Abstract
Introduction
The aim of the present study is to use 3D pseudo-continuous arterial spin labeling (pCASL) to measure changes in normal cerebral blood flow (CBF) values with sex and age.
Methods
Eighty subjects underwent magnetic resonance imaging study on a 3.0-T scanner. 3D pCASL was used to measure CBF. Estradiol and testosterone levels were also assessed. Based on sex and age, the subjects were divided into four groups for statistical analysis.
Results
CBF was higher in young premenopausal women than in young men (P < 0.01), while there was no difference in CBF between older postmenopausal women and older men. CBF was also significantly decreased in older postmenopausal women compared with young women (P < 0.01), whereas no such age-related effect was present in men.
Conclusion
These observations indicate that sex and age differences are important considerations when studying nervous system diseases and that the age range and female-to-male ratio should be considered in the selection of subjects for brain studies in order to eliminate bias. Further, the normal CBF values assessed by pCASL provide a basis for further clinical use of pCASL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Owing to the high metabolic rate and limited substrate storage capacity of brain tissue [1], adequate and constant cerebral blood flow (CBF) is essential for normal brain function. Abnormal CBF can contribute to various nervous system diseases including cerebral ischemic stroke [2]. Previous studies have suggested that premenopausal women experience fewer strokes than men of comparable age, and stroke rates increase among postmenopausal women compared with age-matched men [3]. These sex differences in the prevalence of stroke may be induced by differences in CBF. Thus, understanding the gender-specific brain differences in CBF is a critical step to demonstrate the physiological basis of the mechanisms and outcomes of neurological diseases [4]. Age is also an important factor that can control CBF. Several studies have shown a continuous decrease in resting CBF during adulthood [5–8]. However, decreased CBF was also detected in patients with Alzheimer’s disease (AD) [9], and it was suggested that diagnosis of AD should incorporate a decrease in CBF as an early biomarker [10]. Hence, measuring CBF in different aged healthy subjects is important to differentiate normal versus abnormal changes in CBF.
Although a number of methodologies have been used to assess CBF in healthy subjects [5, 11, 12], only limited studies have used 3D pseudo-continuous arterial spin labeling (pCASL), a non-invasive method that offers a number of advantages over traditional methods. First, pCASL can eliminate the risk of nephrogenic systemic fibrosis in patients with renal dysfunction because pCASL does not require a gadolinium-based tracer [13]. Secondly, as pCASL does not use radiation, it can be used repeatedly to evaluate CBF changes over time. Finally, pCASL provides high reproducibility and reliability [14–17]. Thus, pCASL is increasingly used in the clinical and experimental study of CBF [18, 19]. As different methodologies can provide marked variation in CBF values, pCASL may be useful to quantitatively assess CBF in healthy subjects to allow accurate differentiation of normal and abnormal CBF.
The aims of the present study were to acquire the normal CBF values of healthy subjects using 3D pCASL and to assess the effects of sex and age differences on CBF. We hypothesized that the CBF values of women would be higher than for men and that CBF values would decrease with age.
Materials and methods
Subjects
Eighty healthy subjects were recruited for this study with informed consent obtained. Subjects were 20 young premenopausal women (age 25.4 ± 0.43 years), 20 young men (age 25.45 ± 0.43 years), 20 older postmenopausal women (age 57.05 ± 0.79 years), and 20 older men (age 57.6 ± 1.12 years). Participants were non-smokers, physically fit, and taking no medication. The inclusion criteria of postmenopausal women were over 50 years old and ≥1 year postcomplete cessation of menses. Exclusion criteria were history or clinical evidence of cardiovascular disease, be pregnant, neurologic disease, stroke, known or suspected dementia (mini-mental state examination score <24), and psychiatric disorder. No alcohol, caffeine, or medication that could affect CBF was consumed over the duration of the study. The study was approved by the local medical ethical committee and conformed to standards set by the Declaration of Helsinki.
Experimental design
All subjects received clinical and magnetic resonance (MR) examination between 5–7 pm. For young premenopausal women, the examinations were performed during mid-cycle (when estrogen levels are high).
Laboratory test
Estradiol (E2) and testosterone (T) were measured from the peripheral venous samples using an automatic chemiluminescence immunoassay analyzer UniCel DxI 800 (Beckmancoulter, Brea, CA, USA).
