Abstract
Introduction
CT angiography (CTA) is increasingly used as primary diagnostic tool to replace digital subtraction angiography (DSA) in patients with subarachnoid hemorrhage (SAH). However, 3D rotational angiography (3DRA) has substituted DSA as a reference standard. In this prospective observational study, we compare CTA with 3DRA of all cerebral vessels in a large cohort of patients with SAH.
Methods
Of 179 consecutive patients with SAH admitted between March 2013 and July 2014, 139 underwent 64- to 256-detector row CTA followed by complete cerebral 3DRA within 24 h. In 86 patients (62 %), 3DRA was performed under general anesthesia. Two observers from outside hospitals reviewed CTA data.
Results
In 118 of 139 patients (85 %), 3DRA diagnosed the cause of hemorrhage: 113 ruptured aneurysms, three arterial dissections, one micro-arteriovenous malformation (AVM), and one reversible vasoconstriction syndrome. On CTA, both observers missed all five non-aneurysmal causes of SAH. Sensitivity of CTA in depicting ruptured aneurysms was 0.88–0.91, and accuracy was 0.88–0.92. Of 113 ruptured aneurysms, 28 were ≤3 mm (25 %) and of 95 additional aneurysms, 71 were ≤3 mm (75 %). Sensitivity of depicting aneurysms ≤3 mm was 0.28–0.43. Of 95 additional aneurysms, the two raters missed 65 (68 %) and 58 (61 %). Sensitivity in detection was lower in aneurysms of the internal carotid artery than in other locations.
Conclusion
CTA had some limitations as primary diagnostic tool in patients with SAH. All non-aneurysmal causes for SAH and one in ten ruptured aneurysms were missed. Performance of CTA was poor in aneurysms ≤3 mm. The majority of additional aneurysms were not depicted on CTA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Subarachnoid hemorrhage is a serious disease with high morbidity and mortality. Approximately 80 % of subarachnoid hemorrhages are caused by ruptured intracranial aneurysms [1]. Early endovascular or surgical treatment is advocated to prevent recurrent hemorrhage. The standard of detection and anatomic evaluation of intracranial aneurysms has long been digital subtraction angiography (DSA). With modern multi-detector CT techniques, noninvasive pretreatment assessment of intracranial aneurysms is considered equally diagnostic to DSA by many authors [2]. At some institutions, multi-detector CT angiography (CTA) has replaced DSA in the preoperative evaluations of patients with intracranial aneurysms.
Many previous studies comparing the diagnostic accuracy in depicting intracranial aneurysms of CTA with DSA have been conducted [2]. However, the value of CTA in patients with acute subarachnoid hemorrhage remains controversial because of methodological weaknesses in many studies. For example, most studies excluded CT studies with poor image quality because of patient motion. With modern 3D rotational angiography (3DRA), substantially more aneurysms can be depicted than with conventional 2D DSA but most studies have used 2D DSA as standard reference [3–5]. In most studies, other possible causes of subarachnoid hemorrhage, like arterial dissections, arteriovenous malformations (AVM), moyamoya syndrome, dural fistula, or reversible vasospasm syndrome, are disregarded.
In this study, we evaluate the diagnostic accuracy of CTA in the detection of intracranial aneurysms or other vascular disorders in a large consecutive cohort of patients with acute subarachnoid hemorrhage. In particular, we compare daily practice CTA acquired with three different multi-detector CT scanners with 64-, 128-, and 256-detector rows with 3DRA under optimal conditions, including general anesthesia in intubated and uncooperative patients.
Materials and methods
Patient population
This observational study with prospectively collected data was compliant with institutional privacy policy. The Institutional Review Board gave exempt status for approval and informed consent.
Between March 2013 and July 2014, 179 patients with SAH were admitted. Diagnosis of SAH was established with CT scan or lumbar puncture. Four patients with SAH directly proceeded to angiography without CTA. Of the remaining 175 patients, 139 underwent CTA followed by 3DRA within 24 h. In 36 patients, 3DRA was not performed because of perimesencephalic hemorrhage pattern (n = 15), moribund clinical condition (n = 10), trauma and SAH (n = 9), or doubtful positive liquor puncture (n = 2). There were 99 women (71 %) and 40 men (29 %) with a mean age of 58 ± 12 years. In 86 patients (62 %), 3DRA was performed under general anesthesia. CTA was done under general anesthesia in 1 patient.
