion the related signs of contrast-enhanced MRI/MRA, namely early enhancement and presence of dilated medullary or pial vein. AUC of non-contrast MRI/MRA was 0.80 (95%CI: 0.71–0.90). As compared with CTA and contrast-enhanced MRI/MRA, P values were 0.01 and 0.03, respectively. Other subgroup analyses were performed according to the disease location, clinical aggressiveness, and angiographic grade (Table 3). Locations were classified as cavernous sinus, transverse to sigmoid sinuses, and others. In cavernous sinus group there were 22 patients, and the CTA AUC was 0.92 and the MRI/MRA AUC was 0.88 (P = 0.5). In the transverse to sigmoid sinuses group there were 39 patients, and the CTA AUC was 0.96 and the MRI/MRA AUC was 0.93 (P = 0.16). In the other locations group there were 31 patients, and the CTA AUC was 0.86 and the MRI/MRA AUC was 0.82 (P = 0.18). In the less advanced disease group, there were 68 patients and the CTA AUC was 0.91 and the MRI/MRA AUC was 0.89 (P = 0.2). In the advanced disease group there were 59 patients, and the CTA AUC was 0.97 and the MRI/MRA AUC was 0.90 (P = 0.05). In the aggressive disease group there were 34 patients, and the CTA AUC was 0.70 and the MRI/MRA AUC was 0.70 (P = 1). In the non-aggressive disease group there were 58 patients, and the CTA AUC was 0.90 and the MRI/MRA AUC was 0.82 (P = 0.04).
The ROC curves and analysis for comparison between CTA, contrast-enhanced MR, and non-enhanced MR for the detection of cortical venous reflux in intracranial DAVF were shown as Supplementary material 2 and 3.
Discussion
The results of this study showed that both CTA and MRI/MRA can detect CVR in intracranial DAVFs, but that CTA may be slightly better than MRI/MRA. CTA had better sensitivities than MRI/MRA for imaging signs, whereas the specificities were similar. For MRI/MRA, contrast medium use can improve accuracy. In subgroup analysis, CTA outperformed MRI/MRA in patients with advanced DAVFs with CVR. CTA was also superior to MRI/MRA in patients with non-aggressive disease. The accuracy of the two modalities was similar irrespective of disease location.
The results can be partially explained by the technical differences of the two modalities. CTA had higher spatial resolution in the rapid acquisition time used in our protocol, whereas temporal and spatial resolution is usually a trade-off in MRI/MRA. These differences result in higher sensitivities of CTA than MRI/MRA for the corresponding individual imaging signs. CTA was better than MRI/MRA for detection of cortical venous dilatation, which can be ascribed to the higher spatial resolution of CTA. For detection of early cortical venous enhancement, CTA also exhibited higher sensitivity than MRI/MRA, which is due to the relatively short acquisition time and optimal scanning timing in the CTA protocol. Flow-related enhancement in MRI/MRA is read by time-of-flight (TOF) MRA, reflecting the rapid, high flow in the cortical vein. It is worth mentioning that the source images of thin-slice TOF MRA are pertinent and important for the interpretation of arteriovenous shunting. There is no corresponding CTA sign, and this is useful in clinical practice, but this sign is not more sensitive than cortical venous dilatation. The presence of cortical venous flow void on MRI/MRA exhibited good diagnostic accuracy, and is a result of rapid flow. However, this sign is usually used in conjunction with other imaging signs. The presence of a medullary and pial vein had a high kappa value in both CTA and MRI/MRA, suggestive of its robust clinical use. Nevertheless, it comes from the recruitment of a potential venous outflow route, and tends to occur in more severe disease, and the overall sensitivity is not high. In terms of inter-observer agreement, CTA performed better than MRI/MRA in corresponding items, meaning CTA is actual more robust in general use. The results of the ROC analysis provide overall accuracy data, and the trade-off between sensitivities and specificities. Since the sensitivities of CTA are higher than MRI/MRA in corresponding image signs, it is not surprising that CTA outperforms MRI/MRA.
