Abstract
Introduction
Thromboembolic complications are the most frequent complications of endovascular treatment of ruptured intracranial aneurysms. The optimal protocol to prevent thromboembolic complications during coil embolization does not yet exist. The aim of this study was to investigate the effectiveness and safety of eptifibatide for the prevention of thromboembolic complications during elective coil embolization of ruptured cerebral aneurysms.
Methods
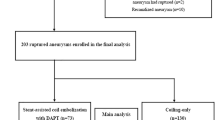
A consecutive series of 100 patients (group 1) with ruptured intracranial aneurysm were treated using endovascular coil embolization. At the beginning of the procedure, all patients received an intra-arterial bolus (0.2 mg/kg) of eptifibatide. The following data were collected: degree of aneurysmal occlusion after treatment, thromboembolic and hemorrhagic complications and other intraoperative adverse events. The results were compared with those from a control group (group 2) which were analyzed retrospectively. Group 2 consisted of 100 previous patients with ruptured aneurysm managed with coil embolization who had received heparin and/or aspirin at the beginning of the procedure.
Results
(1) Patient populations in groups 1 and 2 were considered statistically comparable, except that group 1 (eptifibatide) included more wide-necked aneurysms (p = 0.011). (2) There were less thromboembolic complications in group 1 (p = 0.011): seven intraoperative complications in group 1 versus 20 in group 2. (3) Intraoperative hemorrhagic complications were statistically comparable in both groups (p = 1).
Conclusion
Eptifibatide was effective in lowering the intraoperative thromboembolic complication rate in ruptured aneurysms treated with coil embolization and did not increase the hemorrhagic risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At present, there is no consensus on an optimal protocol to prevent thromboembolic complications during coil embolization of ruptured intracranial aneurysms. Despite IV administration of heparin and/or aspirin intraoperatively, they still remain the most frequent complications of coiling procedures. Considering the increasing complexity of endovascular procedures, the increasing number of high-risk patients (i.e., elderly patients, large aneurysms, fusiform, or wide-necked aneurysms) and the fact that these procedures sometimes require the use of devices suspected of promoting thrombosis (e.g., stents), it is high time that an effective and safe prophylactic therapy protocol should be established.
According to the literature, the use of glycoprotein IIb/IIIa inhibitors (GPIs) is a safe and effective rescue therapy [1, 2] for thromboembolic complications occurring during endovascular procedures. This is why we decided to use eptifibatide prophylactically in patients treated for ruptured cerebral aneurysm with endovascular coiling. A consecutive series of 100 patients with ruptured intracranial aneurysm who had an emergency endovascular treatment with intraoperative eptifibatide were included in a prospective study with the approval of the ethics committee of the institution. All intraoperative and perioperative bleeding and ischemic complications were recorded. The results were compared with those achieved in a control group consisting of 100 previous patients with ruptured aneurysm who had endovascular treatment.
Patients and methods
Study design
The study compares two groups of consecutive patients with ruptured aneurysms treated with coil embolization. One group received an intra-arterial bolus (0.2 mg/kg) of eptifibatide at the beginning of the procedure. The other group did not receive eptifibatide but heparin and/or aspirin. This study was designed, conducted, analyzed, and written independently of industry or any other financial support.
Group 1—population and procedures (Tables 1 and 2)
Between January 2012 and August 2013, 100 patients with subarachnoid hemorrhage due to a ruptured aneurysm were treated using coil embolization. These patients will be referred to as group 1 later on in our study. All of them received an intra-arterial bolus (0.2 mg/kg) of eptifibatide at the beginning of the procedure.
Group 1 consisted of 39 male and 61 female patients.
Age ranged from 27 to 85 years (mean, 53 years; median, 55 years). Forty, out of these 100 patients, were smokers. Associated comorbidities included chronic arterial hypertension (22), obesity, and overweight (9). At the time of the hemorrhagic event, seven patients were on aspirin and two on coumadin for atrial fibrillation. This had no impact on the eptifibatide protocol.
Patients were classified according to the World Federation of Neurosurgeons (WFNS) classification [3] and Fisher’s classification (FC) [4] which uses computerized tomography scans. Forty-four patients were WFNS 1, 25 WFNS 2, four WFNS 3, 13 WFNS 4, and 14 WFNS 5. Five patients were rated Fisher I, 26 Fisher II, 20 Fisher III, and 49 Fisher IV. In 27 patients (27 %), subarachnoid hemorrhage was associated with a cerebral hematoma.
