Abstract
Purpose
Idiopathic intracranial hypertension (IIH) is a disorder of increased intracranial pressure in the absence of any known causative factor. Sinus stenosis is common in these patients. Stenting of stenotic dural sinuses has gained popularity as a treatment option, since these stenoses may contribute to an obstruction of the venous return, and, thereby may contribute to IIH via an increase in venous sinus pressure. We evaluated the safety and efficacy of endovascular treatment in IIH with venous sinus stenosis.
Methods
Fifty-one patients with IIH underwent stenting. Median age was 40 years. Clinical manifestation was headache in 74.5% of the patients and visual obscurations in 78.5%. Papilledema was present in 50/51 patients (98%), and lumbar puncture documented elevated CSF opening pressure in all but one patient (98%). Sinus stenoses were observed in all patients.
Results
Endovascular treatment was successfully performed in all patients. There were no major complications encountered (i.e., live threatening or causing a deterioration of a patient’s condition equivalent to mRS 3–6). Improvement or resolution of papilledema was observed in 88% of the patients, and 84% reported improvement or resolution of the headache. Follow-up angiographies were performed in 48 patients at a median interval of 49 months and demonstrated in stent-stenosis or a de novo stenosis in 12 patients, eight of them needed re-treatment.
Conclusion
Venous sinus stenting is a safe and effective alternative to other invasive treatments (e.g., optic nerve sheath fenestration, CSF diversion) in patients with IIH. The majority of patients have a persistent clinical benefit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Idiopathic intracranial hypertension (IIH), also known as pseudotumor cerebri or benign intracranial hypertension is a syndrome of increased intracranial pressure (ICP) in the absence of any known causative factor, mainly affecting overweight or obese women of childbearing age.
Signs and symptoms associated with IIH include persistent daily headache, visual disturbances, diplopia, and pulsatile tinnitus. Papilledema is normally present, which if left untreated may be a cause of insidious visual loss becoming permanent in up to 10% of patients secondary to irreversible optic nerve atrophy [1].
Imaging of patients with IIH is traditionally performed to exclude lesions that produce intracranial hypertension. MR imaging features of IIH include posterior globe flattening, a protrusion of the subarachnoid space in the cavum sellae (“empty sella”), distension of the perioptic subarachnoid space, enhancement of the prelaminar optic nerve, vertical tortuosity of the orbital optic nerve, and intraocular protrusion of the prelaminar optic nerve [2, 3]. Although these findings support the presence of elevated ICP and, thus, the diagnosis of IIH, they are not predictive for the severity of visual loss, and their absence does not exclude the diagnosis and should not guide specific management of patients with IIH [4].
Besides the established diagnostic criteria (papilledema, normal neurological examination except cranial nerve abnormalities, neuroimaging showing normal brain parenchyma, and normal cerebrospinal fluid (CSF) composition, the key diagnostic feature is a raised lumbar puncture CSF opening pressure (>25 cm H2O in lateral decubitus). The term of “idiopathic” intracranial hypertension refers to the unclear cause of raised intracranial pressure in these patients, and despite the number of pathogenic theories that have been proposed, the final cause of IIH is still unknown.
With the advent of MR venography and increased use of cerebral angiography, there has been emphasis on the role of venous disease in the etiology of IIH with a high proportion of patients presenting nonthrombotic uni- or bilateral dural venous sinus stenosis [5]. Based on this theory, endovascular stenting of stenotic dural sinuses in cases of medically refractory IIH has recently gained popularity. Nevertheless, whether the dural venous stenoses are the cause or a consequence of increased ICP is still debated.
The treatment of IIH regularly starts with a lumbar puncture in the lateral decubitus position with a withdrawal of 40 cm3 of CSF. The procedure may have to be repeated several times depending on the clinical short-term course and the opening pressure. Medical treatment is mainly based on Azetazolamid and Topiramat. Much less data is available for Furosemide. Further, patients are advised to lose weight via dietary interventions. In patients with progressive visual loss despite CSF withdrawal, dietary measures, and medication, surgical treatment options including shunt procedures and optic nerve fenestration have been traditionally discussed. However, given the promising risk-benefit ratio, balloon angioplasty and stent implantation into stenotic segments of the intracranial venous sinus(es) have become an accepted treatment modality [6]. Therefore, this has been included in the German treatment guidelines but appears both underperformed and underreported.