Subgroup analyses were used in search for the source of differences, and may provide information regarding appropriate clinical application. We found that CTA was better than MRI/MRA in patients with advanced DAVFs and with non-aggressive disease. In comparison to angiographic findings, we found that MRI/MRA is prone to miss the diagnosis of type III and V disease, which is probably due to its lower temporal resolution in non-ectatic venous structures. In subgroup of aggressive disease, both methods had only modest accuracy with AUCs of 0.70, as well as a wide confidence interval (0.36 to 1). This result is likely because there were a limited number of cases with aggressive disease without CVR. Thus, result about this subgroup cannot be considered conclusive and further research is required.
Diagnosis of intracranial DAVF by CTA and MRI/MRA should take different aspects into account. A prior meta-analysis showed MRI/MRA is better than CTA in diagnosing symptomatic DAVF, and contrast medium use may not improve the accuracy [8]. It is important to know that CVR has been shown to be associated with an aggressive clinical course and poorer outcomes in cohort studies. [4, 13] A study by Letourneau-Guillon et al. [14] demonstrated the potential role of CTA and MRI/MRA in the detection CVR. In this study, we further explored the diagnostic accuracy of the two modalities in difference situations. Summarizing the current evidence, we have developed an algorithm for choosing non-invasive imaging modalities (Fig. 3). CTA is reserved for endovascular treatment planning because of the potential adverse effects of radiation exposure. In patients with non-aggressive disease, we recommend CTA when the suspicion of a cavernous DAVF is high. This is because the relatively low prevalence of CVR in CS (34%, as compared with 54% overall in our database) may justify the use of CTA with better accuracy at the expense of radiation exposure. For non-cavernous DAVF, MRI/MRA is likely appropriate. In this group of patients, primary diagnosis and follow-up can be done without further catheter angiography given the low possibility of an aggressive clinical course and the good accuracy of CTA and MRI/MRA [15]. In patients with aggressive disease, MRI/MRA is recommended for primary diagnosis and detection of associated brain lesion, such as intracranial hemorrhage, venous hypertension, and ischemia. However, contrast medium is warranted in MRI/MRA. Catheter angiography is still indicated in patients with equivocal non-invasive imaging results, to avoid poor clinical outcomes if misdiagnosis occurs in advanced disease.
The current study was retrospective in which the MRI/MRA protocol was that used for routine clinical use, which may be more realistic for daily practice. Advanced MRI/MRA techniques, namely time-resolved MRA (tr-MRA), perfusion MRI/MRA, susceptibility-weighted image (SWI), and arterial spin-labeling (ASL) are expected to increase the diagnostic accuracy for detection of CVR. Unfortunately, these methods were rarely performed in our patients and therefore not considered in this study. Among these techniques, tr-MRA provides dynamic information which can help capture the direction of flow and some studies have reported its potential diagnostic value [16, 17]. However, the added diagnostic accuracy beyond conventional MRA needs further evaluation due to the trade-off between temporal and spatial resolution. Perfusion imaging is able to detect hemodynamic changes, especially increased cerebral blood volume, which can occur in patients with venous congestion. [18] Arterial spin-labeling, a method of non-contrast perfusion imaging, is also promising in CVR detection [19]. The relationship between CVR and perfusion changes needs further investigation. We believe it is currently more a research tool than an alternative clinical diagnostic technique. SWI is also a promising technique for detection of dilated cortical and medullary veins on its minimal intensity projection. Studies have also described the feasibility of SWI for diagnosis of CRV [20, 21]. These studies, however, were of a small sample size with a case-control design; more large-scale studies to support it use is warranted.
The CTA protocol in this study was pre-specified. Advanced CT techniques to increase the diagnostic accuracy are also promising, but were not included in this study. For example, perfusion CT imaging, as its MRI/MRA counterpart, has been studied for its potential role in evaluation of DAVF. [22] Williams et al. [23] also investigated the role of 4D-CTA for DAVF diagnosis and classification. The diagnostic accuracy of above techniques needs more data to support their routine clinical use. On the other hand, radiation exposure is an issue in the use of perfusion and 4D-CTA. In this study, we used a standard imaging protocol that can be performed in the majority of imaging centers.
We compared the diagnostic accuracy of CTA and MRI/MRA, and proposed an algorithm for diagnostic evaluation in clinical practice. In the future, prospective validation of our algorithm is warranted. Further investigation of subgroups with larger numbers of patients is needed. The cost-effectiveness of the two modalities should also be investigated.