MRI examination
A 3.0-T scanner (GE Discovery MR 750, Fairfield, CT, USA) with an eight-channel head coil (in vivo) was used. The MR protocol included 3D pCASL and T1-weighted anatomical images. The parameters for 3D pCASL were as follows: eight spiral arms with 512 points on each arm, spatial resolution = 3.64 mm, postlabeling delay (PLD) = 2.0 s, repetition time = 4844 ms, echo time = 10.5 ms, bandwidth = ±62.5 kHz, slice thickness = 4 mm, number of slices = 36, field of view = 24 cm, number of excitations = 3, and acquisition time = 4 min 41 s. A high-resolution T1-weighted dataset using a 3D fast-spoiled gradient-recalled echo pulse sequence was acquired and used to generate a mask to separate grey matter and white matter. The parameters were as follows: repetition time = 6.9 ms, echo time = 3.0 ms, inversion time = 450 ms, bandwidth = ±31.25 kHz, field of view = 25.6 cm, slice thickness = 1 mm, matrix = 256 × 256, number of excitations = 1, and acquisition time = 4 min 47 s.
Data processing
The 3D pCASL data were processed using ASL toolbox (http://www.fundacioncien.es/areas/asl_toolbox.asp), SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) running with FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/), and MATLAB (MathWorks Inc., Natick, MA, USA). First, DICOM images were imported into SPM, and the T1-weighted images were then co-registered to 3D ASL NIFTI data after resampling. To exclude the signal from non-brain tissue, CBF images were processed using the skull-stripping BET algorithm from FSL. Registration of CBF images was performed using the segmentation transformation matrix generated from T1-weighted images and was spatially normalized to the MNI template of Montreal Neurological Institute. Finally, these normalized CBF data were smoothed with a 4-mm (FWHM) Gaussian kernel (Fig. 1). Image quality controls were performed after every step by visual inspection.
Statistics
Statistical analysis was performed using SPSS software (version 17.0, SPSS, Chicago, IL, USA). Origin (version 8.0; OriginLab, Hampton, MA, USA) was used to create the artwork. Comparisons between young women and young men, older women and older men, young and older women, and young and older men were performed by independent samples t test. A P value of <0.05 was regarded as statistically significant. Data are presented as mean ± SEM.
Results
Laboratory data
The obtained clinical data are shown in Table 1. E2 in older women was significantly decreased compared with young women (P < 0.01). T was significantly lower in older men compared with young men (P < 0.01).
Cerebral blood flow
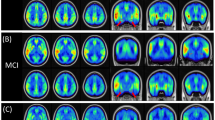
Differences in CBF between young women and young men, and older women and older men, are shown in Fig. 2. There was a significantly higher global brain (GB) CBF in young women (60.44 ± 1.15 ml, 100 g−1 min−1) compared with young men (49.53 ± 1.2 ml, 100 g−1 min−1) (P < 0.01). CBF values in both the grey matter (GM) and white matter (WM) of young women were also significantly higher than in young men (P < 0.01). There were no differences in CBF in the GB, GM, or WM between older women and older men.
CBF in the GB of the young women was significantly higher than that of older women (P < 0.01), whereas no such age-related effect was present in men (Fig. 3). Although CBF in the GB of young men (49.53 ± 1.2 ml, 100 g−1 min−1) was higher than that for older men (46.34 ± 1.74 ml, 100 g−1 min−1), the difference was not significant (P > 0.05). Similar trends were observed for CBF in the GM and WM.
Discussion
Sex differences in CBF
Using pCASL, we found that young women exhibited significantly higher CBF values in the GB, GM, and WM compared with young men, which is consistent with the previous CBF studies using positron emission tomography or single positron emission-computed tomography [20, 21]. These differences may relate to the differences in sex hormones between women and men. For example, E2 was much higher in young women than in men in the present study. In women, E2 is the main circulating sex hormone, while circulating levels of T are relatively low. In men, T is the main circulating sex hormone, while circulating E2 levels are much lower than in women [22].