We report a single center experience with the clinical presentation and outcome of a series of selected patients with IIH who underwent intracranial stent placement after failure of the medical treatment and/or surgical procedures. We sought to evaluate through both short- and long-term follow-up examinations, whether the endovascular therapy (EVT) is suitable to reduce the elevated CSF pressure and improve the signs and symptoms of IIH.
Patients and methods
Patient population
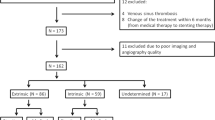
Fifty-one consecutive patients (41 women, 10 men) with a median age of 40 years (range, 5–66 years) were admitted to our hospital for MRI and DSA examinations and endovascular treatment of a diagnosed IIH between March 2007 and July 2016. These patients were retrospectively identified and analyzed. Forty patients were referred with an already established diagnosis of IIH, while in 11 patients, this diagnosis was the result of our work-up. The diagnosis “IIH” was based on clinical signs and symptoms in all patients (e.g., papilledema, headache, transient visual obscurations, diplopia due to sixth cranial nerve palsy, pulse-synchronous tinnitus). Patients with an identifiable cause of their intracranial hypertension were excluded from this series.
All patients were discussed before the endovascular treatment in a multidisciplinary team including neuroradiologists, neurologists, neurosurgeons, and neuroophthalmologists.
Indication for endovascular treatment was persistent or progressive papilledema and/or intractable headaches despite maximally tolerated conservative therapy (attempted weight loss, repeated lumbar punction with CSF withdrawal and carbonic anhydrase inhibitors, such as acetazolamide and/or topiramate). Stenting was also offered to patients with severe side-effects secondary to carbonic anhydrase inhibitors and after failed surgical procedures.
One patient was directly considered for endovascular treatment due to noncompliance with medical treatment. Three patients (6%) had ventriculo-peritoneal shunts placed prior to stent placement. One patient presented an Eagle syndrome (elongated styloid process) causing bilateral jugular vein stenosis and was previously treated by surgery. None of our patients had been treated with optic nerve sheath fenestration or subtemporal decompression.
Fifty patients (98%) fulfilled the updated modified Dandy criteria for IIH [7]. One patient (4%) had headache without papilledema and without elevated opening CSF pressure but had all the other clinical features of IIH and a venous pressure gradient confirmed by venous manometry via a microcatheter. Five patients (10%) had a history of thrombophilia and deep venous thrombosis, and two patients (4%) harbored a heterozygous factor V Leiden mutation.
Clinical manifestations included daily headache in 38 patients (74.5%), visual obscuration in 40 patients (78.5%), and pulsatile tinnitus in nine patients (18%). All but one patient presented bilateral papilledema (98%) and lumbar puncture documented elevated CSF opening pressure (> 25 cm H2O) in all but one patient (98%), ranging from 26 to >50 cm H2O.
MR imaging (MRI) was carried out in all patients before the treatment to exclude other possible causes of increased ICP (e.g., mass lesion and/or hydrocephalus). MR venogram was also performed in order to evaluate the venous sinuses for stenosis. Our standard MR protocol for the work-up of patients with IIH includes T1 TSE axial 5 mm (TR 550, TE 8.9, FoV 186*230, axial), T2 TSE 3 mm (TR 3440, TE 96, FoV 194*230, sagittal Hypophysis), DIF 5 mm (TR 5500, TE 103, FoV 229*229, axial), TOF 2D (TR 26, TE 2.15, FoV 201*230, coronal with MIP reconstruction), T2 IR 3 mm (TR 4300, TE 52, TI 145, FoV 208*230, coronal orbital), T1 3D–MPRAGE after Gd-DTPA IV 1 mm (TR 1530, TE 3.42, TI 950, FoV 250*250 sagittal), and T1 reph SE 5 mm after Gd-DPTA IV (TR 453, TE 17, FoV 186*230 transversal). Neither contrast enhanced MRA nor phase contrast MRA were used for the imaging of intracranial sinuses.
MR imaging showed typical findings of IIH such as enlargement and tortuosity of the perioptic subarachnoid space of both optic nerves in all but one of the patients (98%) and an “empty sella” in 45 out of 51 patients (88%). Stenotic dural sinuses were observed in all patients. In 48 out of 51 (94%) patients, the stenosis was typically located at the transverse-sigmoid junction. Thirty-two of them (67%) presented bilateral stenosis, ten patients (21%) unilateral stenosis with contralateral hypoplastic/aplastic sinus, two patients (4%) presented concomitant stenosis in another location, and four patients (8%) presented unilateral transverse sinus stenosis with the contralateral sinus showing a normal caliber. One patient presented an isolated stenosis of the straight sinus. One patient presented stenosis of the right jugular bulb and of the proximal part of the superior sagittal sinus. One patient presented stenoses of the jugular veins due to an Eagle syndrome which persisted after surgery. Resection of the styloid processus was only partial on both sides. The remnant of both styloid processus apparently still compromised the venous drainage requiring endovascular stent treatment.