CT angiography
CTA was performed on either one of three CT scanners: Philips Brilliance iCT 256-detector row, Philips Ingenuity 128-detector row (Philips Healthcare, Best, The Netherlands), and Siemens SOMATOM Definition AS 64-detector row (Siemens, Erlangen, Germany). Volume CT was routinely performed at 130 mA and 100–120 kVp. Collimation, rotation time, and pitch were optimized for the individual CT scanner according to recommendations of the manufacturer. A 90-mL dose of iodinated contrast medium (iopromide, 270 mg of iodine/mL, Visipaque 270; GE Healthcare, Cork, Ireland) was injected at a rate of 4.0 mL/s into an antecubital vein via a 20-gauge catheter, followed by 40 mL of saline solution. CT scanning was triggered by using a bolus-tracking technique, with the region of interest placed in the aortic arch. Image acquisition started 8 s after the attenuation reached the predefined threshold of 130–150 HU. The scanning time was approximately 5.0–7.0 s. Images were reconstructed with a 0.9- to 1.0-mm section thickness and a 0.45- to 0.5-mm increment with an UB or I26f kernel. Volume CT dose index and dose-length product were 25.3–26.7 mGy and 475–576 mGy · cm, respectively.
3D rotational angiography
Angiography was performed on a biplane angiographic system (Allura Xper FD 20/10, Philips Healthcare, Best, The Netherlands). In 86 uncooperative or intubated patients (62 %), 3DRA was performed under general anesthesia. A single 3D rotational angiographic run was acquired of both internal carotid arteries and one vertebral artery with hand injection of 12–20 mL of contrast material. When the contralateral distal vertebral artery was not visualized, an additional 2D biplane run was performed of this vertebral artery. The tube rotation arc was 240° (one rotation used), with a rotation time of 4.1 s. The images were reconstructed in a 256 × 256 matrix. The rotational angiographic data were transferred to an independent workstation (Integris 3D, Philips Healthcare) for instant generation of 3D reformatted images. When possible, angiography was followed immediately by endovascular treatment under general anesthesia. Patients with aneurysms not suitable for coiling were scheduled for surgery.
Analysis of CT angiography
CTA data were reformatted on an independent 3D workstation (Philips IntelliSpace Portal). Maximum intensity projection, volume rendering, and multiplanar reformatting was performed by three neuroradiologists (WR, JP, and MS with 12, 10, and 12 years of post-processing experience). Source images, post-processed images, and 3D reconstructions with bone removal were transferred to a picture archiving and communication system (PACS) (IDS7, Sectra, Linköping, Sweden). Native CTs and CTA data were collected in a dedicated work list in PACS and were reviewed by two observers from outside hospitals (SR with 4 years experience and MES with 15 years experience). Both observers had all raw data and reconstructions available and could make new reconstructions on the 3D workstation. Both observers were blinded for additional imaging or clinical findings. Aneurysms were allocated according to 18 predefined locations and in four vascular territories: anterior cerebral artery, middle cerebral artery, internal carotid artery, and posterior circulation. Aneurysms were classified as ruptured or as additional unruptured. Other vascular disorders were separately recorded.
Analysis of 3D rotational angiography
3D reformatted images were reviewed on the workstation by the senior author (WR with 26 years experience in neuroradiology) and served as reference standard. Presence, location, and size of aneurysms were recorded in a database. Aneurysms were allocated to one of 18 predefined locations. In patients with multiple aneurysms, the ruptured aneurysm was identified based on bleeding pattern on CT, aneurysm size, and angiographic morphology. Other vascular disorders that might be responsible for the subarachnoid hemorrhage such as arterial dissections, arteriovenous malformations, moyamoya phenomenon, or reversible vasospasm syndrome were separately recorded. In patients without an obvious vascular disorder that might be responsible for the SAH, 3DRA was repeated after 1 week.