There are several limitations to this study. First, the series contained more advanced disease cases, and less cavernous DAVF cases, as compared with prior reported series, partly due to inclusion was based on angiographic diagnosis [24, 25]. However, the distribution of the different groups was still diverse, making generalization to clinical practice feasible. Second, the heterogeneity of the MRI/MRA protocols, and lack of advanced pulse sequence data make it possible that there was underestimation of its accuracy and that the role of the individual pulse sequence was indeterminate. This issue is inherently drawback for retrospective study. However, although our study involved MRI/MRA with multiple different machines, sensitivity analysis for studies in our institute only did not change our results (not shown). A well-designed, prospective study in the future can overcome this issue. Third, the overall case number was relatively low, especially in the subgroups. A multi-centers study can help to overcome this weakness. Fourth, the accuracy of readers was not evaluated in this study. We did not use a multiple-reader, multiple comparison study design. Different interpretation of result between readers may need further evaluation.
Conclusions
Both CTA and MRI/MRA are useful in detection of CVR in patients with intracranial DAVFs. There is modest evidence that CTA has slightly better overall diagnostic accuracy than MRI/MRA, and maybe performs better in the subgroups of patients with advanced disease and non-aggressive disease.
References
Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, Chiras J, Merland JJ (1995) Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 194(3):671–680. https://doi.org/10.1148/radiology.194.3.7862961
Borden JA, Wu JK, Shucart WA (1995) A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 82(2):166–179. https://doi.org/10.3171/jns.1995.82.2.0166
Kobayashi A, Al-Shahi Salman R (2014) Prognosis and treatment of intracranial dural arteriovenous fistulae: a systematic review and meta-analysis. International journal of stroke : official journal of the International Stroke Society 9(6):670–677. https://doi.org/10.1111/ijs.12337
van Dijk JMC, terBrugge KG, Willinsky RA, Wallace MC (2002) Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke 33(5):1233–1236. https://doi.org/10.1161/01.str.0000014772.02908.44
van Rooij WJ, Sluzewski M, Beute GN (2007) Dural arteriovenous fistulas with cortical venous drainage: incidence, clinical presentation, and treatment. AJNR Am J Neuroradiol 28(4):651–655
Lee CW, Huang A, Wang YH, Yang CY, Chen YF, Liu HM (2010) Intracranial dural arteriovenous fistulas: diagnosis and evaluation with 64-detector row CT angiography. Radiology 256(1):219–228. https://doi.org/10.1148/radiol.10091835
Shweel M, Hamdy B (2013) Diagnostic utility of magnetic resonance imaging and magnetic resonance angiography in the radiological evaluation of pulsatile tinnitus. Am J Otolaryngol 34(6):710–717. https://doi.org/10.1016/j.amjoto.2013.08.001
Lin YH, Lin HH, Liu HM, Lee CW, Chen YF (2016) Diagnostic performance of CT and MRI on the detection of symptomatic intracranial dural arteriovenous fistula: a meta-analysis with indirect comparison. Neuroradiology 58(8):753–763. https://doi.org/10.1007/s00234-016-1696-8
Bulters DO, Mathad N, Culliford D, Millar J, Sparrow OC (2012) The natural history of cranial dural arteriovenous fistulae with cortical venous reflux--the significance of venous ectasia. Neurosurgery 70(2):312–318; discussion 318-319. https://doi.org/10.1227/NEU.0b013e318230966f
Kitajima M, Hirai T, Korogi Y, Yamura M, Kawanaka K, Ikushima I, Hayashida Y, Yamashita Y, Kuratsu J (2005) Retrograde cortical and deep venous drainage in patients with intracranial dural arteriovenous fistulas: comparison of MR imaging and angiographic findings. Am J Neuroradiol 26(6):1532–1538
Willinsky R, Terbrugge K, Montanera W, Mikulis D, Wallace MC (1994) Venous congestion: an MR finding in dural arteriovenous malformations with cortical venous drainage. Am J Neuroradiol 15(8):1501–1507