Endovascular treatment protocol
All procedures were routinely performed under general anesthesia by four experienced interventional neuroradiologists as a single operator procedure.
Prior to the endovascular treatment, all patients underwent a three-vessel catheter angiography via a 4F arterial femoral access with injection of both internal carotid arteries and one vertebral artery with long runs visualizing the cerebral veins and intracranial venous sinuses. Femoral venous access was gained with an 8F sheath, and an 8F guiding catheter (e.g., Vista brite tip™, Cordis Corporation) was positioned into the right or left jugular vein just below the jugular bulb. Access into the superior sagittal sinus was usually straightforward with a 0.027″ microcatheter (e.g., Rebar-027™, Medtronic) over a 0.016″ or 0.014″ microguidewire (e.g., SilverSpeed16™, Medtronic; Traxcess14™, Microvention). Intracranial direct venography was performed by selective contrast injections through the microcatheter to confirm the presence and location of a stenosis. Cerebral venous pressure was measured at the superior sagittal sinus, transverse sinus, sigmoid sinus, and jugular vein in order to confirm or rule out the presence of a pressure gradient between pre-stenotic and post-stenotic sinus segment. For dural sinus pressure measurements, we usually use an invasive pressure monitor (IntelliVue, Philips) in venous mode attached to the 0.027″ microcatheter. The pressure gradient is only one aspect in the process of decision-making. In this scenario, a pressure gradient of 5 mmHg can be considered as an argument pro stent treatment. We learned, however, that despite identical anatomic conditions, a pressure gradient recorded under conscious sedation may vanish after induction of general anesthesia. A missing pressure gradient would therefore not constitute an absolute contraindication against stent treatment in a patient with sinus stenosis and elevated CSF opening pressure.
After pressure measurements, the stenosis was again crossed with the microguidewire and the microcatheter; thereafter, the microguidewire was exchanged over the microcatheter for an exchange-length 0.014″ microguidewire (Hi-Torque All-Star™, Abbott Vascular) with the tip of this wire placed sufficiently distal in the superior sagittal sinus or, if possible, in the contralateral jugular vein, allowing a reasonable backup for the balloon and stent catheter. The access for both balloon angioplasty and stent deployment was either transfemoral or via a direct retrograde puncture of the internal jugular vein, based on the discretion of the operator. In a series of 84 procedures, the access was transfemoral in 50 and transjugular in 34.
According to the diameter of the sinus adjacent to the stenosis, a 6–8 mm balloon (e.g., Submarine Rapido™, Invatec,) was then navigated over the microguidewire and inflated across the stenosis followed by the implantation of a self-expanding stent (e.g., Wallstent™, Boston Scientific; Protégé™, Medtronic). Postdilatation was not required. Given the tortuous anatomy of the sigmoid sinus, the use of a self-expanding stent (e.g., Wallstent™, Boston Scientific) is recommended because of their flexibility and atraumatic implantation. Balloon-expandable stents were used but have no apparent advantage and are more rigid.
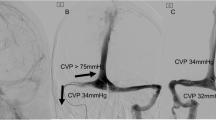
After stent deployment, direct venography and pressure measurement were repeated in order to compare pre- and post-stent values (Fig. 1).
Direct DSA venography with contrast injection into the superior sagittal sinus in a 38-year-old female patient with IIH. A circumscribed stenosis at the transition from the right transverse to sigmoid sinus is visible (a). Intra-sinus pressure readings are given, confirming a pressure gradient over the stenosis of 11 mmHg (b). Balloon angioplasty and stent deployment (c) result in a drop of this pressure gradient (d). Headache and visual disturbance in this patient improved for the last 9 years
After the treatment, the patients were monitored and kept in the hospital for a minimum of 3 days prior to discharge. All patients underwent a neurological examination after the endovascular treatment, and we performed MRI examinations after the procedure and prior to discharge in 35/51 patients. These examinations ruled out any adverse effect of the stent treatment in all cases.