Statistical analysis
Quantitative variables were expressed as mean ± standard deviation, and categorical variables were expressed as frequencies or percentages. In this study, 3DRA was considered a diagnostic standard for the evaluation of cerebral aneurysms and other vascular disorders. Sensitivity, specificity, and accuracy of CTA in depicting the cause of SAH and aneurysms (overall, ≤3 and 4 mm and larger) were calculated on per-patient basis. Agreement between the two raters was assessed using kappa statistics (poor agreement, κ = 0; slight agreement, κ = 0.01–0.20; fair agreement, κ = 0.21–0.40; moderate agreement, κ = 0.41–0.60; good agreement, κ = 0.61–0.80; and excellent agreement, κ = 0.81–1.00). Statistical analysis was performed with MedCalc statistical software version 14.12.0 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014).
Results
Results of 3DRA
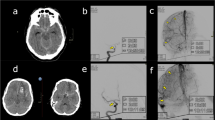
In 118 of 139 patients (85 %), 3DRA diagnosed the cause of hemorrhage in 114 patients at initial 3DRA and in 4 after repeated 3DRA. There were 113 patients with a ruptured aneurysm, 2 patients with a supraclinoid carotid artery dissection, 1 patient with a vertebral dissection, 1 patient with a cervical micro-AVM, and 1 patient with a reversible vasoconstriction syndrome (this patient also had a 3-mm middle cerebral artery aneurysm not considered the cause of hemorrhage). In 25 patients, initial 3DRA was negative. In 9 patients with probable perimesencephalic bleeding pattern, 3DRA was not repeated. In 16 patients with definite aneurysmal bleeding pattern, repeated 3DRA showed a ruptured aneurysm that was not visible on first 3DRA in four: 8-mm supraclinoid dissection aneurysm (Fig. 1), 1-mm distal superior cerebellar artery aneurysm, 2-mm posterior communicating aneurysm, and 1-mm A1 aneurysm (Fig. 2). 3DRA detected 208 aneurysms (113 ruptured, 94 additional, and one unruptured) in 114 patients. Sixty-two patients had one aneurysm (54 %), 24 patients had two aneurysms (21 %), 16 patients had three aneurysms (14 %), 11 patients had four aneurysms (10 %), and 1 patient had five aneurysms (0.9 %). Of 113 ruptured aneurysms, 28 were ≤3 mm (25 %), and of 95 additional aneurysms, 71 were ≤3 mm (75 %).
Diagnostic performance of CTA
Results of CTA of both readers are summarized in Tables 1 and 2.
Results of CTA in detecting the cause of SAH
Of 139 patients with a SAH who underwent 3DRA and CTA, a cause for the SAH was established in 118 patients.
Both raters missed all five non-aneurysmal causes (cervical micro-arteriovenous malformation (AVM), vertebral dissection, two supraclinoid carotid artery dissections, and reversible vasoconstriction syndrome).
Rater 1 missed ten of 113 ruptured aneurysms (9 %): two posterior communicating artery aneurysms (2 and 6 mm), two anterior communicating aneurysms (2 and 3 mm), two superior cerebellar artery aneurysms of 1 mm (Fig. 3) and 2 mm, one posterior inferior cerebellar artery (PICA) aneurysm (2 mm), one A1 aneurysm (1 mm), one pericallosal artery aneurysm (2 mm), and one basilar tip aneurysm (7 mm). Rater 1 diagnosed three false-positive findings: one dural fistula, one arteriovenous malformation, and one middle cerebral artery aneurysm.
Rater 2 missed 14 of 113 ruptured aneurysms (12 %): five anterior communicating artery aneurysms (2, 2, 3, 3, and 6 mm), four posterior communicating aneurysms (2, 2, 5, and 5 mm), two superior cerebellar artery aneurysms (1 and 2 mm), one A1 aneurysm (1 mm), one PICA aneurysm (2 mm), and one pericallosal artery aneurysm (2 mm). Rater 2 diagnosed three false-positive aneurysms: one basilar tip aneurysm, one posterior communicating aneurysm, and one PICA aneurysm.
The kappa coefficient between the two raters in establishing the cause of SAH was 0.83 (95 % CI, 0.73–0.93), indicating excellent agreement.
