Abstract
Introduction
Parry–Romberg syndrome (PRS) and en coup de sabre (ECS) are variants of morphea. Although numerous findings on central nervous system (CNS) imaging of PRS and ECS have been reported, the spectrum and frequency of CNS imaging findings and relation to cutaneous and neurologic abnormalities have not been fully characterized.
Methods
We retrospectively reviewed patients younger than 50 years at our institution over a 16-year interval who had clinical diagnosis of PRS and ECS by a skin or facial subspecialist. Two neuroradiologists evaluated available imaging and characterized CNS imaging findings.
Results
Eighty-eight patients with PRS or ECS were identified (62 women [70.4 %]; mean age 28.8 years). Of the 43 patients with CNS imaging, 19 (44 %) had abnormal findings. The only finding in 1 of these 19 patients was lateral ventricle asymmetry; of the other 18, findings were bilateral in 11 (61 %), ipsilateral to the side of facial involvement in 6 (33 %), and contralateral in 1 (6 %). Sixteen patients had serial imaging examinations over an average of 632 days; 13 (81 %) had stable imaging findings, and 3 (19 %) had change over time. Of six patients with progressive cutaneous findings, five (83 %) had stable imaging findings over time. Among the 23 patients with clinical neurologic abnormality and imaging, 12 (52 %) had abnormal imaging findings. All seven patients with seizures (100 %) had abnormal imaging studies.
Conclusions
In PRS and ECS, imaging findings often are bilateral and often do not progress, regardless of cutaneous disease activity. Findings are inconsistently associated with clinical abnormalities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Morphea is a sclerosing disorder of the skin and underlying tissues [1]. One subtype of morphea, en coup de sabre (ECS), is considered to be on a clinical spectrum with progressive facial hemiatrophy or Parry–Romberg syndrome (PRS) [2]. ECS involves the face or scalp; PRS results in progressive, self-limited facial hemiatrophy. PRS and ECS are most commonly present in teenagers and young adults [3–7]. The goal of treatment—often, systemic therapy with corticosteroids and methotrexate—is to halt disease progression [2, 8].
The investigation of central nervous system (CNS) imaging findings in PRS and ECS is important to ensure that findings can be attributed appropriately to this condition, to define appropriate indications for CNS imaging, and to further explore the pathogenesis. PRS and ECS are reportedly associated with various clinical neurologic abnormalities and numerous variable neuroimaging findings. Most of these findings originate from case reports and case series with relatively few data from analysis of studies of consecutive patients [2, 9, 10]. Therefore, current understanding of purported imaging findings could reflect substantial selection bias for rare or even coincidental findings. Additional dedicated characterization of the imaging findings of PRS and ECS with a larger number of consecutive patients was needed. The present study characterized the neuroimaging findings of patients evaluated at our institution over a 16-year interval, with correlation to cutaneous and clinical neurologic abnormalities.
Materials and methods
Patient identification and inclusion criteria
Institutional review board approval was obtained for this study, which was in compliance with the Health Insurance Portability and Accountability Act. All patients with a diagnosis of PRS or ECS, or both, between January 1, 1997, and May 15, 2013, at our institution were identified through clinical database search. Age at initial diagnosis of PRS or ECS and at the most recent imaging examination, if performed, were determined for each patient. Eighteen of our patients with imaging had been included in a previous clinical evaluation of PRS and ECS [2], and imaging findings in one patient from our cohort had been published previously as an isolated case report [11]. Since no established criteria are available to direct neuroimaging in PRS or ECS, neuroimaging was not available for all patients.
Relevant clinical notes from each patient chart were reviewed in detail by a radiology resident with the assistance of a pediatric dermatologist who is board certified in both pediatrics and dermatology and has 3 years of postfellowship experience. Inclusion criteria contained a definite diagnosis of PRS or ECS, or both, by a dermatologist, rheumatologist, or craniofacial plastic surgeon. Patients were classified as having ECS morphea when they had linear scleroderma involving the frontoparietal scalp or the medial or paramedial forehead with or without extension into the scalp, nasal sidewall, and maxillary area. These patients were included in the category PRS when they had idiopathic hemifacial atrophy. Patients older than 50 years at the most recent imaging were excluded from analysis, to decrease the likelihood of age-related changes, such as white matter T2 hyperintensity and cerebral volume loss seen on imaging. When patients were age 50 years or older at any point during the study interval (i.e., by May 15, 2013), they were also excluded.
Documentation of clinical neurologic and pathologic abnormalities
The presence or absence of patient evaluation by a neurologist on chart review was documented by the radiology resident, with the assistance of a board-certified pediatric neurologist who had 3 years of postfellowship experience. For patients evaluated by a neurologist, the relevant clinical notes and tests were reviewed to characterize neurologic symptoms and deficits. Specifically, we documented headache, seizure, swallowing difficulty, sleep disruption, cognitive abnormality, motor deficit, sensory deficit, abnormal reflex, abnormal gait, and miscellaneous findings (e.g., trigeminal neuralgia, vision problems, and facial spasms). Results of electroencephalography (EEG), electromyography, swallow studies, and sleep studies were recorded if performed. If brain biopsies were done, the results were documented.