Ninety-six of the 100 emergency patients had one aneurysm, and four had two aneurysms. Ninety-three aneurysms were located in the anterior part of the circle of Willis and 11 in the posterior circulation. The aneurysm size ranged from 1.5 mm to 30 mm: maximal diameter was less than 10 mm in 79 aneurysms and more than 10 mm in 25. Neck width ranged from 1.5 mm to 10 mm; 55 aneurysms had a broad neck (dome-to-neck ratio <1.5 and/or neck ≥4 mm); and 49 a small neck. Nineteen patients underwent simple endovascular coiling, 73 a balloon remodeling procedure, and 12 endovascular coiling using stent assistance. In the latter cases, 11 stents were required in a broad neck and one as a rescue treatment.
In all patients, even those managed with simple coiling, a remodeling balloon was routinely placed in the parent artery, ready to be immediately inflated if aneurysm bleeding occurs.
All 100 patients were given the intra-arterial bolus of eptifibatide after placement of the introducers. Three patients who underwent a procedure longer than 5 h were given a second bolus (0.2 mg/kg). The 12 patients in whom a stent was used were loaded intraoperatively with 600 mg of clopidogrel using a gastric tube, after the stent was deployed.
External ventricular drainage was used in 32 patients: before coil embolization in 23 patients, within 4 h of eptifibatide infusion in three, and during hospital stay in six.
After the procedure, the 12 stented patients were placed on 75 mg/day clopidogrel and 75 mg/day aspirin (started the next day after the procedure). Among the other patients, 83 were given 75 mg of aspirin once daily, three did not receive any antiplatelet agent at all, and one was given heparin intraoperatively.
All the patients had a CT scan of the head 24 h post-surgery.
Group 2—population and procedures (Tables 1 and 2)
Group 2 consisted of 100 consecutive patients with subarachnoid hemorrhage due to a ruptured aneurysm who were treated using coil embolization before January 2012. In 98 patients, an IV bolus of heparin (5000 units) and aspirin (260 mg) was given after placement of the introducers. Two patients were given a bolus of heparin alone. As in group 1, the five stented patients were loaded intraoperatively with 600 mg of plavix using a gastric tube, after the stent was deployed.
Group 2 consisted of 36 male and 64 female patients with age ranging from 17 to 79 years (mean, 53 years; median, 52 years). Forty-four, out of the 100 patients, were smokers. The most commonly associated comorbidity was chronic arterial hypertension (19). At the time of the hemorrhagic event, seven patients were on aspirin and one on coumadin for a history of pulmonary embolism.
Fifty-nine patients were WFNS 1, 13 WFNS 2, five WFNS 3, 14 WFNS 4, and nine WFNS 5. Three patients were rated Fisher I, 32 Fisher II, 16 Fisher III, and 49 Fisher IV (19 of whom had a cerebral hematoma).
In group 2, emergency procedures were performed in 103 aneurysms (three patients had two intracranial aneurysms that were potentially responsible for the hemorrhagic event).
Ninety-seven aneurysms were located in the anterior part of the circle of Willis and six in the posterior circulation. The aneurysm size ranged from 1.5 mm to 45 mm: maximal diameter was less than 10 mm in 87 and more than 10 mm in 16. Neck width ranged from 1 mm to 8 mm; 36 aneurysms had a broad neck (dome-to-neck ratio <1.5 and/or neck ≥4 mm), and 67 had a small neck.
Twenty-seven patients underwent simple endovascular coiling, five endovascular coiling using stent assistance (two stents were used as a rescue treatment), and 71 a balloon remodeling procedure.
External ventricular drainage was used in 17 patients: before coil embolization in 10 patients, immediately after the procedure in two and during hospital stay in five.
After the procedure, eight patients were placed on a 75-mg/day clopidogrel and 75-mg/day aspirin, 44 patients were given 75 mg of aspirin once daily, and 48 did not receive any antiplatelet agent at all.
All patients had a CT scan of the head during their hospital stay.
All patients in both groups were operated on by the same two senior surgeons. Endovascular treatment in both groups was performed within 48 h after diagnosis.
Analyzed parameters
The following parameters were analyzed in both groups:
-
Intraoperative thromboembolic complications—these complications were assessed using angiograms obtained during the procedure or at the end of the procedure.
Angiographic findings were classified as follows: (1) type I: partly occlusive thrombus at the coil-parent artery junction or non-occlusive in-stent thrombus; (2) type II: complete occlusion of a proximal arterial trunk (i.e. internal carotid artery (ICA), anterior communicating artery (AComA), or middle cerebral artery (MCA)) or in-stent thrombosis; and (3) type III: occlusion of distal arteries.