Antiplatelet and anticoagulation regimen
From 2007 to 2012, the patients were placed on dual antiplatelet treatment receiving a loading dose of 500 mg acetylsalicylic acid (ASA) and 600 mg Clopidogrel at least 24 h prior to treatment in order to avoid intraprocedural thromboembolic complications. As we observed that dual platelet inhibition is not necessary for venous stenting, our loading regimen was changed and replaced by a periprocedural medication with 5000 units of heparin and 500 mg ASA, both given intravenously after groin puncture.
Our current postprocedural medication includes permanent medication with 100 mg ASA daily and total anticoagulation with LMW Heparin s.c. daily for 3 months, followed by oral anticoagulation with vitamin K antagonists (target INR 2.5) or dabigatran for 6 months. Only 100 mg of ASA daily for life is continued thereafter. Despite only three patients presented delayed minor complications related to the combination of ASA and heparin or vitamin K antagonists as described before, many patients complained about the self-injection of heparin and the frequent INR measurements. The combination of ASA and dabigatran is more convenient; data on efficacy and safety is, however, limited so far.
Follow-up schedule
In the beginning of our experience, we proposed follow-up DSAs for 3, 9, 24, and 36 months after the treatment, without measurement of the CSF opening pressure. We gradually shifted to a more individualized approach. Since 2014, we recommend DSA follow-up examinations at 3, 9, and 24 months, always combined with a lumbar tap. In the case of complete resolution of the clinical signs and symptoms of IIH and if the CSF opening pressure is below 25 cm H2O, no further examinations are recommended, unless the patient presents with a clinical recurrence.
Catheter angiography is performed in all the patients as the only reliable examination to confirm complete patency of the stent lumen and to rule out in-stent stenosis. In this cohort of patients who underwent endovascular treatment of IIH, a total of 231 postprocedural DSA examinations has been carried out, and no permanent sequelae of these examinations were encountered.
The clinical examination alone is not sufficient, and control of the CSF opening pressure was also routinely performed since 2014. With a chronic increase of the ICP, papilledema may disappear despite an elevated ICP. The description of headache can be quite vague, and many patients describe a changed character of their headache. In patients with ophthalmological manifestations of IIH, a formal visual field assessment and the measurement of the CSF opening pressure are required.
An ophthalmological examination was recommended within 1 month and routinely thereafter. Due to the variety of clinical manifestations of IHH, the available clinical data early after treatment are inconsistent. In patient 40 for instance, tinnitus was the only manifestation of IIH. Tinnitus disappeared immediately after stent treatment, and there was little reason of an ophthalmological examination.
No neuroimaging studies were routinely performed in the follow-up. Neither the MRI prior to discharge nor mid- and long-term follow-up examinations correspond with the clinical condition of the patients. Even in patients with complete resolution of their clinical manifestations of IIH, MRI findings like enlarged optic nerve sheaths and an “empty sella” usually persist.
Results
Endovascular treatment was successfully performed in all 51 patients (technical success rate, 100%) with a total of 85 stenoses treated in 78 procedures. Thirty patients received bilateral treatment (58.8%), 17 unilateral (33.3%), two patients received treatments for transverse sinuses and superior sagittal sinus stenoses (4%), one patient received treatment for bilateral transverse sinuses and straight sinus (2%), and one patient received treatment for an isolated stenose of the straight sinus (2%).
In patients who were treated for more than one stenosis (n = 34), the treatment was separately performed in 27 patients (79.4%), and in seven patients (20.6%), both stenoses were treated in the same procedure.
Direct manometry before stent placement was performed in 45 patients (88%), and a significant pressure gradient (≥5 mmHg) across the stenosis was observed in 38 patients (84.5%). The significance of the pressure gradient is essentially unknown. This parameter is only an aspect in the process of therapeutic decision-making. In our series, and based on individual circumstances, seven patients (15.5%) underwent stent implantation despite no pressure gradient was found. These patients had persistent clinical signs and symptoms of IIH and presented an elevated CSF opening pressure. Follow-up examinations confirmed a clinical improvement in 6/7 patients. In patients who received bilateral treatment a direct manometry was not routinely performed when the second stenosis was treated. In our experience, a significant pressure gradient is normally not observed in these patients after the first stent placement despite they may still remain symptomatic.
A single balloon dilatation was performed in three out of 85 stenoses (3.5%); 82 stenoses (96.5%) were treated with balloon dilatation and stent implantation, 75 of them using self-expanding carotid stents and three using a Solitaire Stent (Medtronic) (Fig. 2). Seven stenoses (8.2%) were treated using balloon-expandable stents. In 19 out of 85 stenoses (22%), more than one stent was necessary. The fact that a lumbar puncture with withdrawal of 30 cm3 of CSF may result in an instantaneous disappearance of a previous sinus stenosis underlines the fact that we are dealing with a functional stenosis. Therefore, balloon angioplasty without stent deployment will unlikely result in a removal of a sinus stenosis. We implanted at least one stent in all patients subject to this report.