Imaging analysis
Two neuroradiologists with a certificate of added qualification in neuroradiology and a radiology resident analyzed the imaging studies by consensus. Clinical charts were masked to the neuroradiologists at image interpretation, although they could not be masked to incidental imaging evidence of PRS or ECS involvement of the subcutaneous tissues.
Patient age at imaging was recorded. For serial examinations, the interval between the first and the last imaging examination was determined. All available scans from computed tomography (CT), magnetic resonance imaging (MRI), computed tomographic angiography (CTA), magnetic resonance angiography (MRA), and conventional angiography were evaluated, and use of contrast medium was recorded. Numerous imaging findings derived from those reportedly associated with PRS and ECS [8] were evaluated in each patient, with notation of location, multiplicity, and laterality. Specifically, the presence and location were assessed individually and documented regarding cortical T2 hyperintensity, white matter T2 hyperintensity (on MRI) or hypodensity (on CT), cerebral atrophy, focal encephalomalacia, calcification (on CT), T2 hypointensity (on MRI), enhancing lesions (on MRI or CT), sulcal effacement, blurring of the gray-white junction, malformations of cortical development, subdural fluid collection, porencephalic cysts, cavernoma, arteriovenous malformation, aneurysm, areas of vascular narrowing, and asymmetrical lateral ventricular size.
Since the specific pattern and distribution of white matter T2 hyperintensity may be of particular benefit in helping discriminate white matter disease, T2 hyperintense lesions were scrutinized further. Specifically, the location (i.e., subcortical, deep hemispheric, periventricular, corpus callosum, posterior fossa, and associated cerebral lobe), number (0, 1–5, 6–10, 11–20, and >20), and size (greatest diameter of the smallest and the largest discrete lesion) of lesions in each hemisphere were documented.
Identification of a definite cavernoma required a typical appearance on T1- and T2-weighted images, with a thin T2 hypointense rim and internal “popcorn” signal pattern, rather than a purely hypointense susceptibility signal with no other signal abnormality on T1- or T2-weighted images, since PRS and ECS may be associated with cerebral calcifications that result in susceptibility signal on gradient or susceptibility weighted images. Because sulcal asymmetry may represent either sulcal effacement in one area or volume loss in another, the radiologists were required to commit to one of these two processes on the basis of overall CNS appearance for patient age and with consideration of other findings of volume loss. The neuroradiologists also could document descriptively any additional CNS abnormality identified.
When serial examinations (defined as two or more CNS imaging examinations of any type on different occasions) of a patient had been performed, the most recent of each type was used for the formal analysis, outlined herein. In addition, each serial examination was analyzed and the imaging findings were categorized as either stable or evolving. For cases that evolved, a descriptive account of the evolution was generated. In patients with serial imaging, we recorded the use of immunomodulatory and antiseizure medications, since treatment with these medications could affect the change of cutaneous or neurologic symptoms over time.
Data analysis
We recorded demographic information of all patients. For those patients who underwent CNS imaging studies, each study was globally designated as either normal or abnormal. For patients with imaging, the radiologic findings were correlated with both the cutaneous and the clinical neurologic findings.
Specifically, the relation of the side of each imaging finding for all affected patients to the side of facial or scalp involvement was determined and tabulated. For simplification, imaging findings in patients with bilateral cutaneous involvement were considered to only be ipsilateral to the respective cutaneous findings; otherwise, each imaging finding would have been considered both ipsilateral and contralateral for these patients (for example, a patient with bilateral PRS or ECS and imaging findings only on the left would be classified as having ipsilateral imaging findings). Any patient with bilateral imaging findings was considered to have bilateral imaging findings regardless of whether the cutaneous findings were unilateral or bilateral. Asymmetrical lateral ventricles were noted but were reported descriptively and not used for this laterality tabulation since it might not be clear which side (larger or smaller) was abnormal. Because PRS and ECS are considered part of a spectrum and commonly coexist, these characteristics were not differentiated for comparison to the imaging findings.
Clinical neurologic history and examinations were abstracted from the neurology consultations. Patients evaluated by the neurology service were divided into two subgroups: those with and those without a clinical neurologic abnormality. The frequency of normal versus abnormal imaging was determined for each of these two groups. Furthermore, the frequency of each imaging finding was determined for each specific clinical neurologic abnormality. Finally, for patients with consecutive imaging examinations, the stability or change of imaging, cutaneous, and neurologic findings over this interval were correlated. We also noted any change in disease-modulating medication over this interval.
Results
Patients
Eighty-eight patients with PRS or ECS were identified, of whom 62 (70 %) were female. Of the 88 patients, 43 (49 %) had imaging examinations. Of the 43 patients with imaging, 18 had been included in a previous clinical evaluation of PRS and ECS [2]. Fifteen additional patients were excluded because they were older than 50 years. No excluded patient had imaging before age 50 years during the study period. The average age at PRS or ECS diagnosis was 11.9 years (range 1–46 years), although age at diagnosis was unavailable for three patients. The average age of disease onset was 12.2 years (range 1–46 years) for the group with imaging and 11.8 years (range 1–27 years) for the group without imaging. For patients with imaging, the average interval between disease onset and first imaging examination was 7.9 years (range 0–31 years).