Both E2 and T exert profound actions on cerebrovascular reactivity and CBF [23]. Indeed, E2 was reported to (1) enhance function of endothelial nitric oxide synthase and subsequently increase production of nitric oxide, (2) alter the balance of prostanoids by enhancing the production of vasodilating prostacyclin, and (3) manipulate the effect of endothelium-derived hyperpolarizing factor [24]. These compounds all promote cerebral vascular dilation and can increase CBF. In contrast to E2, T alters the balance of prostanoids by enhancing the production of vasoconstricting thromboxane and attenuating the effect of endothelium-derived hyperpolarizing factor, which promotes cerebral vascular constriction and subsequently decreases CBF [25]. These opposing effects of E2 and T may underlie the CBF differences in young women and young men. In the present study, premenopausal women were tested during mid-cycle when the E2 levels were high. Further studies on the CBF of premenopausal women during different phases of the menstrual cycle are needed to have a better understanding of the effect of E2. Other factors that may contribute to the sex difference in CBF of young subjects include heart rate, blood pressure, and cardiac index, which have all been reported to be higher in young women than in age-matched men [26].
By contrast, there were no differences in CBF between postmenopausal older women and age-matched older men. This may relate to the low sex hormone level in older subjects. Indeed, there was a significant decrease of E2 in older women compared with young women and a decrease in T in older men compared with young men. With such low levels of sex hormones, the cerebral vasodilatory effect of E2 and the vasoconstrictor effect of T might disappear, resulting in similar CBF values in older women and older men. This might also reflect that sex hormones play a dominant role in the sex difference of CBF in young subjects.
Age differences in CBF
The decrease in CBF with age is consistent with previous studies [7, 8, 27, 28]. However, the reported extent of age-related CBF differences varies between studies because of differences in the age and sex of the patients and the measurement methods [7, 29]. In the present study, pCASL was used to measure CBF of young premenopausal women, young men, older postmenopausal women, and older men. The decrease in CBF observed in older subjects (older postmenopausal women and older men) compared with young subjects (young premenopausal women and young men) may relate to deterioration of nervous system and cardiovascular function. Previous studies have shown that aging is accompanied by increasing atrophy of brain tissues [30], progressive loss of cerebral neurons, degeneration of dendritic branches and synaptic connections, and an increase of senile plaques [7]. This age-related deterioration of nervous system function may result in a decreased requirement for cerebral blood supply. Age-related deterioration of cardiovascular function may also contribute to decreased blood supply to organs including the brain. Indeed, previous studies have reported that even without coronary artery disease, aging is still accompanied with a decline of maximal heart rate, cardiac output, and cardiorespiratory fitness, such as aerobic capacity [31].
Although there were no differences in CBF between older women and older men, the change of CBF between older and young women differed from that of men. There was a marked drop in CBF in older women compared with young women, whereas the decrease of CBF in older men compared with young men was not significant. As discussed, E2 can promote cerebrovascular dilation leading to increased CBF. Thus, with decreased E2 levels in older women, the cerebrovascular dilating effect may disappear, thus accentuating the decrease of CBF caused by deterioration of nervous and cerebrovascular function. By contrast, with decreased levels of T in older men, the cerebral vasoconstrictor effect may disappear, thus increasing CBF. This increase in CBF caused by reduced T in older men may attenuate the decrease of CBF caused by deterioration of nervous and cerebrovascular function.
Clinical and experimental implications
In the present study, we used pCASL to measure CBF values of healthy subjects. This provides a basis for use of pCASL in further studies examining abnormal CBF. Awareness of the sex and age differences in normal CBF values may also help to understand the sex- and age-related differences in the prevalence of various nervous system diseases [4]. For example, the higher CBF in young premenopausal women than in young men might reduce the incidence of stroke in young women [3]. Further, stroke rates increase among postmenopausal women compared with age-matched men, which may be related to the marked drop in CBF observed in older postmenopausal women compared with young women. Our findings of normal sex- or age-related CBF changes may also help to identify CBF changes related to various diseases. For example, decreased CBF was reported in patients with AD [9]. However, as CBF can also decrease with normal aging, especially in postmenopausal women, this decrease in CBF should be interpreted carefully to separate disease states from normal aging. Finally, our findings highlight the importance of considering age range and female-to-male ratio in the selection of subjects for studies of the brain in order to eliminate bias.
Limitations
This study has some potential limitations. We used the same PLD for young and older subjects for comparison between the two groups. However, arterial transit time might be different between young and older subjects because of blood velocity differences in the arteries [32]. Although the PLD used in the present study was applicable to both young and older healthy subjects, multi-PLD can provide more accurate CBF assessment. Another limitation is that CBF in young women was measured during the mid-cycle, when the E2 level is high. Assessment of CBF in premenopausal women during different phases of the menstrual cycle might provide a better understanding of the sex hormones effects on CBF.
