Abstract
Introduction
We investigated whether MR diffusion tensor imaging (DTI) analysis of the cervical spinal cord could aid the (differential) diagnosis of sensory neuronopathies, an underdiagnosed group of diseases of the peripheral nervous system.
Methods
We obtained spinal cord DTI and T2WI at 3 T from 28 patients, 14 diabetic subjects with sensory-motor distal polyneuropathy, and 20 healthy controls. We quantified DTI-based parameters and looked at the hyperintense T2W signal at the spinal cord posterior columns. Fractional anisotropy and mean diffusivity values at C2–C3 and C3–C4 levels were compared between groups. We also compared average fractional anisotropy (mean of values at C2–C3 and C3–C4 levels). A receiver operating characteristic (ROC) curve was used to determine diagnostic accuracy of average fractional anisotropy, and we compared its sensitivity against the hyperintense signal in segregating patients from the other subjects.
Results
Mean age and disease duration were 52 ± 10 and 11.4 ± 9.3 years in the patient group. Eighteen subjects had idiopathic disease and 6 dysimmune etiology. Fractional anisotropy at C3–C4 level and average fractional anisotropy were significantly different between patients and healthy controls (p < 0.001 and <0.001) and between patients and diabetic subjects (p = 0.019 and 0.027). Average fractional anisotropy presented an area under the curve of 0.838. Moreover, it had higher sensitivity than visual detection of the hyperintense signal (0.86 vs. 0.54), particularly for patients with short disease duration.
Conclusion
DTI-based analysis enables in vivo detection of posterior column damage in sensory neuronopathy patients and is a useful diagnostic test for this condition. It also helps the differential diagnosis between sensory neuronopathy and distal polyneuropathies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sensory neuronopathies are characterized by primary degeneration of the dorsal root ganglia and projections. This condition leads to widespread sensory deficits and afferent ataxia. On clinical grounds, it is often difficult to distinguish sensory neuronopathy and length-dependent sensory polyneuropathies [1]. This distinction has obvious clinical implications regarding management and prognosis.
In this scenario, a few markers [2] have been investigated to aid this differential diagnosis, such as MRI of the spinal cord. The typical imaging finding is a non-enhancing hyperintense T2-weighted signal at the posterior columns of the spinal cord, which has been included in recently published diagnostic criteria [1]. Despite that, there is still no consensus regarding its frequency, particularly for subjects with short disease duration. Thus, we hypothesize that diffusion tensor imaging (DTI) of the spinal cord might be more sensitive to detect posterior column damage in sensory neuronopathy [3, 4]. DTI indirectly evaluates the integrity of myelin and white fiber tracts through alterations on water diffusivity parameters [5]. Previous spinal cord DTI studies have proven useful and reliable in other neurodegenerative diseases, such as multiple sclerosis [6] and amyotrophic lateral sclerosis [7].
Therefore, we prospectively investigated whether DTI of the posterior column separates sensory neuronopathy from other polyneuropathies and healthy controls. We also compared its sensitivity with the visual detection of the hyperintense T2W signal in the posterior spinal cord, to evaluate if it could serve as an auxiliary for diagnosis.
Methods
This was a prospectively designed case-control study.
Subjects
Between 2013 and 2015, we enrolled 28 consecutive sensory neuronopathy patients (52 ± 10 years), 20 healthy subjects (46 ± 11 years), and 14 individuals (53 ± 10 years) with diabetic sensory-motor distal polyneuropathy at our university hospital. Diagnosis of both conditions relied upon published criteria [1, 8], which included clinical and/or electrophysiological investigations. We did not include patients with concomitant neurological disorders or compressive myelopathy. Twenty-two out of the 28 sensory neuronopathy patients underwent clinical evaluation (INCAT Sensory Sum Score (ISSS) [9], Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) [10], and Scale for the Assessment and Rating of Ataxia (SARA) [11]). This study was approved by our Research Ethics Committee, and all participants signed a written informed consent.