Results of CTA in aneurysm detection
Diagnostic performance of CTA in aneurysms ≤3 and >3 mm is displayed in Table 1 and diagnostic performance per location in Table 2. Sensitivity and specificity of detecting aneurysms larger than 3 mm was good especially in the anterior and middle cerebral artery territory. Sensitivity in detection was lower in aneurysms of the internal carotid artery than in other locations (Fig. 4). Diagnostic value was low in detecting aneurysms ≤3 mm (Fig. 3). Diagnostic value was also low in detecting additional aneurysms, as the majority of additional aneurysms were ≤3 mm. The kappa coefficient between the two raters in detecting all aneurysms was 0.75 (95 % CI, 0.67–0.83), indicating good agreement, and in detecting additional aneurysms, kappa coefficient was 0.40 (95 % CI, 0.19–0.60), indicating fair agreement.
A 63-year-old woman with a ruptured large middle cerebral artery aneurysm. a CTA demonstrates the large right middle cerebral artery aneurysm; there are no visible additional aneurysms. b 3DRA of the left internal carotid artery reveals a 4-mm additional para-ophthalmic carotid artery aneurysm (arrow), not visible on CTA
Clinical relevance of missed vascular disorders on CTA
Rater 1 missed all five non-aneurysmal causes of SAH and ten ruptured aneurysms. Furthermore, 58 of 95 (61 %) additional aneurysms were missed on CTA. Twelve of 55 missed additional aneurysms in 10 patients were treated either by coiling or clipping. Five additional aneurysms in 4 patients were not treated because these patients died during hospital admission. Thirteen aneurysms in 14 patients are monitored by MR angiography (MRA). Treatment was judged not necessary in 25 aneurysms in 21 patients.
Rater 2 missed all five non-aneurysmal causes of SAH and 14 ruptured aneurysms. Furthermore, 65 of 95 (68 %) additional aneurysms were missed on CTA. Seventeen of 68 missed additional aneurysms in 14 patients were treated either by coiling or clipping. Six additional aneurysms in 4 patients were not treated because these patients died during hospital admission. Twenty aneurysms in 14 patients are monitored by MRA. Treatment was judged not necessary in 28 aneurysms in 22 patients.
Discussion
In this prospective study comparing CTA and 3DRA, we found that diagnostic performance of CTA in a clinical setting of patients with SAH is hampered by limitations. In 139 patients, all five non-aneurysmal causes of SAH and one in ten ruptured aneurysms were missed. Moreover, the majority of aneurysms that were additionally found on 3DRA were missed on CTA. Although positive predictive value of CTA seems acceptable both in very small and larger aneurysms, the exclusion of vascular pathology with CTA is unreliable. Overall, 30–40 % of aneurysms diagnosed on 3DRA were missed on CTA. Most missed aneurysms were ≤3 mm but some missed aneurysms were 6–7 mm large in patients with poor image quality.