Conclusion
We found sex- and age-related CBF differences in healthy subjects using pCASL. These differences may be causative in the distinct prevalence and outcomes of various nervous system diseases in subjects with different sex and ages. Thus, selection of subjects for neuroscience studies should consider sample size, as well as age and female-to-male ratio. Further studies with larger sample size are necessary to investigate the effects of sex and age on CBF with pCASL, in order to set a range of normal reference values to differentiate normal changes with disease states. Meanwhile, studies with the consideration of menstrual stage are still needed to have a better understanding of the effect of sex hormones on CBF.
References
Brown AM, Ransom BR (2007) Astrocyte glycogen and brain energy metabolism. Glia 55(12):1263–1271. doi:10.1002/glia.20557
Gibson CL (2013) Cerebral ischemic stroke: is gender important? J Cereb Blood Flow Metab 33(9):1355–1361. doi:10.1038/jcbfm.2013.102
Haast RA, Gustafson DR, Kiliaan AJ (2012) Sex differences in stroke. J Cereb Blood Flow Metab 32(12):2100–2107. doi:10.1038/jcbfm.2012.141
Cosgrove KP, Mazure CM, Staley JK (2007) Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 62(8):847–855. doi:10.1016/j.biopsych.2007.03.001
Parkes LM, Rashid W, Chard DT, Tofts PS (2004) Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med 51(4):736–743. doi:10.1002/mrm.20023
Pagani M, Salmaso D, Jonsson C, Hatherly R, Jacobsson H, Larsson SA, Wägner A (2014) Regional cerebral blood flow as assessed by principal component analysis and 99mTc-HMPAO SPET in healthy subjects at rest: normal distribution and effect of age and gender. Eur J Nucl Med Mol Imaging 29(1):67–75. doi:10.1007/s00259-001-0676-2
Bertsch K, Hagemann D, Hermes M, Walter C, Khan R, Naumann E (2009) Resting cerebral blood flow, attention, and aging. Brain Res 1267:77–88. doi:10.1016/j.brainres.2009.02.053
Schmid Daners M, Knobloch V, Soellinger M, Boesiger P, Seifert B, Guzzella L, Kurtcuoglu V (2012) Age-specific characteristics and coupling of cerebral arterial inflow and cerebrospinal fluid dynamics. PLoS One 7(5):e37502. doi:10.1371/journal.pone.0037502
Austin BP, Nair VA, Meier TB, Xu G, Rowley HA, Carlsson CM, Johnson SC, Prabhakaran V (2011) Effects of hypoperfusion in Alzheimer’s disease. J Alzheimers Dis 26(Suppl 3):123–133. doi:10.3233/jad-2011-0010
Wierenga CE, Hays CC, Zlatar ZZ (2014) Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J Alzheimers Dis 42(Suppl 4):S411–419. doi:10.3233/jad-141467
Jones K, Johnson KA, Becker JA, Spiers PA, Albert MS, Holman BL (1998) Use of singular value decomposition to characterize age and gender differences in SPECT cerebral perfusion. J Nucl Med Off Publ Soc Nucl Med 39(6):965–973
Wintermark M, Sesay M, Barbier E, Borbely K, Dillon WP, Eastwood JD, Glenn TC, Grandin CB, Pedraza S, Soustiel JF, Nariai T, Zaharchuk G, Caille JM, Dousset V, Yonas H (2005) Comparative overview of brain perfusion imaging techniques. Stroke 36(9):e83–99. doi:10.1161/01.STR.0000177884.72657.8b
Sadowski EA, Bennett LK, Chan MR, Wentland AL, Garrett AL, Garrett RW, Djamali A (2007) Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiologe 243(1):148–157. doi:10.1148/radiol.2431062144
Mutsaerts HJ, Steketee RM, Heijtel DF, Kuijer JP, van Osch MJ, Majoie CB, Smits M, Nederveen AJ (2014) Inter-vendor reproducibility of pseudo-continuous arterial spin labeling at 3 Tesla. PLoS One 9(8):e104108. doi:10.1371/journal.pone.0104108
Wang Y, Saykin AJ, Pfeuffer J, Lin C, Mosier KM, Shen L, Kim S, Hutchins GD (2011) Regional reproducibility of pulsed arterial spin labeling perfusion imaging at 3T. Neuroimage 54(2):1188–1195. doi:10.1016/j.neuroimage.2010.08.043