MRI acquisition
All participants performed a MR scan (3 T Achieva; Philips Medical Systems, Best, The Netherlands) to acquire the following images with an 18-channel coil (SENSE Neurovascular, Philips Medical Systems, Best, The Netherlands):
-
1.
Axial structural T2W image: cervical level (C1–C7). SENSE factor = 1.5; reconstructed voxel size = 0.29 × 0.29 × 3 mm3; field of view (FOV) = 150 × 150 × 108 mm3; image matrix = 512 × 512; 36 slices; repetition time (TR)/echo time (TE) = shortest/100 ms; flip angle = 90°
-
2.
Sagittal structural T2W image: SENSE factor = 1.5; reconstructed voxel size = 3 × 0.49 × 0.49 mm3; slice gap = 0.3 mm; FOV = 36 × 220 × 220 mm3; image matrix = 11 × 448 × 448; TR/TE = shortest/120 ms; flip angle = 90°
-
3.
Diffusion tensor image (DTI): cervical level (C1–C6). SENSE factor = 2; 15 gradient directions with b = 600 s/mm2 and one image with b = 0; TR/TE = shortest/75 ms; FOV = 224 × 224 × 105 mm3; image matrix = 448 × 448 × 306; reconstructed voxel size = 0.5 × 0.5 × 3 mm3; slice gap = 3 mm; number of samples acquired = 1
Analysis
A blind evaluator (JLRP) measured fractional anisotropy (FA) and mean diffusivity (MD) values at two levels (C2–C3 and C3–C4) in the posterior cord of all subjects. The average FA value for each person was also calculated. We then tested these DTI measurements for group differences after regressing out age and gender. We obtained a receiver operating characteristic (ROC) curve for average FA value and determined the best cutoff to distinguish sensory neuronopathy (SN) from non-SN subjects.
Another blind evaluator (FR) assessed T2W images for the presence of the hyperintense T2W signal in the posterior funiculi of all participants. Subsequently, we compared the sensitivity and specificity of both approaches.
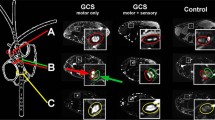
DT images were analyzed using the Main Explore DTI software (http://exploredti.com/), a MATLAB toolbox (MATLAB 7.1, The MathWorks Inc., Natick, MA, 2010). Sagittal images were used to select two slices at the level of the intervertebral discs: one between C2 and C3 vertebrae and another between C3 and C4. We used a circular region of interest (ROI) of 1.8 mm2, which was placed approximately one voxel away from the spine-CSF interface (Fig. 1a, b).
Statistics
We used the analysis of variance (ANOVA) test to assess between-group differences regarding DTI parameters, taking age/gender as covariates and setting a significance level of 95 %. P values were corrected for multiple testing via the Bonferroni adjustment.
We also compared age and gender between groups using ANOVA and chi-square contingency table test, respectively. For the analysis of the hyperintense T2W signal presence, we used Fisher’s exact test.
Subsequently, we created a ROC curve to assess DTI diagnostic accuracy in separating sensory neuronopathy patients from the other groups. Regarding the hyperintense T2W signal, sensitivity and specificity were directly calculated from the classifications provided by visual inspection. We also computed Spearman’s rank correlation coefficient between clinical scales and DTI parameters. All the statistical analyses were performed in SPSS (version 22).
Results
Demographics and clinical assessment
Detailed demographic data of subjects are shown in Table 1. Mean disease duration of sensory neuronopathy was 11.4 ± 9.3 years, and 18 % of our patients were wheelchair bound. Eighteen out of 28 (64 %) of the patients were idiopathic, 4 (14 %) were related to Sjögren’s syndrome, and other more infrequent etiologies were related to human T cell lymphotropic virus (HTLV), autoimmune hepatitis, paraneoplasia, monoclonal gammopathy of undetermined significance, and vitamin B12 deficiency. Clinically assessed patients (22 out of 28) had mean ISSS, SARA, and LANSS scores of 17.7 ± 3.7, 11.1 ± 5.7, and 10.9 ± 7.1, respectively. The six remaining patients also had a complete clinical evaluation, but these were not paired with the MRI exam. Therefore, they were excluded from the correlation analyses but included in the group comparisons of DTI-based parameters.