Our findings are in contrast to many previous studies. Several meta-analyses of the accuracy of CTA for diagnosing intracranial aneurysms have been published [2, 6–8] with pooled sensitivities and specificities of >95 % in the most recent studies [2, 7]. There are several factors that can explain this discrepancy in sensitivity to detect the ruptured aneurysm in our study and in previous studies. First, in our study, we used 3DRA under optimal clinical circumstances as a reference standard. It has been shown that 3DRA detects substantially more aneurysms than conventional 2D DSA, the reference used in almost all previous studies [3–5]. The complete cerebral vasculature was imaged with 3DRA in all patients. The majority of 3DRAs were performed under general anesthesia thereby eliminating image degradation by patient motion. This resulted in excellent 3D image quality with high aneurysm conspicuity; even 1-mm aneurysms were easily detected (Figs. 2 and 3). For example, in our patient cohort, we found mean 1.8 aneurysm per patient (208 aneurysms in 114 patients) while in a comparable, although retrospective, study by Lu et al. [9], a mean 1.1 aneurysm per patient was found (459 aneurysms in 407 patients), which is substantially lower than in our study. Second, in patients with aneurysmal SAH and initial 3DRA negative findings, 3DRA was repeated 1 week later. Third, CTA was performed 24/7 in a daily clinical setting on three scanners with 64-, 128-, and 256-detector rows. Some CTA studies had poor image quality because of patient motion or suboptimal contrast bolus timing, and these studies were not excluded. This explains why both observers missed some larger aneurysms. Our findings suggest that previous data in general overestimate the diagnostic performance of CTA in patients with SAH. Next to publication bias [2], methodology in many studies favors good results of CTA, for example, by using suboptimal reference standards such as 2D DSA and operative findings [14, 15], exclusion of poor quality CTAs [6], exclusion of previously coiled or clipped patients [6], retrospective study design [14, 16], selection of patients with aneurysms only [17], only performing angiography in patients with negative or inconclusive CTA [15], or comparing DSA and CTA in CT-negative but CSF-positive patients only [18]
Performance of CTA in our study was poor in detecting non-aneurysmal causes of SAH: both observers missed one cervical micro-AVM, one reversible vasospasm syndrome, and three arterial dissections. Little is known in literature about sensitivity of CTA in arterial dissections since most studies of CTA in patients with SAH excluded other causes than aneurysms. In one study, CTA missed most carotid artery dissections causing SAH [10]. There are no data about CTA and SAH from vertebral artery dissections.
CTA was also poor in depicting aneurysms of ≤3 mm. In our patient cohort, a quarter (28 of 113) of ruptured aneurysms was ≤3 mm. Most additional aneurysms were ≤3 mm, and most of these additional aneurysms were missed on CTA (Fig. 4). Previous studies also found low sensitivities for detecting small aneurysms on CTA [11, 12], especially, as in our study, aneurysms located on the internal carotid artery [13]. This poor performance of CTA in small aneurysms makes CTA less suitable as a primary diagnostic tool in patients with SAH. Detecting all aneurysms that are present is important in clinical practice. Additional aneurysms can be treated at the same time as the ruptured aneurysm, either by a surgical or endovascular approach. Given the morbidity and costs associated with cerebral aneurysm treatment, accurate detection of all aneurysms before making a treatment decision is essential. Many missed additional aneurysms on CTA in our study were actually treated with clipping or coiling, either in the same session as the ruptured aneurysm or later after evaluation in a multidisciplinary team of neurosurgeons, neuroradiologists, and neurologists.
Our data indicate that CTA is not suitable to replace 3DRA in the diagnostic work-up of patients with SAH. Too many aneurysms remain undetected, and other causes of SAH besides aneurysms are generally missed. Ruling out vascular pathology with CTA is impossible. At best, CTA can be useful to obtain a quick general impression of the vascular pathology, for instance, in patients who need emergency evacuation of a cerebral hematoma before angiography can be performed. When CTA identifies the ruptured aneurysm, 3DRA may be limited to the vessel harboring the aneurysm in selected patients for example very old patients in poor clinical condition. The use of CTA is even questionable in patients with SAH and a low a priori chance of having an aneurysm such as patients with trauma and SAH and patients with a perimesencephalic bleeding pattern. In these patients, CTA as a sole diagnostic tool may provide a false sense of security.
We agree with several of our colleagues who question the role of CTA in patients with SAH because both in patients with negative and positive CTA, catheter angiography still needs to be performed [19–21]. Although CTA is considered a noninvasive investigation, the extra load of contrast material can have a negative effect on renal function. In patients with SAH, CTA should be interpreted with restraint and 3DRA of at least the one cerebral vessel with the ruptured aneurysm should follow in all patients. Exclusion of vascular pathology with CTA is impossible.