Thromboembolic complications were also categorized into two groups: those requiring rescue therapy (mechanical or chemical thrombectomy) and those which did not require rescue therapy: small-size thrombus, non-occlusive thrombus remaining stable over time, and/or thrombus not involving an eloquent region of the brain.
-
Cerebral and extracerebral hemorrhage after endovascular treatment—intraoperative intracranial hemorrhage was evidenced either by contrast extravasation on intraoperative angiography or by worsening of the subarachnoid hemorrhage on the postoperative CT scan.
-
Other intraoperative complications related to the procedure or occurring during the procedure (e.g. coil migration…).
-
Rescue treatments for complications.
-
Post-procedure aneurysm occlusion rate was assessed using the modified Raymond classification [5].
CORELAB
Post-treatment CT scans were obtained for each patient and compared by two vascular neurologists with the initial preoperative scans. The aim was to evaluate the intracranial bleeding status.
Both the absence or occurrence of intraoperative thromboembolic complications and the type of complications were validated by a non-interventional neuroradiologist and a neurovascular surgeon who reviewed and analyzed the angiograms obtained during the embolization procedure.
Statistics
The initial comparison of the two groups used univariate analyses. The qualitative variables were compared using the Pearson Chi-square test (or non-parametric Fisher’s exact test), the quantitative variables were compared using Student’s t test (or non-parametric Wilcoxon test).
Thromboembolic and hemorrhagic complication risk factors were assessed using univariate analyses.
All statistical analyses were performed by using the R3.0.2 software (Copyright (C) 2013. The R Foundation for Statistical Computing, Vienna, Austria).
Results
Initial comparability of groups 1 and 2 (Tables 1 and 2)
The vast majority of initial factors analyzed in the two groups did not show any significant difference. Nevertheless, there were more wide-necked aneurysms (p = 0.011), and more patients requiring ventriculostomy (p = 0.02) in group 1. There were more WFNS 2 patients in group 1 (p = 0.046) and more WFNS 1 patients in group 2 (p = 0.047).
Intraoperative thromboembolic complications were less frequent in group 1 (p = 0.011) (Table 3)
There were seven thromboembolic complications in group 1 (eptifibatide). Four of them were related to small-size thrombi located distally in a non-eloquent region of the brain and did not require rescue therapy. Three were related to non-occlusive in-stent thrombi in two cases and to occlusion of a middle cerebral artery trunk in one case. In all three cases, an additional bolus of eptifibatide was given; in one of them, it was associated with mechanical thrombectomy. It should be pointed out that three of these seven complications occurred during placement of a stent (altogether, 12 stents were used).
Twenty thromboembolic complications occurred in group 2: eight occlusions of a proximal trunk, eight distal occlusions, and fur thrombi formed at the coil-parent artery junction. Two complications occurred intraoperatively during placement of a stent (two in five deployed stents).
In 19 out of the 20 complications, rescue treatments were required consisting of in situ infusion of a bolus of eptifibatide (0.2 mg/kg), plus mechanical thrombectomy in four patients.
Intra- and extracranial hemorrhagic complications were comparable in both groups (p = 1) (Table 3)
In group 1, four patients had intraoperative intracranial hemorrhage, which was evidenced by contrast extravasation on intraoperative angiography. Bleeding was not spontaneous; it occurred during coiling in three cases and resulted from mechanical perforation by the microcatheter in one case. In all the patients, inflation of the remodeling balloon and placement of a coil stopped the hemorrhage. However, in one patient, bleeding caused the development of a cerebral hematoma, which led to patient’s death. In the other three patients, the initial clinical condition remained unchanged.
In addition to these cerebral hemorrhagic complications, three patients had groin hematoma with false arterial aneurysm. No patient had retroperitoneal, gastrointestinal, or genitourinary hemorrhage. The biological tests performed during hospital stay did not reveal thrombocythemia.
In group 2, five patients had intraoperative bleeding. As in group 1, rescue treatments consisted of inflation of the remodeling balloon and immediate coiling. One patient died from intraoperative hemorrhage. In a further patient, the use of the balloon allowed to stop bleeding but caused distal occlusion of an artery. In the other three patients, intraoperative bleeding was clinically inconsequential.
Two patients, in this group, had groin hematoma with pseudoaneurysm of artery.