Overall, 43 patients (49 %) had ECS only, 30 (34 %) had PRS only, and 15 (17 %) had both. In total, PRS or ECS was present on the right side in 41 patients (47 %), on the left side in 42 (48 %), and bilaterally in 5 (6 %). Of the patients with imaging, PRS or ECS was present on the right in 21 patients (49 %), on the left in 18 patients (42 %), and bilaterally in 4 patients (9 %). Of the 45 patients without imaging, PRS or ECS was present on the right in 20 patients (44 %), on the left in 24 patients (53 %), and bilaterally in 1 patient (2 %).
Among the 43 patients with imaging, 38 patients (88 %) had magnetic resonance examination; 31 (72 %) had at least 1 magnetic resonance examination with contrast. Fourteen patients (33 %) had CT and 9 (21 %) had both MRI and CT. Ten patients (23 %) had MRA, CTA, and digital subtraction angiography examinations and underwent MRI; 5 patients (12 %) had MRI and CT, as well as MRA, CTA, and digital subtraction angiography. The average age at the most recent available imaging was 22.4 years (range 2–46 years).
Imaging and cutaneous findings
The major clinical and CNS imaging findings are detailed in Table 1. Of the 43 patients with imaging examinations, 19 (44 %) had abnormal imaging findings. Asymmetry of the lateral ventricles was the only imaging abnormality in 1 (2 %) of these 43 patients. Of the other 18 patients with imaging abnormalities, 11 (61 %) had bilateral findings, 6 (33 %) had findings strictly ipsilateral to the cutaneous involvement, and 1 (6 %) had findings strictly contralateral to cutaneous findings. One of these patients had bilateral facial involvement and unilateral neuroimaging findings and was included in the ipsilateral category, in accordance with methods.
The most common CNS imaging finding was white matter T2 hyperintensity, found in 14 (33 %) of the 43 patients. Areas of T2 hyperintensity were subcortical in these 14 patients (100 %), periventricular in 10 (71 %), and in the central white matter in 8 (57 %). Association with atrophy or encephalomalacia was seen in five patients (36 %). White matter lesions were present with the following frequency by location: frontal lobe, 13 patients (93 %); parietal lobe, 12 (86 %); temporal lobe, 10 (71 %); occipital lobe, 7 (50 %); corpus callosum, 1 (7 %); subinsular region, 6 (43 %); and posterior fossa, 1 (7 %). The finding in the posterior fossa was likely due to a cerebellar hemorrhage. One patient with unilateral PRS and ECS had too many lesions to count, with more than 20 lesions on both sides. Two patients with white matter T2 hyperintense lesions had bilateral PRS and ECS. Of the other 11 patients, 8 (73 %) had more lesions in the ipsilateral cerebral hemisphere, 2 (18 %) had more lesions in the contralateral hemisphere, and 1 (9 %) had an equal number on both sides. When present, lesions per hemisphere ranged in quantity from 1 to more than 20. Per patient, the smallest lesion ranged from 0.1 to 0.5 cm and the largest lesion ranged from 0.4 to 2.6 cm.
Other imaging findings were less common, including atrophy and encephalomalacia (n = 5; 12 %), deep gray matter T2 hyperintensity (n = 3; 7 %), sulcal effacement (n = 3; 7 %), and asymmetrical lateral ventricles (n = 3; 7 %). Other findings were present in only 1 or 2 patients (Table 1). The only finding identified on CT and not on MRI was parenchymal calcifications.
One patient with left-sided cutaneous involvement had three aneurysms, including bilateral ophthalmic arteries and a single left supraclinoid ICA aneurysm. This was the only patient (10 %) of ten patients with an abnormal angiogram; nine (90 %) of the ten patients with angiography had normal vascular findings. One patient presented with cerebellar hemorrhage; its cause was still undetected after two conventional angiograms and surgical evacuation. Otherwise, no posterior fossa abnormalities were detected in any patient.
Sixteen (37 %) of 43 patients had serial imaging examinations; 12 (28 %), serial MRI; 3 (7 %), serial CT; and 1 (2 %), serial MRI, CT, and angiography, obtained over an average interval of 3.0 years (range 0.33–9.40 years). Three (19 %) of the 16 patients with serial imaging had change in their imaging findings over time. The first patient was monitored with imaging for 2.5 years and received methotrexate therapy that was discontinued and restarted. The patient had progressive skin changes, a stable headache, and an increasing number of T2 hyperintense white matter lesions. The second patient was monitored with imaging for 5.6 years and treated with methotrexate and antiseizure medication. This patient had stable to improved skin findings, fluctuating seizure frequency, and decreasing white matter T2 hyperintensity with development of encephalomalacia in the basal ganglia, as well as enlargement of a porencephalic cyst. The third patient was monitored with imaging for 9.4 years and received intermittent therapy with cyclophosphamide, systemic corticosteroids, and rituximab treatments. This patient had stable cutaneous findings; fluctuating frequency and severity of seizures, including status epilepticus; and progression of cerebral atrophy and cortical T2 hyperintensity, with overall decreased white matter T2 hyperintensity.