Jiang L, Kim M, Chodkowski B, Donahue MJ, Pekar JJ, Van Zijl PC, Albert M (2010) Reliability and reproducibility of perfusion MRI in cognitively normal subjects. Magn Reson Imaging 28(9):1283–1289. doi:10.1016/j.mri.2010.05.002
Wu B, Lou X, Wu X, Ma L (2014) Intra- and interscanner reliability and reproducibility of 3D whole-brain pseudo-continuous arterial spin-labeling MR perfusion at 3T. J Magn Reson Imaging 39(2):402–409. doi:10.1002/jmri.24175
Williams DS, Detre JA, Leigh JS, Koretsky AP (1992) Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A 89(1):212–216
Steketee RM, Bron EE, Meijboom R, Houston GC, Klein S, Mutsaerts HJ, Mendez Orellana CP, de Jong FJ, van Swieten JC, van der Lugt A, Smits M (2016) Early-stage differentiation between presenile Alzheimer’s disease and frontotemporal dementia using arterial spin labeling MRI. Eur Radiol 26(1):244–253. doi:10.1007/s00330-015-3789-x
Devous MD Sr, Stokely EM, Chehabi HH, Bonte FJ (1986) Normal distribution of regional cerebral blood flow measured by dynamic single-photon emission tomography. J Cereb Blood Flow Metab 6(1):95–104. doi:10.1038/jcbfm.1986.12
Podreka I, Baumgartner C, Suess E, Muller C, Brucke T, Lang W, Holzner F, Steiner M, Deecke L (1989) Quantification of regional cerebral blood flow with IMP-SPECT. Reproducibility and clinical relevance of flow values. Stroke 20(2):183–191
Miller VM, Duckles SP (2008) Vascular actions of estrogens: functional implications. Pharmacol Rev 60(2):210–241. doi:10.1124/pr.107.08002
Krause DN, Duckles SP, Pelligrino DA (2006) Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol 101(4):1252–1261. doi:10.1152/japplphysiol.01095.2005
Orshal JM, Khalil RA (2004) Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physioly 286(2):R233–249. doi:10.1152/ajpregu.00338.2003
Turtzo LC, McCullough LD (2008) Sex differences in stroke. Cerebrovasc Dis 26(5):462–474. doi:10.1159/000155983
Gur RE, Gur RC (1990) Gender differences in regional cerebral blood flow. Schizophr Bull 16(2):247–254
Tarumi T, Ayaz Khan M, Liu J, Tseng BY, Parker R, Riley J, Tinajero C, Zhang R (2014) Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab 34(6):971–978. doi:10.1038/jcbfm.2014.44
Waldstein SR, Lefkowitz DM, Siegel EL, Rosenberger WF, Spencer RJ, Tankard CF, Manukyan Z, Gerber EJ, Katzel L (2010) Reduced cerebral blood flow in older men with higher levels of blood pressure. J Hypertens 28(5):993–998
Van Laere K, Versijpt J, Audenaert K, Koole M, Goethals I, Achten E, Dierckx R (2001) 99mTc-ECD brain perfusion SPET: variability, asymmetry and effects of age and gender in healthy adults. Eur J Nucl Med 28(7):873–887
Andres P, Parmentier FB, Escera C (2006) The effect of age on involuntary capture of attention by irrelevant sounds: a test of the frontal hypothesis of aging. Neuropsychologia 44(12):2564–2568. doi:10.1016/j.neuropsychologia.2006.05.005
Dustman RE, Emmerson R, Shearer D (1994) Physical activity, age, and cognitive-neuropsychological function. J Aging Phys Activ
Grade M, Hernandez Tamames JA, Pizzini FB, Achten E, Golay X, Smits M (2015) A neuroradiologist’s guide to arterial spin labeling MRI in clinical practice. Neuroradiology 57(12):1181–1202. doi:10.1007/s00234-015-1571-z
Acknowledgments
We thank all the subjects for participating in the study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
We declare that all human and animal studies have been approved by the ethics committee of Chinese PLA General Hospital (Registration Number: S2015-014-02) and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, W., Lou, X. & Ma, L. Use of 3D pseudo-continuous arterial spin labeling to characterize sex and age differences in cerebral blood flow. Neuroradiology 58, 943–948 (2016). https://doi.org/10.1007/s00234-016-1713-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1713-y