Other intraoperative events were very similar in both groups (p = 1) (Table 3)
Intraoperative events in the eptifibatide group included severe anaphylaxis reaction to anesthetic agents (1), acute neurogenic pulmonary edema (1), mild dissection of the cervical carotid artery (1), coil migration (1), coil fracture (1), coil herniation (2, one of which resulted in a thromboembolic complication).
Intraoperative events in group 2 included acute neurogenic pulmonary edema (1), coil herniation (3), and coil migration (2). One of the three herniations and one of the two coil migrations resulted in thromboembolic complications.
Aneurysmal occlusion
Aneurysmal occlusion rates were identical in both groups. Overall, there were 26 Raymond grade 2 aneurysms and 77 Raymond grade 1. In group 1 which involved 104 aneurysms (versus 103 in the control group), one patient was rated Raymond grade 3 after treatment.
Discussion
Thromboembolic complications are the most frequent complications of endovascular treatment of intracranial aneurysms. [6–8]. The rate of thromboembolic events is heterogeneously reported in the series because of the different methods of detection used: at clinical examination, at angiography, or at diffusion-weighted MR imaging. The reported rate of thromboembolic complications usually ranges from 4.7 [8, 9] to 12.5 % [10]. Actually, if one considered the silent ischemic injuries which would be detected postoperatively in more than 60 % of the patients on diffusion-weighted MR images, this rate would likely be much higher [11, 12]. Intraoperative thromboembolic complications may have extremely severe clinical consequences. Recently, in a large series, they have been found to be associated with 3.8 % permanent morbidity and mortality [10]. According to Park et al. [13], in established thromboembolic events, the mortality and permanent morbidity rate could even exceed 20 and 40 %, respectively. Using a large administrative database, Brinjiski et al. [1] have demonstrated an intraoperative rescue therapy rate of approximately 7–8 % in endovascular coiling procedures.
The risk of intraoperative thromboembolic complications is highly variable according to patients and aneurysms. Smoking, diabetes [14, 15], age exceeding 65 years [16], maximal aneurysm diameter >10 mm, broad-necked aneurysm [10], and subarachnoid hemorrhage [17–19] (due to activation of the coagulation system) are all risk factors.
It is also related to the technique used. In recent series [20], remodeling balloons did not seem to be associated with an increase in thromboembolic events. In contrast, emergency stenting was associated with a higher risk of intraoperative complications [21–23] including in-stent thrombosis, hemorrhagic complications which, according to Adamanta et al., would be attributable to the combined use of clopidogrel and aspirin during the procedure [24].
Chalouhi [25] and Yi et al. [26] already used GPI prophylaxis (IIb/IIIa inhibitors) intraoperatively: Chalouhi when coiling and stenting were associated in the same procedure, and Yi et al. during endovascular coil embolization of ruptured and non-ruptured aneurysms. But the reported outcomes are contradictory: Yi et al. [26] who gave a bolus of eptifibatide to their patients stated that the use of IIb/IIIa inhibitors was most hazardous, whereas Chalouhi et al. [25] claimed that the use of tirofibran had not been associated with morbidity or mortality.
Our study is the first prospective study about the effects of GPI prophylaxis in endovascular treatment of ruptured aneurysms. We had several good reasons to conduct this study:
-
The absence of consensus on an optimal protocol to prevent thromboembolic complications during coil embolization of ruptured intracranial aneurysms
-
Good results of the prophylactic use of antiplatelet agents such as aspirin and clopidogrel in endovascular treatment of non-ruptured aneurysms [27, 28]
-
The major role played by platelet aggregation in the development of thromboembolic complications and the fact that GP IIb/IIIa are typically receptor antagonists [29, 30]
-
Effectiveness of IIb/IIIa inhibitors in the treatment of thromboembolic complications occurring during interventional neurovascular procedures [1], with complete arterial recanalization in between 40 % [31] and 96 % [32] of the patients
-
Low hemorrhagic risk during infusion of IIb/IIIa inhibitors in these rescue therapies [1, 2]
Eptifibatide is a selective and competitive GP IIb/IIIa receptor antagonist. Our deliberate choice of eptifibatide for our study owes to its fast action following infusion. Eptifibatide has a lower affinity for the GP IIb/IIIa receptor as compared to abciximab. Furthermore, it shows rapid reversal of effects (4 h) after infusion [33], which allows to perform more invasive procedures (e.g. ventriculostomy) after endovascular treatment.