Among six patients with serial imaging who had progressive skin changes during the interval, five (83 %) had stable imaging findings. Three patients had change of neurologic abnormalities over time: one (33 %) with worsening headaches had stable normal imaging findings, and two (66 %) with fluctuating seizures had evolution of imaging findings. A comparison of the key imaging findings in this study with the other major studies is detailed in Table 2.
Clinical neurologic and pathologic findings
Eight (18 %) of 45 patients without imaging were evaluated by neurology. Of these eight patients, three (38 %) had headaches, two (25 %) had motor weakness (one with lower extremity weakness and one with facial nerve palsy), and one (13 %) had sensory abnormality in the lower extremities. Twenty-seven (63 %) of the 43 patients with imaging were evaluated by neurology. Twenty-three (85 %) of these 27 patients had neurologic abnormalities. One (6 %) of 16 patients with imaging who were not evaluated by neurology at our institution had a neurologic abnormality—a history of seizures—documented in the medical record.
The most commonly reported symptoms were headache and seizure (Table 1). Of 23 patients with a clinical neurologic abnormality and neuroimaging, 12 (52 %) had abnormal neuroimaging findings. Of these 12 patients with neurologic abnormalities and neuroimaging findings, 3 (25 %) presented with headache alone, 4 (33 %) with seizure alone, 3 (25 %) with headache and seizure, 1 (8 %) with facial spasms, and 1 (8 %) with hemiparesis. All seven patients with seizures had imaging abnormalities (Table 1). Fifteen patients with headache had neuroimaging, of whom six (40 %) had neuroimaging abnormalities. These abnormalities included four patients (27 %) with white matter T2 hyperintensity, two (13 %) with sulcal effacement, two (13 %) with atrophy or encephalomalacia, one (7 %) with cortical T2 hyperintensity, one (7 %) with deep gray matter hyperintensity, and one (7 %) with asymmetrical lateral ventricles. Only two (20 %) had abnormal imaging among the ten patients with headache as their only neurologic symptom. Other neurologic abnormalities were less common, with one to three patients in each category, and had variable association with the presence and type of imaging abnormality.
Eleven patients had EEG. Six (86 %) of seven patients with seizures had EEG abnormalities, including focal slowing, epileptiform discharges, and recorded seizures; one patient without seizures had an EEG that showed focal slowing. This latter patient had bilateral aneurysms.
Six patients with PRS or ECS were evaluated by the neurology service and found to have no neurologic abnormality. Of these patients, four (67 %) had imaging and two (33 %) had imaging findings—one with bilateral cerebral aneurysms and one with a left cerebellar hemorrhage, prominent deep and subcortical WM T2 hyperintensities, and a T2 hyperintensity in the thalamus (i.e., other than neurologic findings directly attributed to the hemorrhage itself). Examples of MRI findings correlated with skin findings and clinical neurologic symptoms are shown in Figs. 1, 2, 3, 4, 5, 6, and 7.
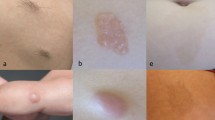
Woman who had unilateral right Parry–Romberg syndrome and en coup de sabre morphea diagnosed at age 14 years, with headaches and new bilateral magnetic resonance imaging abnormalities years after initial clinical diagnosis. a The linear morphea of the right forehead at age 17 years. b Axial T2 fluid-attenuated inversion recovery (FLAIR) images at age 18 years were unremarkable. c Axial T2 FLAIR at age 19 years, showing a new right parietal white matter area of T2 hyperintensity and a new small area of T2 hyperintensity in the left aspect of the body of the corpus callosum. These abnormal areas all showed faint contrast medium enhancement on the latter examination, too subtle to show on single images. During the interval between imaging examinations, the morphea progressed, methotrexate treatment was stopped and restarted, and the headaches continued to be stable
Woman with bilateral morphea onset at age 6 years, seizures, and bilateral, but asymmetrical, magnetic resonance imaging abnormalities. a Clinical photograph at age 13 years showing bilateral morphea of the forehead. Axial computed tomographic images showing multifocal left subcortical calcifications with b subtle surrounding white matter hypointensity and c subtle global left cerebral volume loss (the consensus was volume loss on the left rather than sulcal effacement on the right). d Axial T2 fluid-attenuated inversion recovery performed 1 day after computed tomography showing multifocal subcortical T2 hyperintensity, predominantly on the left but also in the right parietal periventricular region
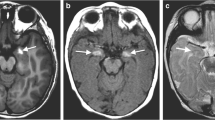
Man with left Parry–Romberg syndrome onset at age 5 years, bilateral magnetic resonance imaging (MRI) abnormalities that are most marked on the right, and seizures. a Clinical photograph at age 13 years showing left facial hemiatrophy. b Axial T2 MRI at age 24 years showing bilateral, but right predominant, cerebral atrophy. c Axial T2 fluid-attenuated inversion recovery from the same examination shown in b, at the level of the lateral ventricles, further shows right predominant atrophy, as well as bilateral, but right predominant, white matter T2 hyperintensity