Indirect DSA venography in a 44-year-old female patient with IIH, showing a tight stenosis of the superior sagittal sinus (a, arrow). The patient was previously treated for a stenosis at the transition from the right transverse to sigmoid sinus without total improvement of the IIH symptoms. After the deployment of a 5-mm self-expanding stent, the stenosis of the superior sagittal sinus was removed (b, arrow), and the patient experienced full recovery from previous papilledema and visual disturbance
The results are summarized in Table 1.
Complications
No major acute peri- and postprocedural neurological complications were encountered in a total of 84 procedures. Twenty patients experienced retro-orbital pain ipsilateral to the site of stent placement after treatment, which resolved within the first week and was likely due to dural stretching during the stent placement. Access-site adverse events occurred in six patients in a total of 84 procedures (7%). Of those, three patients required surgery for a femoral artery pseudoaneurysm, two patients presented a minor neck hematoma after direct puncture of the internal jugular vein and were treated conservatively, and one patient presented a subacute thrombosis of the femoral vein despite anticoagulation. Delayed minor complications related to the antiplatelet and anticoagulation regimens were observed in three patients (6%); one presenting melena, one hypermenorrhea, and one epistaxis; all were treated conservatively.
Overall, we encountered no procedure-related neurological morbidity and mortality at 30 days and beyond.
Clinical outcome
Clinical follow-up examinations were obtained in 49 out of 51 patients (96%). Angiographic follow-up was performed in 48 out of 51 patients (94%). One patient was lost to angiographic follow up, but the clinical course of this patient was assessed via telephone interview. The median follow-up interval was 49 months (cummulative 1879 months).
Thirty-seven out of 38 patients presenting with headache prior to EVT were available for clinical follow-up (97%). Fourteen of these patients (38%) reported complete resolution of the previous headache, and 17 of them (46%) had at least a remarkable improvement (total rate for improvement or resolution of headache: 84%). In six patients (16%), the headache did not change after the stent placement.
Thirty-eight out of 40 patients presenting with visual disturbances were available for follow-up (95%). Nineteen of these patients (50%) reported complete resolution of these disturbances, and 12 of them (31.5%) had an improvement (total rate for improvement or resolution of visual symptoms: 81.5%). Seven patients (18%) still presented severe visual disturbances at follow-up. All of them showed chronic changes of the optic nerve due to the chronic elevation of the intracranial pressure prior to EVT.
All patients presenting with papilledema but two were available for follow-up (96%). Papilledema completely resolved in 22 patients (46%) and improved in 20 patients (42%) (total rate for improvement or resolution of papilledema: 88%). No change was observed in six patients (12.5%). Nevertheless, these six patients presented with chronic atrophic optic nerve changes before EVT and at least did not show further worsening at follow-up.
Tinnitus disappeared in all the nine affected patients (100%).
Angiographic follow-up and retreatment
Angiographic follow-up examinations were available for 48 out of 51 treated patients (94%). The median follow-up was 49 months; the cumulative follow-up was 1879 months. In-stent stenoses were encountered in 5/48 patients with DSA follow-up (10.4%). These in-stent stenoses were found 22, 8, 18, 4, and 11 months after the deployment of the concerning stent. All five patients with in-stent stenoses were symptomatic. Recurrent symptoms were found in three patients, and two patients with in-stent stenosis had persistent symptoms. In all five patients balloon angioplasty of the in-stent stenosis was performed, which resulted in a significant enlargement of the sinus lumen in all cases. Recurrent in-stent stenosis was observed and treated in two patients. In the first patient, in-stent stenosis was observed 18 and 79 months after the stent-deployment and 61 months after the first balloon angioplasty, and in the second patient, in-stent stenosis was observed 4, 48, and 68 months after the stent-deployment and 42 and 64 months after the first balloon angioplasty. De novo stenoses distal to the previously implanted stent were observed in seven of the 48 patients (14.6%). Of these seven patients, four were asymptomatic despite the de novo stenosis. Recurrent symptoms were found in three patients and a new stenting was performed in all of them. No peri- and postprocedural complications occurred during a total of 12 retreatments.