In our study, eptifibatide significantly decreased the rate of thromboembolic complications. And yet, there were more wide-necked aneurysms in the eptifibatide group in which the best results were achieved. Management of these complications was also very different in the two groups. In group 1, only three out of seven thromboembolic events required rescue therapy and four events were considered potentially inconsequential. In group 2, 19 out of 20 thromboembolic events were judged severe enough to justify an attempt at recanalization of the parent artery. This suggests that thromboembolic events were less severe in patients who received eptifibatide, which is confirmed by the analysis of the types of thromboembolic events: a smaller proportion of group 1 patients had complete occlusion of a proximal artery (Table 3; p = 0.034).
In our study, the prophylactic infusion of eptifibatide did not increase the incidence or the severity of intraoperative intracranial hemorrhage. None of the four aneurysmal perforations in group 1 occurred spontaneously after infusion of eptifibatide. No bleeding complication was observed in group 1 after ventriculostomy, even for those for which external ventricular drainage was performed within 4 h after injection of eptifibatide (three patients). Despite these results, it makes sense to preferentially perform ventriculostomy, prior to the endovascular procedure, or more than 4 h after the eptifibatide bolus. Similarly, extracerebral hemorrhagic complications were not statistically more significant in the eptifibatide group.
Obviously, there are several limitations to our study: study population combines both retrospective and prospective data, there are no short- and long-term clinical evaluations, analysis of thromboembolic events is only based on intraoperative angiography findings, and there is no postoperative MRI evaluation. Additional studies are needed to confirm these results to define what GPI to use and what is the best way to administer it. Confirmation of our results would thus provide a preventive treatment protocol that could be applied to endovascular treatment of intracranial aneurysms ruptured, and perhaps to the other brain endovascular procedures.
Conclusion
Prophylactic intraoperative use of eptifibatide in the endovascular treatment of ruptured aneurysms allows to significantly decrease the rate of thromboembolic complications associated with this type of procedure and does not, in any way, increases the rate of intraoperative hemorrhagic complications. Should further studies confirm these successful outcomes, a standard treatment protocol could be developed and guidelines could be established based on current practices.
References
Brinjikji W, McDonald JS, Kallmes DF et al (2013) Rescue treatment of thromboembolic complications during endovascular treatment of cerebral aneurysms. Stroke 44:1343–1347
Sedat J, Chau Y, Mondot L et al (2014) Is eptifibatide a safe and effective rescue therapy in thromboembolic events complicating cerebral aneurysm coil embolization? Single-center experience in 42 cases and review of the literature. Neuroradiology 56:145–153
Teasdale GM, Drake CG, Hunt W et al (1988) A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry 51:1457
Fisher CM, Kistler JP, Davis JM (1980) Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computed tomographic scanning. Neurosurgery 6:1–9
Raymond J, Guibert F, Weill A et al (2003) Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 33:1398–1403
Brilstra EH, Rinkel GJ, van der Graaf Y et al (1999) Treatment of intracranial aneurysms with coils: a systematic review. Stroke 30:470–476
Lanterna LA, Tredici G, Dimitrov BD et al (2004) Treatment of unruptured cerebral aneurysms by embolization with guglielmi detachable coils: case-fatality, morbidity, and effectiveness in preventing bleeding—a systematic review of the literature. Neurosurgery 55:767–775
van Rooij WJ, Sluzewski M, Beute GN et al (2006) Procedural complications of coiling of ruptured intracranial aneurysms: incidence and risk factors in a consecutive series of 681 patients. AJNR Am J Neuroradiol 27:1498–1501
Henkes H, Fischer S, Weber W et al (2004) Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery 54:268–280
Pierot L, Cognard C, Anxionnat R et al (2010) CLARITY investigators. Ruptured intracranial aneurysms: factors affecting the rate and outcome of endovascular treatment complications in a series of 782 patients (CLARITY study). Radiology 256:916–923
Rordorf G, Bellon RJ, Budzik RE Jr et al (2001) Silent thromboembolic events associated with the treatment of unruptured cerebral aneurysms by use of Guglielmi detachable coils: prospective study applying diffusion-weighted imaging. AJNR Am J Neuroradiol 22:5–10