A 21-year-old woman with onset of left en coup de sabre morphea (ECS) at age 4 years who had seizures. Axial T2-weighted magnetic resonance imaging showing focal encephalomalacia centered in the left parietooccipital region with hemosiderin deposition. The patient also had subcortical T2 hyperintensity in all left lobes and subinsular region and a small area of subcortical T2 hyperintensity in the right frontal lobe
Woman who had left Parry–Romberg syndrome at age 10 years without neurologic abnormalities, with bilateral carotid ophthalmic aneurysms and additional left supraclinoid internal carotid artery aneurysm. a Cerebral angiogram showing a right carotid ophthalmic aneurysm. b Cerebral angiogram showing a left carotid ophthalmic aneurysm with an additional left supraclinoid aneurysm
A 25-year-old woman with onset of left Parry–Romberg syndrome and en coup de sabre (ECS) morphea at age 10 years who had headache. Axial T2-weighted magnetic resonance imaging showing diffuse left sulcal effacement. The patient also had deep and subcortical white matter hyperintensity in the left frontal, parietal, and temporal lobes and left lentiform nucleus
A 9-year-old girl with left Parry–Romberg syndrome and en coup de sabre morphea and seizures. a Axial T2-weighted magnetic resonance imaging (MRI) showing a porencephalic cyst in the right frontal white matter. b Axial T2-weighted MRI showing a small area of focal encephalomalacia in the left caudate and adjacent corona radiata. A facial photo clearly depicting the facial abnormality was not available
Two patients had brain biopsy; one biopsy obtained from the ipsilateral (to facial lesion) occipital lobe demonstrated T cell infiltrates, and the second biopsy, obtained from the contralateral (to facial lesion) temporal lobe, demonstrated mild to focally moderate gliosis.
Discussion
To our knowledge, this is the largest study on neuroimaging findings in a consecutive cohort of patients with PRS and ECS and is distinctive in that imaging findings were analyzed systematically and correlated with cutaneous and neurologic deficits over time. Imaging abnormalities are frequently bilateral, highly variable, and inconsistently associated with a neurologic abnormality; frequently present in the clinical setting of seizure; usually are stable; and often do not correlate with cutaneous disease activity. These results have implications for exploring the pathogenesis, for image interpretation, and for understanding the clinical utility of imaging examinations.
Although the present study was not designed to determine the pathogenesis of PRS and ECS, its findings allow interesting comparison with other lateralizing neurocutaneous syndromes and support certain prior hypotheses. For example, key CNS imaging findings are strongly lateralized to the ipsilateral cerebral hemisphere in most patients who have several other neurocutaneous syndromes with unilateral facial cutaneous involvement, such as Sturge–Weber syndrome, posterior fossa malformations–hemangiomas–arterial anomalies–cardiac defects–eye abnormalities (PHACE) [14, 15], cerebrofacial arteriovenous metameric syndrome [16], linear epidermal nevus syndrome, and encephalocraniocutaneous lipomatosis [17]. Developmental and genetic causes, such as abnormal vascular development [18], mosaic genetic mutations [19, 20], and abnormal neural crest development [21, 22], are proposed to underlie these syndromes, accounting for ipsilateral cutaneous CNS abnormalities with common developmental origins. The presence of frequent bilateral CNS findings observed in our study suggests that the pathomechanism of PRS and ECS does not arise strictly from a unilateral developmental insult and likely differs from these other neurocutaneous syndromes with unilateral facial involvement.
Numerous potential pathomechanisms of PRS and ECS have been pondered, including autoimmune, genetic, traumatic, sympathetic dysregulation, and infectious mechanisms [8, 23–26]. The pathomechanism may be multifactorial, resulting from a genetic predisposition to certain environmental insults that, when encountered, exaggerate an inflammatory response [26]. Indeed, inflammation has been found on skin and brain pathologic specimens [27–34]. An autoimmune etiologic basis has been proposed and could explain the coexistence of autoimmune antibodies [35], which is an association with other autoimmune disorders [26], and could account for the relatively uncommon association with Rasmussen encephalitis [24].
Although we can only speculate, these mechanisms could constitute a regional or systemic process and could account for the bilateral findings, including areas of T2 hyperintensity and, when associated with active inflammation, enhancement. A regional inflammatory process could explain the tendency of findings to be more prevalent in the cerebral hemisphere ipsilateral to the cutaneous involvement. However, the genetic predisposition to environmental insults and the autoimmune hypotheses would not account for the less common existence of structural abnormalities, such as malformations of cortical development or porencephalic cysts, unless some manifestation of morphea or a predisposing factor is present during development.