Discussion
IIH is defined as a syndrome of raised intracranial pressure of unknown etiology without ventricular enlargement or an intracranial mass on imaging, with no evidence of venous sinus thrombosis, and with normal CSF constituents.
Catheter studies of the venous sinuses (cerebral venography) and measurement of intrasinus pressures (manometry) have documented that most patients with IIH have an elevated pressure in the superior sagittal sinus and proximal transverse sinuses, with a significant drop of venous pressure at the level of the distal transverse sinus [8]. This increased pressure in the dural sinuses may be due to systemic venous hypertension but more often is a result of stenoses of either dominant or both transverse sinuses, causing partial obstruction to cranial venous outflow. This theory is supported by the fact that more than 90% of patients with IIH have transverse sinus stenotic appearance compared with those of healthy patients. Therefore, these stenoses could be considered a reliable radiologic marker of intracranial hypertension, with a high specificity (93%) and sensitivity (93%) [5].
The etiology of venous abnormalities in IIH remains unclear. The most intriguing question is whether the venous narrowing is a cause or consequence of increased intracranial pressure. Considering the venous stenoses as consequence, elevated intracranial CSF pressure could lead to a secondary narrowing of the sinus lumen by compression (extrinsic compression), which can be reversed by CSF removal [9, 10] or diversion procedures [11]. On the other hand, an acutely marginated filling defect due to arachnoid granulations or fibrous septae (intrinsic obstruction) could obstruct the venous outflow, increase intracranial venous pressure proximal to the stenosis, and lead to increased CSF pressure as a result of a reduction in CSF absorption via the arachnoid granulations [5]. In this setting, a pressure gradient across the stenosis should be expected and, hence, reconstruction of the venous lumen with stents would be effective in lowering elevated CSF pressure [11].
Most patients with IIH respond to maximal medical therapy. Conservative treatment comprises a significant loss of body weight and drugs such as acetazolamide or topiramate, which act against carbonic anhydrase and reduce the rate of CSF production [12]. Therapeutic lumbar puncture with withdrawal of about 40 cm3 CSF is another common treatment method, but the benefit is mostly only short-term [13].
Indications for more invasive measures, such as CSF shunt surgery, optic nerve sheath fenestration, or subtemporal decompression, are reserved for patients with failure of or noncompliance with medical treatment, with new or worsening visual deficits, persistent headache, or fulminant IIH [14]. There are no evidence-based guidelines for the management of IIH, and randomized controlled trials supporting one of the treatment strategies are missing [15]. In this cohort of 51 patients, four patients had previously undergone ventricular shunting, which resulted in a temporary improvement in two patients. All four patients with CSF diversion were seen by a neurosurgeon and evaluated for an improvement of the shunt function prior to stent implantation. Shunts had been either explanted on a previous occasion (n = 2) or were left in place (n = 2). One of our patients was treated before endovascular stenting by surgery of both styloid processes due to an Eagle syndrome (Fig. 3). Resection of the styloid processus was only partial on both sides, and the remnant apparently still compromised the venous drainage. Only one of our patients was directly considered for endovascular treatment due to noncompliance with medical treatment, and no patient had been treated with optic nerve sheath fenestration or subtemporal decompression.
DynaCT showing giant styloid processes causing compression of both internal jugular veins (a, dotted circle). Surgical shortening of these processes was not sufficient to improve venous drainage (b, dotted circle; c, arrow). Deployment of balloon expandable stents improved the venous drainage (d). Despite this angiographic result, long-standing increase of the ICP caused a severe optic nerve atrophy, which caused a persistent visual field reduction and reduced visual acuity
Stenting for IIH was first performed by Higgins et al. [16] in 2002. Since then, several individual case reports and case series have demonstrated the clinical benefit of this procedure [17–23].
In 2013, Puffer et al. reviewed the current literature on venous sinus stenting for the management of IIH [24]. A total of 143 published patients were analyzed concluding that 88% of patients had improvement in headache, 97% had resolution of papilledema, and 87% had improvement or resolution of visual symptoms. The largest single-center experience published until now includes 52 patients with IIH treated with venous stent placement [25]. In all 52 patients (100%), stent placement immediately eliminated the pressure gradient, with striking symptomatic improvement and abolished papilledema. Our series presents similar efficacy with 84% of patients showing complete relief or improvement of headache after endovascular stenting and resolution or improvement of visual symptoms in 81.5% of patients. We also observed resolution or improvement of papilledema in 88% of the patients, demonstrating that endovascular treatment of IIH can be a useful treatment option for these patients.