Willinsky R, Goyal M, terBrugge K, Montanera W (1999) Tortuous, engorged pial veins in intracranial dural arteriovenous fistulas: correlations with presentation, location, and MR findings in 122 patients. AJNR Am J Neuroradiol 20(6):1031–1036
Kwon BJ, Han MH, Kang HS, Chang KH (2005) MR imaging findings of intracranial dural arteriovenous fistulas: relations with venous drainage patterns. AJNR Am J Neuroradiol 26(10):2500–2507
Letourneau-Guillon L, Cruz JP, Krings T (2015) CT and MR imaging of non-cavernous cranial dural arteriovenous fistulas: findings associated with cortical venous reflux. Eur J Radiol 84(8):1555–1563. https://doi.org/10.1016/j.ejrad.2015.04.019
Miller TR, Gandhi D (2015) Intracranial dural arteriovenous fistulae: clinical presentation and management strategies. Stroke 46(7):2017–2025. https://doi.org/10.1161/strokeaha.115.008228
Farb RI, Agid R, Willinsky RA, Johnstone DM, Terbrugge KG (2009) Cranial dural arteriovenous fistula: diagnosis and classification with time-resolved MR angiography at 3T. AJNR Am J Neuroradiol 30(8):1546–1551. https://doi.org/10.3174/ajnr.A1646
Noguchi K, Melhem ER, Kanazawa T, Kubo M, Kuwayama N, Seto H (2004) Intracranial dural arteriovenous fistulas: evaluation with combined 3D time-of-flight MR angiography and MR digital subtraction angiography. AJR Am J Roentgenol 182(1):183–190. https://doi.org/10.2214/ajr.182.1.1820183
Noguchi K, Kubo M, Kuwayama N, Kamisaki Y, Tomizawa G, Kameda K, Kawabe H, Ogawa S, Watanabe N, Endo S, Seto H (2006) Intracranial dural arteriovenous fistulas with retrograde cortical venous drainage: assessment with cerebral blood volume by dynamic susceptibility contrast magnetic resonance imaging. AJNR Am J Neuroradiol 27(6):1252–1256
Amukotuwa SA, Heit JJ, Marks MP, Fischbein N, Bammer R (2016) Detection of cortical venous drainage and determination of the Borden type of dural arteriovenous fistula by means of 3D pseudocontinuous arterial spin-labeling MRI. Am J Roentgenol 207(1):163–169. https://doi.org/10.2214/AJR.15.15171
Nakagawa I, Taoka T, Wada T, Nakagawa H, Sakamoto M, Kichikawa K, Hironaka Y, Motoyama Y, Park YS, Nakase H (2013) The use of susceptibility-weighted imaging as an indicator of retrograde leptomeningeal venous drainage and venous congestion with dural arteriovenous fistula: diagnosis and follow-up after treatment. Neurosurgery 72(1):47–54; discussion 55. https://doi.org/10.1227/NEU.0b013e318276f7cc
Letourneau-Guillon L, Krings T (2012) Simultaneous arteriovenous shunting and venous congestion identification in dural arteriovenous fistulas using susceptibility-weighted imaging: initial experience. AJNR Am J Neuroradiol 33(2):301–307. https://doi.org/10.3174/ajnr.A2777
Lagares A, Millan JM, Ramos A, Alen JA, Gallego JH (2010) Perfusion computed tomography in a dural arteriovenous fistula presenting with focal signs: vascular congestion as a cause of reversible neurologic dysfunction. Neurosurgery 66(1):E226–E227; discussion E227. https://doi.org/10.1227/01.neu.0000361996.27921.6c
Willems PW, Brouwer PA, Barfett JJ, terBrugge KG, Krings T (2011) Detection and classification of cranial dural arteriovenous fistulas using 4D-CT angiography: initial experience. AJNR Am J Neuroradiol 32(1):49–53. https://doi.org/10.3174/ajnr.A2248
Kim MS, Han DH, Kwon OK, Oh CW, Han MH (2002) Clinical characteristics of dural arteriovenous fistula. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 9(2):147–155. https://doi.org/10.1054/jocn.2001.1029
McDougall CG, Halbach VV, Higashida RT, Larsen DW, Dowd CF, Hieshima GB, Wilson CB (1997) Treatment of dural arteriovenous fistulas. Neurosurg Q 7(2):110–134
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
For this type of retrospective study formal consent is not required.
Rights and permissions
About this article
Cite this article
Lin, YH., Wang, YF., Liu, HM. et al. Diagnostic accuracy of CTA and MRI/MRA in the evaluation of the cortical venous reflux in the intracranial dural arteriovenous fistula DAVF. Neuroradiology 60, 7–15 (2018). https://doi.org/10.1007/s00234-017-1948-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1948-2