Our results add to those of the prior studies of PRS and ECS. Chiu et al. [10] reported CNS imaging findings in less than one fifth of consecutively evaluated patients, about one-half the percentage in our study. This difference may reflect a larger proportion (66 %) of patients undergoing CNS imaging in their study, more than half without clinical neurologic deficit. It could also reflect differences in methods of image analysis. The literature review by Chiu et al. reports CNS findings in greater than two thirds of patients, which likely represents selective publication of positive findings. Careta et al. [12] and Blasczyk et al. [13] reported CNS imaging findings in 75 and 42 % of patients, respectively. Comparison with these results is limited since the methods of these two studies do not specify whether all consecutive patients with PRS or ECS evaluated at the respective institutions were considered for inclusion. Although a large spectrum of findings have been reported, areas of white matter T2 hyperintensity are the most common finding in both our study and these prior studies [10, 12, 13]. In contrast, our series of consecutive patients indicates that findings such as encephalomalacia or atrophy, developmental abnormalities, and vascular abnormalities are much less common. Therefore, characterization of such white matter lesions is important to ensure that these can be appropriately attributed to PRS or ECS rather than other white matter disease during image interpretation.
There is little prior characterization of the number, size, and distribution of T2 hyperintense white matter lesions. Such analysis is important since this finding is seen in a large number of conditions, although many have suggestive features or distribution. For example, both multiple sclerosis and PRS or ECS can show white matter T2 hyperintense lesions. However, the present study shows that posterior fossa lesions and corpus callosal lesions that may be present in multiple sclerosis are relatively infrequent, but do occur, in PRS and ECS.
Our finding of frequent bilateral imaging abnormalities contrasts the results of prior literature reviews, which determined that findings are unilateral predominantly [10, 36]. This discrepancy may reflect differing capabilities of older imaging techniques in the literature reviews and the dedicated systematic analysis by neuroradiologists in the current study. In addition, most patients had a greater number and size of T2 hyperintense areas ipsilateral to the cutaneous involvement. In some prior reports, it is possible that small or subtle bilateral findings were present but unrecognized because of lack of systematic analysis or use of older technologies, or both. To avert an unnecessary workup, it is important for radiologists and clinicians to recognize that bilateral findings commonly exist, to avoid bilateral findings being misattributed to another process such as demyelination or vasculitis.
Although the reported imaging associations identified in prior literature reviews [8, 10] of these case reports are manifold, many of these reported associations were not identified in the present study. For example, there are previous case reports of cavernomas in association with PRS and ECS [37], but we did not identify a single definite example. Since cavernomas frequently are detected as incidental findings in the pediatric population [38], this association may be coincidental. Other previously reported associations not identified in this study include teratoma [39], hamartoma [8], arteriovenous malformation [40], and narrowed, constricted, or hypoplastic intracranial arteries [40, 41], which are suggestive that these findings are either rare or coincidental.
The majority of patients with serial imaging had stable imaging findings. This result is similar to the results of a study by Careta et al. [12], which found that the included 12 patients had stable imaging findings, but it differs from a literature review that found change over time in more than 50 % of patients [36]. Careta et al. also suggest that neuroimaging findings may represent an early manifestation of neurologic involvement, citing this as a rationale to commence aggressive medical treatment in patients with imaging abnormalities. Our results challenge this recommendation because evolution of imaging findings did not correlate predictably with evolution of neurologic or dermatologic findings. Our results also indicate that use of serial imaging to monitor disease activity is of uncertain value, given the inconsistent correlation to cutaneous and neurologic progression.
The current study confirms an inconsistent relation between clinical neurologic abnormalities and neuroimaging findings, a belief previously based predominantly on literature review of case reports [6, 10, 12, 13]. Prior authors have recommended baseline neuroimaging studies in patients with PRS or ECS [10]. However, no conclusive evidence supports MRI or CT screening in the absence of neurologic abnormality, since the importance of asymptomatic findings are unknown and no conclusive evidence shows that any particular findings are useful for directing disease-modifying medication. All patients in the present study—and most of the patients previously reported—with cerebral atrophy or encephalomalacia had seizures, a patient subgroup likely to undergo neuroimaging for evaluation of this neurologic deficit [24, 28, 29, 42–49]. Further study is needed to evaluate the utility of screening MRA for intracranial aneurysm since determining the frequency of this association is difficult with only one patient who had an abnormal angiogram in the present study.
Limitations of this study include its retrospective study design and the associated reliance on the clinical diagnosis of specialist physicians. The side of cutaneous involvement was masked to the radiologists, but evidence of involvement incidentally visualized in the subcutaneous soft tissues and osseous structures of the imaging examinations could not be masked. Imaging findings due to PRS or ECS cannot be separated from any that could relate more directly to the clinical neurologic symptoms themselves since, outside the clinical setting of PRS and ECS, seizures can be associated with atrophy with underlying white matter T2 hyperintensity [50, 51] and headaches can be associated with white matter T2 hyperintense areas [52]. Imaging findings in the early stages of PRS and ECS may not have been observed in all patients, since the average delay between disease onset and first imaging was 7.9 years. This study was conducted at a quaternary care referral center, which may select for relatively severe cases. Furthermore, not all patients underwent CNS imaging, potentially selecting for patients with neurologic involvement.
Conclusion
A high percentage of patients with PRS and ECS who undergo CNS imaging have CNS imaging abnormalities. Imaging findings are often bilateral and often do not progress, regardless of cutaneous disease activity. Although all patients with seizures in the present series had imaging findings, the imaging findings overall were inconsistently associated with clinical abnormalities.