DTI-based parameters
DTI measurements and results for group comparisons are shown in Table 1 and Fig. 2a–e. In brief, we found statistically significant differences between groups for all DTI parameters after regression of age and gender, except for MD at C3–C4 level (Table 1). Regarding FA at C2–C3 and MD at C2–C3 level, we found significant differences between SN patients and healthy controls (p < 0.001 and p = 0.004, respectively) and between diabetic subjects and healthy controls (p = 0.034 and p = 0.039). Moreover, FA at C3–C4 level and average FA were significantly different between SN patients and healthy controls (p < 0.001 for both) and between SN patients and diabetic subjects (p = 0.019 and p = 0.027).
The receiver operating characteristic curve showed an optimum cutoff for average FA value of 0.744 for segregating sensory neuronopathy patients from the other subjects (diabetics + healthy controls), with a sensitivity of 0.86, 95 % CI [0.67,0.96], and a specificity of 0.71, 95 % CI [0.53, 0.85]. The area under the curve was 0.838 (Fig. 2f). Table 2 summarizes subject classification (SN vs. non-SN) using the derived cutoff value.
Considering only sensory neuronopathy patients who were clinically evaluated, we found significant correlations between average FA and LANSS scale (rho = 0.572; uncorrected p = 0.011) and FA at C2–C3 level and LANSS (rho = 0.502; uncorrected p = 0.029). There was no correlation between DTI parameters and other measures of clinical disability (ISSS and SARA). All the correlation values are given in Table 3.
Hyperintense T2W signal
Hyperintense T2W signal was visually assessed along the entire cervical spinal cord, identified in 18 cases (15 sensory neuronopathy patients, 1 diabetic subject, and 2 healthy subjects) (Table 2). This resulted in a sensitivity of 0.54 and a specificity of 0.91 for the binary logistic regression of sensory neuronopathy patients vs. non-patients (diabetics + healthy controls).
We then divided patients with SN into two groups: those with abnormal T2 (n = 15) and those with normal T2 (n = 13). As expected, all but one in the group with abnormal T2 also had reduced FA values (according to the threshold obtained using the ROC curve). Interestingly, we found that 10 out of 13 in the normal T2 group also had reduced FA values (76 %).
Discussion
We showed that spinal cord DTI enables non-invasively in vivo identification of damage to the central projections of the dorsal root ganglia neurons in sensory neuronopathy patients. Axonal loss and gliosis of the cuneate and gracile fasciculi probably underlie the FA and MD abnormalities found. This may also be the same substrate for hyperintense T2W signal [12]. It seems, however, that DTI abnormalities take place earlier than the hyperintense signal. Indeed, four out of the five patients with disease duration shorter than 1 year presented average FA values below the suggested cutoff point, whereas only one had detectable hyperintense signal. Furthermore, most patients without T2W hyperintensity had reduced FA values (10/13). Taken together, these data suggest that DTI may be more sensitive than isolated visual analysis, particularly early in the disease course. The unexpected T2W hyperintensity found in two entirely normal controls was focal (encompassing one vertebral body, in striking contrast to that found in SN, which was longitudinally extensive) and probably related to artifacts.
Out of the proven DTI parameters, FA seems to be the most useful measure, since MD failed to separate SN patients from non-SN patients. Our findings indeed showed that average FA and FA at C3–C4 level distinguish patients from subjects with diabetic polyneuropathy and healthy controls. Furthermore, these parameters had greater sensitivity than the visual detection of the hyperintense T2W signal, what turns to be an important result given that sensory neuronopathies are frequently underdiagnosed. We failed to identify correlations between FA and measures of clinical disability. This might be due to the fact that the employed scales do not adequately assess impairment related to sensory neuronopathy. SARA and ISSS were originally designed for patients with distal polyneuropathies and cerebellar ataxias, not afferent ataxias. Unexpectedly, spinal cord FA only correlated with pain score, thus suggesting that posterior column damage is somehow associated with neuropathic pain in this condition.