Despite these very encouraging results, the role of venous stenting in the armamentarium for the treatment of IIH remains controversial. Rohr [11] proposed that it is crucial to differentiate between intrinsic versus extrinsic compression of venous sinuses aiding in the choice of therapy—shunt surgery for those with secondary reversible stenoses (extrinsic compression) and stent placement for primary fixed stenoses (intrinsic compression). On the other hand, Ahmed [25] observed that regardless of whether the stenosis was secondary or primary, the absence of even one normal low-resistance transverse sinus resulted in venous hypertension. Therefore, dilatation of just one of these stenoses produces a reduction in intracranial venous pressures abolishing papilledema and improving symptoms. Lenck [26] also reported that irrespective of the type of stenosis, stenting of lateral sinus stenoses is an effective treatment for IIH symptoms. The clear symptomatic response in stented patients with IIH confirms in any case the role of transverse sinus stenosis in this pathology.
Possible procedure-related complications are also a matter of concern, including perforation of the vessel, stent migration, increased risk of thrombus formation, and restenosis [27]. The reported complication rates vary between 5 and 15%. Ahmed et al. [25] observed two significant neurologic complications, one subdural hematoma due to guidewire perforation and one complex hemorrhage; both patients underwent craniotomy and made full recovery. We did not observe major peri- or postprocedural neurological complications in our series. Minor access-site adverse events occurred in six patients, and complications related with the anticoagulation regimen were observed in two.
Data concerning the long-term results after venous sinus stenting is still limited. Some series [25, 28] have already confirmed long-term clinical improvement after EVT, and despite up to 12% of patients required a repeated procedure [25], this rate is less than that of the surgical options. A recent cost comparison between sinus stenting and CSF shunting [29] concluded that there is no significant difference in the cost of the initial procedure; however, long-term data showed that venous sinus stenting incurred significantly less cost than CSF shunting because of the higher revision and complication rates associated with this last one.
Limitations
This series is subject to several limitations. The number of treated patients is still small. Data collection and analysis were performed retrospectively, and the potential biases may limit generalization. Evolution of treatment details (e.g., routine performance of CSF opening pressure measurement) during the last 9 years caused some data inconsistency. Our findings and those presented in the literature might justify a randomized controlled trial comparing medical treatment, shunt procedures, and EVT, with long-term follow-up to confirm the effectiveness of these treatment modalities. The enrollment rate of such a trial would most likely be small. The antiplatelet and anticoagulant regimen should also be evaluated under controlled conditions.
Conclusions
Since venous sinus stenoses have become a common finding in many patients suffering from IIH, it has become evident that there is a potential role for venous sinus stenting in the management of these patients. Results of several patient series have shown resolution of papilledema and improvement of symptoms that is comparable to other treatment modalities. Despite these promising results, venous sinus stenting for the treatment of IIH remains controversial.
Based on our results, sinus stent placement is a safe and effective alternative to other invasive treatments (e.g., shunt surgery). Patients who have failed conservative therapy should undergo MR venography to confirm the presence of any venous sinus stenoses. EVT should then be considered as an alternative treatment. A significant pressure gradient across the stenosis confirms the indication but is not a compulsory finding.