Abbreviations
- CNS:
-
Central nervous system
- CT:
-
Computed tomography
- CTA:
-
Computed tomographic angiography
- ECS:
-
En coup de sabre
- EEG:
-
Electroencephalography
- MRA:
-
Magnetic resonance angiography
- MRI:
-
Magnetic resonance imaging
- PRS:
-
Parry–Romberg syndrome
References
Fett N, Werth VP (2011) Update on morphea: part I. Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol 64:217–228
Tollefson MM, Witman PM (2007) En coup de sabre morphea and Parry-Romberg syndrome: a retrospective review of 54 patients. J Am Acad Dermatol 56:257–263
Duymaz A, Karabekmez FE, Keskin M (2009) Parry-Romberg syndrome: facial atrophy and its relationship with other regions of the body. Ann Plast Surg 63:457–461
Palmero ML, Uziel Y, Laxer RM (2010) En coup de sabre scleroderma and Parry-Romberg syndrome in adolescents: surgical options and patient-related outcomes. J Rheumatol 37:2174–2179, Erratum in: J Rheumatol 37: 2444
Peterson LS, Nelson AM, Su WP (1995) Classification of morphea (localized scleroderma). Mayo Clin Proc 70:1068–1076
Sommer A, Gambichler T, Bacharach-Buhles M (2006) Clinical and serological characteristics of progressive facial hemiatrophy: a case series of 12 patients. J Am Acad Dermatol 54:227–233
Stone J (2003) Parry-Romberg syndrome: a global survey of 205 patients using the Internet. Neurology 61:674–676
El-Kehdy J, Abbas O, Rubeiz N (2012) A review of Parry-Romberg syndrome. J Am Acad Dermatol 67:769–784
Lehman VT, Doolittle DA, Hunt CH (2014) Intracranial imaging of uncommon diseases is more frequently reported in clinical publications than in radiology publications. AJNR Am J Neuroradiol 35:45–48
Chiu YE, Vora S, Kwon EK (2012) A significant proportion of children with morphea en coup de sabre and Parry-Romberg syndrome have neuroimaging findings. Pediatr Dermatol 29:738–748
Moseley BD, Burrus TM, Mason TG (2010) Neurological picture: contralateral cutaneous and MRI findings in a patient with Parry-Romberg syndrome. J Neurol Neurosurg Psychiatry 81:1400–1401
Careta MF, Leite Cda C, Cresta F (2013) Prospective study to evaluate the clinical and radiological outcome of patients with scleroderma of the face. Autoimmun Rev 12:1064–1069
Blaszczyk M, Krolicki L, Krasu M (2003) Progressive facial hemiatrophy: central nervous system involvement and relationship with scleroderma en coup de sabre. J Rheumatol 30:1997–2004
Hess CP, Fullerton HJ, Metry DW (2010) Cervical and intracranial arterial anomalies in 70 patients with PHACE syndrome. AJNR Am J Neuroradiol 31:1980–1986
Metry D, Heyer G, Hess C (2009) PHACE Syndrome Research Conference (2009) Consensus Statement on Diagnostic Criteria for PHACE Syndrome. Pediatrics 124:1447–1456
Bhattacharya JJ, Luo CB, Suh DC (2001) Wyburn-Mason or Bonnet-Dechaume-Blanc as cerebrofacial arteriovenous metameric syndromes (CAMS): a new concept and a new classification. Interv Neuroradiol 7:5–17
Parazzini C, Triulzi F, Russo G (1999) Encephalocraniocutaneous lipomatosis: complete neuroradiologic evaluation and follow-up of two cases. AJNR Am J Neuroradiol 20:173–176
Comi AM (2003) Pathophysiology of Sturge-Weber syndrome. J Child Neurol 18:509–516
Hafner C, van Oers JM, Vogt T (2006) Mosaicism of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest 116:2201–2207
Moog U, Roelens F, Mortier GR (2007) Encephalocraniocutaneous lipomatosis accompanied by the formation of bone cysts: harboring clues to pathogenesis? Am J Med Genet A 143A:2973–2980
Castillo M (2008) PHACES syndrome: from the brain to the face via the neural crest cells. AJNR Am J Neuroradiol 29:814–815
Moog U (2009) Encephalocraniocutaneous lipomatosis. J Med Genet 46:721–729
Dupont S, Catala M, Hasboun D (1997) Progressive facial hemiatrophy and epilepsy: a common underlying dysgenetic mechanism. Neurology 48:1013–1018
Longo D, Paonessa A, Specchio N (2011) Parry-Romberg syndrome and Rasmussen encephalitis: possible association. Clinical and neuroimaging features. J Neuroimaging 21:188–193
Pichiecchio A, Uggetti C, Grazia Egitto M (2002) Parry-Romberg syndrome with migraine and intracranial aneurysm. Neurology 59:606–608
Fett N (2013) Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol 31:432–437
Woolfenden AR, Tong DC, Norbash AM (1998) Progressive facial hemiatrophy: abnormality of intracranial vasculature. Neurology 50:1915–1917