References
Byyny RL, Mower WR, Shum N et al (2008) Sensitivity of noncontrast cranial computed tomography for the emergency department diagnosis of subarachnoid hemorrhage. Ann Emerg Med 51:697–703
Westerlaan HE, van Dijk JM, Jansen-van der Weide MC et al (2011) Intracranial aneurysms in patients with subarachnoid hemorrhage: CT angiography as a primary examination tool for diagnosis-systematic review and meta-analysis. Radiology 258:134–145
van Rooij WJ, Sprengers ME, de Gast AN et al (2008) 3D rotational angiography: the new gold standard in the detection of additional intracranial aneurysms. AJNR Am J Neuroradiol 29:976–979
van Rooij WJ, Peluso JP, Sluzewski M et al (2008) Additional value of 3D rotational angiography in angiographically negative aneurysmal subarachnoid hemorrhage: how negative is negative? AJNR Am J Neuroradiol 29:962–966
Ishihara H, Kato S, Akimura T et al (2007) Angiogram-negative subarachnoid hemorrhage in the era of three dimensional rotational angiography. J Clin Neurosci 14:252–255
Chappell ET, Moure FC, Good MC (2003) Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: a meta-analysis. Neurosurgery 52:624–631
Menke J, Larsen J, Kallenberg K (2011) Diagnosing cerebral aneurysms by computed tomographic angiography: meta-analysis. Ann Neurol 69:646–654
van Gelder JM (2003) Computed tomographic angiography for detecting cerebral aneurysms: implications of aneurysm size distribution for the sensitivity, specificity, and likelihood ratios. Neurosurgery 53:597–605
Lu L, Zhang LJ, Poon CS et al (2012) Digital subtraction CT angiography for detection of intracranial aneurysms: comparison with three-dimensional digital subtraction angiography. Radiology 262:605–612
Gonzalez AM, Narata AP, Yilmaz H et al (2014) Blood blister-like aneurysms: single center experience and systematic literature review. Eur J Radiol 83:197–205
Dammert S, Krings T, Moller-Hartmann W et al (2004) Detection of intracranial aneurysms with multi-slice CT: comparison with conventional angiography. Neuroradiology 46:427–434
White PM, Wardlaw JM, Easton V (2000) Can non-invasive imaging accurately depict intracranial aneurysms? A systematic review. Radiology 217:361–370
Romijn M, Gratama van Andel HA, van Walderveen MA et al (2008) Diagnostic accuracy of CT angiography with matched mask bone elimination for detection of intracranial aneurysms: comparison with digital subtraction angiography and 3D rotational angiography. Am J Neuroradiol 29:134–139
Nijjar S, Patel B, McGinn G et al (2007) Computed tomographic angiography as the primary diagnostic study in spontaneous subarachnoid hemorrhage. J Neuroimaging 17:295–299
Westerlaan HE, Gravendeel J, Fiore D et al (2007) Multislice CT angiography in the selection of patients with ruptured intracranial aneurysms suitable for clipping or coiling. Neuroradiology 49:997–1007
Colen TW, Wang LC, Ghodke BV et al (2007) Effectiveness of MDCT angiography for the detection of intracranial aneurysms in patients with nontraumatic subarachnoid hemorrhage. AJR Am J Roentgenol 189:898–903
Zhang LJ, Wu SY, Niu JB et al (2010) Dual-energy CT angiography in the evaluation of intracranial aneurysms: image quality, radiation dose, and comparison with 3D rotational digital subtraction angiography. AJR Am J Roentgenol 194:23–30
Lim LK, Dowling RJ, Yan B, Mitchell PJ (2014) Can CT angiography rule out aneurysmal subarachnoid haemorrhage in CT scan-negative subarachnoid haemorrhage patients? J Clin Neurosci 21:191–193
Kallmes DF, Layton K, Marx WF et al (2007) Death by nondiagnosis: why emergent CT angiography should not be done for patients with subarachnoid hemorrhage. AJNR Am J Neuroradiol 28:1837–1838
Pradilla G, Wicks RT, Hadelsberg U et al (2013) Accuracy of computed tomography angiography in the diagnosis of intracranial aneurysms. World Neurosurg 80:845–852
Moran CJ (2011) Aneurysmal subarachnoid hemorrhage: DSA versus CT angiography—is the answer available? Radiology 258:15–17
Ethical standards and patient consent
We declare that all human and animal studies have been approved by the Institutional Review Board and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study
Conflict of interest
We declare we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bechan, R.S., van Rooij, S.B., Sprengers, M.E. et al. CT angiography versus 3D rotational angiography in patients with subarachnoid hemorrhage. Neuroradiology 57, 1239–1246 (2015). https://doi.org/10.1007/s00234-015-1590-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1590-9