Soeda A, Sakai N, Murao K et al (2003) Thromboembolic events associated with Guglielmi detachable coil embolization with use of diffusion-weighted MR imaging. Part II. Detection of the microemboli proximal to cerebral aneurysm. AJNR Am J Neuroradiol 24:2035–2038
Park HK, Horowitz M, Jungreis C et al (2005) Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol 26:506–514
Dion JE, Gates PC, Fox AJ et al (1987) Clinical events following neuroangiography: a prospective study. Stroke 18:997–1004
Earnest F IV, Forbes G, Sandok BA et al (1984) Complications of cerebral angiography: prospective assessment of risk. AJR Am J Roentgenol 142:247–253
Cai Y, Spelle L, Wang H et al (2005) Endovascular treatment of intracranial aneurysms in the elderly: single-center experience in 63 consecutive patients. Neurosurgery 57:1096–1102
Vermeulen M, van Vliet HH, Lindsay KW et al (1985) Source of fibrin/fibrinogen degradation products in the CSF after subarachnoid hemorrhage. J Neurosurg 63:573–577
Kasuya H, Shimizu T, Okada T et al (1988) Activation of the coagulation system in the subarachnoid space after subarachnoid hemorrhage: serial measurement of fibrinopeptide A and bradykinin of cerebrospinal fluid and plasma in patients with subarachnoid hemorrhage. Acta Neurochir (Wien) 91:120–125
Ikeda K, Asakura H, Futami K et al (1997) Coagulative and fibrinolytic activation in cerebrospinal fluid and plasma after subarachnoid hemorrhage. Neurosurgery 41:344–350
Pierot L, Cognard L, Spelle L et al (2012) Safety and efficacy of balloon remodelling technique during endovascular treatment of intracranial aneurysms: critical review of the literature. Am J Neuroradiol AJNR 33:12–15
Tahtinen OI, Vanninen RL, Manninen HI et al (2009) Wide-necked intracranial aneurysms: treatment with stent-assisted coil embolization during acute (<72 hours) subarachnoid hemorrhage—experience in 61 consecutive patients. Radiology 253:199–208
Wakhloo AK, Linfante I, Silva CF et al (2012) Closed-cell stent for coil embolization of intracranial aneurysms: clinical and angiographic results. AJNR Am J Neuroradiol 33:1651–1656
Chalouhi N, Jabbour P, Singhal S et al (2013) Stent-assisted coiling of intracranial aneurysms predictors of complications, recanalization, and outcome in 508 cases. Stroke 44:1348–1353
Amenta PS, Dalyai RT, Kung D et al (2012) Stent-assisted coiling of wide-necked aneurysms in the setting of acute subarachnoid hemorrhage: experience in 65 patients. Neurosurgery 70:1415–1429
Chalouhi N, Jabbour P, Kung D et al (2012) Safety and efficacy of tirofibran in stent-assisted coil embolization of intracranial aneurysms. Neurosurgery 71:710–714
Yi HJ, Gupta R, Jovin TG et al (2006) Initial experience with the use of intravenous eptifibatide bolus during endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol 27:1856–1860
Yamada NK, Cross DT 3rd, Pilgram TK et al (2007) Effect of antiplatelet therapy on thromboembolic complications of elective coil embolization of cerebral aneurysms. AJNR Am J Neuroradiol 28:1778–1782
Hwang G, Jung C, Park SQ et al (2010) Thromboembolic complications of elective coil embolization of unruptured aneurysms: the effect of oral antiplatelet preparation on periprocedural thromboembolic complication. Neurosurgery 67:743–748
Qureshi A, Luft A, Sharma M et al (2000) Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures: part I—pathophysiological and pharmacological features. Neurosurgery 46:1344–1359
Coller BS (1997) GPIIb/IIIa antagonists: pathophysiologic and therapeutic insights from studies of c7E3 Fab. Thromb Haemost 78:730–735
Jeon JS, Sheen SH, Hwang G et al (2012) Intraarterial tirofiban thrombolysis for thromboembolisms during coil embolization for ruptured intracranial aneurysms. J Cerebrovasc Endovasc Neurosurg 14:5–10
Kang HS, Kwon BJ, Roh HG et al (2008) Intra-arterial tirofiban infusion for thromboembolism during endovascular treatment of intracranial aneurysms. Neurosurgery 63:230–238
Altenburg A, Haage P (2012) Antiplatelet and anticoagulant drugs in interventional radiology. Cardiovasc Intervent Radiol 35:30–42
Ethical standards and patient consent
We declare that all human and animal studies have been approved by the Ethics Committee of the Nice University Hospital and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sedat, J., Chau, Y., Gaudard, J. et al. Administration of eptifibatide during endovascular treatment of ruptured cerebral aneurysms reduces the rate of thromboembolic events. Neuroradiology 57, 197–203 (2015). https://doi.org/10.1007/s00234-014-1452-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-014-1452-x