Carreno M, Donaire A, Barcelo MI (2007) Parry Romberg syndrome and linear scleroderma in coup de sabre mimicking Rasmussen encephalitis. Neurology 68:1308–1310
Shah JR, Juhasz C, Kupsky WJ (2003) Rasmussen encephalitis associated with Parry-Romberg syndrome. Neurology 61:395–397
Pensler JM, Murphy GF, Mulliken JB (1990) Clinical and ultrastructural studies of Romberg’s hemifacial atrophy. Plast Reconstr Surg 85:669–674
Holland KE, Steffes B, Nocton JJ (2006) Linear scleroderma en coup de sabre with associated neurologic abnormalities. Pediatrics 117:e132–e136
Obermoser G, Pfausler BE, Linder DM (2003) Scleroderma en coup de sabre with central nervous system and ophthalmologic involvement: treatment of ocular symptoms with interferon gamma. J Am Acad Dermatol 49:543–546
Pupillo G, Andermann F, Dubeau F (1996) Linear scleroderma and intractable epilepsy: neuropathologic evidence for a chronic inflammatory process. Ann Neurol 39:277–278
Stone J, Franks AJ, Guthrie JA (2001) Scleroderma “en coup de sabre”: pathological evidence of intracerebral inflammation. J Neurol Neurosurg Psychiatry 70:382–385
Takehara K, Sato S (2005) Localized scleroderma is an autoimmune disorder. Rheumatology (Oxford) 44:274–279, Erratum in: Rheumatology (Oxford) 44: 569
Kister I, Inglese M, Laxer RM (2008) Neurologic manifestations of localized scleroderma: a case report and literature review. Neurology 71:1538–1545
Fain ET, Mannion M, Pope E (2011) Brain cavernomas associated with en coup de sabre linear scleroderma: two case reports. Pediatr Rheumatol Online J 9:18
Schwartz ES, Barkovich AJ (2012) Congenital anomalies of the spine. In: Barkovich AJ, Raybaud C (eds) Pediatric neuroimaging, 5th edn. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, p 884
Bergler-Czop B, Lis-Swiety A, Brzezinska-Wcislo L (2009) Scleroderma linearis: hemiatrophia faciei progressiva (Parry-Romberg syndrome) without any changes in CNS and linear scleroderma “en coup de sabre” with CNS tumor. BMC Neurol 9:39
Bosman T, Van Bei Jnum J, Van Walderveen MA (2009) Giant intracranial aneurysm in a ten-year-old boy with Parry Romberg syndrome: a case report and literature review. Interv Neuroradiol 15:165–173
Aoki T, Tashiro Y, Fujita K (2006) Parry-Romberg syndrome with a giant internal carotid artery aneurysm. Surg Neurol 65:170–173
Chang SE, Huh J, Choi JH (1999) Parry-Romberg syndrome with ipsilateral cerebral atrophy of neonatal onset. Pediatr Dermatol 16:487–488
Duro LA, de Lima JM, dos Reis MM (1982) [Progressive hemifacial atrophy (Parry-Romberg disease): study of a case]. Arq Neuropsiquiatr 40:193–200, Portuguese
Grosso S, Fioravanti A, Biasi G (2003) Linear scleroderma associated with progressive brain atrophy. Brain Dev 25:57–61
Klene C, Massicot P, Ferriere-Fontan I (1989) [“Saber-cut” scleroderma and Parry-Romberg facial hemiatrophy: nosologic problems: neurologic complications]. Ann Pediatr (Paris) 36:123–125, French
Moko SB, Mistry Y, de Chalain TM B (2003) Parry-Romberg syndrome: intracranial MRI appearances. J Craniomaxillofac Surg 31:321–324
Paprocka J, Jamroz E, Adamek D (2006) Difficulties in differentiation of Parry-Romberg syndrome, unilateral facial sclerodermia, and Rasmussen syndrome. Childs Nerv Syst 22:409–415
Sathornsumetee S, Schanberg L, Rabinovich E (2005) Parry-Romberg syndrome with fatal brain stem involvement. J Pediatr 146:429–431
Speciali JG, Resende LA (1984) [Progressive facial hemiatrophy: report of a case]. Arq Neuropsiquiatr 42:166–170, Portuguese
Doherty CP, Cole AJ, Grant PE (2004) Multimodal longitudinal imaging of focal status epilepticus. Can J Neurol Sci 31:276–281
Meierkord H, Wieshmann U, Niehaus L (1997) Structural consequences of status epilepticus demonstrated with serial magnetic resonance imaging. Acta Neurol Scand 96:127–132
Swartz RH, Kern RZ (2004) Migraine is associated with magnetic resonance imaging white matter abnormalities: a meta-analysis. Arch Neurol 61:1366–1368
Ethical Standards and Patient Consent
We declare all human and animal studies have been approved by the Institutional Review Board and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doolittle, D.A., Lehman, V.T., Schwartz, K.M. et al. CNS imaging findings associated with Parry–Romberg syndrome and en coup de sabre: correlation to dermatologic and neurologic abnormalities. Neuroradiology 57, 21–34 (2015). https://doi.org/10.1007/s00234-014-1448-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-014-1448-6