Other studies have also targeted the upper cervical spinal cord in order to evaluate damage associated with neurodegenerative diseases [6, 7]. This is because sensory (and motor) fibers from arms and legs have already converged into the cord at this level, thus allowing a more comprehensive assessment of tracts. There is also a larger cross-sectional area at this level (cervical intumescence), which favors the positioning of the DTI ROI in the posterior funiculus.
Conclusions
Our results highlight the potential usefulness of spinal cord DTI as a diagnostic test for sensory neuronopathy. Further studies with larger and more homogeneous (in terms of etiology) cohorts should be performed to validate our approach. In addition, longitudinal evaluation might prove useful to determine whether spinal cord DTI is also valuable as a prognostic biomarker.
References
Camdessanché J-P, Jousserand G, Ferraud K et al (2009) The pattern and diagnostic criteria of sensory neuronopathy: a case–control study. Brain 132:1723–1733. doi:10.1093/brain/awp136
Bao Y-F, Tang W-J, Zhu D-Q et al (2013) Sensory neuronopathy involves the spinal cord and brachial plexus: a quantitative study employing multiple-echo data image combination (MEDIC) and turbo inversion recovery magnitude (TIRM). Neuroradiology 55:41–48. doi:10.1007/s00234-012-1085-x
Casseb RF, Martinez ARM, de Paiva JLR, França MC Neuroimaging in sensory neuronopathy. J Neuroimaging 25:704–9. doi:10.1111/jon.12210
Paiva JLR de, Casseb RF, Martinez ARM et al (2015) Diffusion tensor imaging (DTI) of the cervical cord in sensory neuronopathies (P5.070). Neurology 84, P5.070
Alexander AL, Hurley SA, Samsonov AA et al (2011) Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect 1:423–446. doi:10.1089/brain.2011.0071
Kearney H, Miller DH, Ciccarelli O (2015) Spinal cord MRI in multiple sclerosis—diagnostic, prognostic and clinical value. Nat Rev Neurol 11:327–338. doi:10.1038/nrneurol.2015.80
El Mendili M-M, Cohen-Adad J, Pelegrini-Issac M et al (2014) Multi-parametric spinal cord MRI as potential progression marker in amyotrophic lateral sclerosis. PLoS ONE 9:e95516. doi:10.1371/journal.pone.0095516
Garcia RU, Ricardo JAG, Horta CA et al (2013) Ulnar sensory-motor amplitude ratio: a new tool to differentiate ganglionopathy from polyneuropathy. Arq Neuropsiquiatr 71:465–469. doi:10.1590/0004-282X20130063
Merkies IS, Schmitz PI, van der Meché FG, van Doorn PA (2000) Psychometric evaluation of a new sensory scale in immune-mediated polyneuropathies. Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Neurology 54:943–949
Bennett M (2001) The LANSS pain scale: the Leeds assessment of neuropathic symptoms and signs. Pain 92:147–157. doi:10.1016/S0304-3959(00)00482-6
Schmitz-Hübsch T, du Montcel ST, Baliko L et al (2006) Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66:1717–1720. doi:10.1212/01.wnl.0000219042.60538.92
Okumura R, Asato R, Shimada T et al Degeneration of the posterior columns of the spinal cord: postmortem MRI and histopathology. J Comput Assist Tomogr 16:865–7
Acknowledgments
We thank Brunno Machado de Campos, Benílton de Sá Carvalho and biomedical technicians for their help in MRI discussions, statistical analysis and data acquisition, respectively. We thank FAPESP (Sao Paulo Research Foundation - Grants 2013/01766-7, 2013/07559-3 – Brazilian governmental agency) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that all human studies have been approved by the research ethics committee of the School of Medical Sciences - UNICAMP and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Casseb, R.F., de Paiva, J.L.R., Branco, L.M.T. et al. Spinal cord diffusion tensor imaging in patients with sensory neuronopathy. Neuroradiology 58, 1103–1108 (2016). https://doi.org/10.1007/s00234-016-1738-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1738-2