References
Corbett JJ, Savino PJ, Thompson HS et al (1982) Visual loss in pseudotumor cerebri: follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol 39:461–474
Brodsky MC, Vaphiades M (1998) Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology 105:1686–1693
Degnan AJ, Levy LM (2011) Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol 32:1986–1993
Saindane AM, Bruce BB, Riggeal BD, Newman NJ, Biousse V (2013) Association of MRI findings and visual outcome in idiopathic intracranial hypertension. AJR Am J Roentgenol 201:412–418
Farb RI, Vanek I, Scott JN et al (2003) Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology 60:1418–1424
Arac A, Lee M, Steinberg GK et al (2009) Efficacy of endovascular stenting in dural venous sinus stenosis for the treatment of idiopathic intracranial hypertension. Neurosurg Focus 27:E14
Friedman DJ, Liu GT, Digre KB (2013) Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 81:1159–1165
King JO, Mitchell PJ, Thomson KR et al (1995) Cerebral venography and manometry in idiopathic intracranial hypertension. Neurology 45:2224–2228
King JO, Mitchell PJ, Thomson KR et al (2002) Manometry combined with cervical puncture in idiopathic intracranial hypertension. Neurology 58:26–30
De Simone R, Marano E, Fiorilio C et al (2005) Sudden re-opening of collapsed transverse sinuses and longstanding clinical remission after a single lumbar puncture in a case of idiopathic intracranial hypertension. Pathogenetic implications. Neurol Sci 25:342–344
Rohr A, Dörner L, Stingele R et al (2007) Reversibility of venous sinus obstruction in idiopathic intracranial hypertension. AJNR Am J Neuroradiol 28:656–659
Celebisoy N, Gokcay F, Sirin H et al (2007) Treatment of idiopathic intracranial hypertension: topiramate vs acetazolamide, an open-label study. Acta Neurol Scand 116:322–327
Johnston I, Paterson A (1974) Benign intracranial hypertension. II CSF pressure and circulation. Brain 97:301–312
Corbett JJ, Thompson HS (1989) The rational management of idiopathic intracranial hypertension. Arch Neurol 1046–51
Lueck C, McIlwaine F (2005) Interventions for idiopahic intrcranial hypertension. Cochrane Database Syst Rev CD003434
Higgins JN, Owler BK, Cousins C et al (2002) Venous sinus stenting for refractory benign intracranial hypertension. Lancet 359:228–230
Ogungbo B, Roy D, Gholkar A et al (2003) Endovascular stenting of the transverse sinus in a patient presenting with benign intracranial hypertension. Br J Neurosurg 17:565–568
Rajpal S, Niemann DB, Turk AS (2005) Transverse venous sinus stent placement as treatment for benign intracranial hypertension in a young male: case report and review of the literature. J Neurosurg 102:342–346
Métellus P, Levrier O, Fuentes S et al (2005) Endovascular treatment of benign intracranial hypertension by stent placement in the transverse sinus: therapeutic and pathophysiological considerations illustrated by a case report. Neurochirurgie 5:113–120
Higgins JN, Cousins C, Owler BK et al (2003) Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry 74:1662–1666
Owler BK, Parker G, Halmagyi GM et al (2003) Pseudotumour cerebri syndrome: venous sinus obstruction and its treatment with stent placement. J Neurosurg 98:1045–1055
Donnet A, Metellus P, Levrier O et al (2008) Endovascular treatment of idiopathic intracranial hypertension: clinical and radiologic outcome of 10 consecutive patients. Neurology 70:641–647
Bussiere M, Falero R, Nicolle D et al (2010) Unilateral transverse sinus stenting of patients with idiopathic intracranial hypertension. AJNR Am J Neuroradiol 31:645–650
Puffer RC, Mustafa W, Lanzino G (2013) Venous sinus stenting for idiopathic intracranial hypertension: a review of the literature. J Neurointerv Surg 5:483–486
Ahmed RM, Wilkinson M, Parker GD et al (2011) Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. AJNR Am J Neuroradiol 32:1408–1414
Lenck S, Vallée F, Labeyrie MA, et al. (2017) Stenting of the lateral sinus in idiopathic intracranial hypertension according to the type of stenosis. Neurosurgery 1–8
Tsumoto T, Miyamoto T, Shimizi M et al (2003) Restenosis of the sigmoid sinus after stenting for treatment of intracranial venous hypertension: case report. Neuroradiology 45:911–915
Kumpe DA, Bennett JL, Seinfeld J et al (2012) Dural sinus stent placement for idiopathic intracranial hypertension. J Neurosurg 116:538–548
Ahmed RM, Zmudzki F, Parker GD, et al. (2014) Transverse sinus stenting for pseudotumor cerebri: A cost comparison with CSF shunting. AJNR Am J Neuroradol 35:952–958
Acknowledgment
The authors are most grateful to James Lago for language revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that this retrospective analysis of a series of patients treated according to clinical routine did not follow a pre-given protocol. The Guidelines of the Declaration of Helsinki of the World Medical Association in its current version (WMA, 2004), the Guidelines of Good Clinical Practice (CPMP/ICH/135/95) and demands of the national medical and data protection laws were followed. We declare that the ethics committee waived the obligation of a legal consultation.
Conflict of Interest
MAP serves as a proctor and consultant for phenox and Medtronic; RMM and WK consult for phenox; HH has proctoring and consulting agreements with Medtronic and Balt, and is co-founder and shareholder of phenox.
Rights and permissions
About this article
Cite this article
Aguilar-Pérez, M., Martinez-Moreno, R., Kurre, W. et al. Endovascular treatment of idiopathic intracranial hypertension: retrospective analysis of immediate and long-term results in 51 patients. Neuroradiology 59, 277–287 (2017). https://doi.org/10.1007/s00234-017-1783-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1783-5