Abstract
Introduction
The purpose of this study was to evaluate the safety and efficacy of the pREset stent retriever in a real-world clinical setting.
Methods
Patients treated with pREset were selected from a prospectively maintained single-center database. A TICI score ≥2b after ≤3 passes was regarded as successful recanalization. All device-related complications and their clinical significance were reported. Parenchymal hematomas (PH) were classified according to ECASS, adding focal and diffuse subarachnoid hemorrhage (SAH) as categories. A 90-day mRS of 0–2 was defined as favorable outcome. In addition, we separately analyzed patients treated with >3 pREset passes and patients receiving other rescue maneuvers.
Results
We included 271 patients. Successful recanalization was achieved in 76.4 %. Device-related complications occurred in 9.2 % of which 2.2 % were clinically significant. PH I, PH II, focal SAH, and diffuse SAH was observed in 5.2, 4.8, 12.2, and 2.2 %, respectively. A total of 39.5 % of patients had favorable clinical outcome. Considering treatments with >3 pREset passes or other rescue procedures, an additional 8.5 and 9.3 % of target vessels were recanalized. The chance of favorable clinical outcome decreased significantly with any kind of rescue therapy. In addition, the rate of PH I was significantly higher in patients treated with >3 pREset passes, whereas all other types of hemorrhage showed no difference.
Conclusion
In terms of safety and effectiveness, pREset performed comparably to other stent retriever devices. To avoid futile recanalization and potential additional harm, escalation of therapy beyond three thrombectomy passes should only be performed after careful individual consideration of each case.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endovascular recanalization is a viable treatment option for acute stroke patients with cerebral large vessel occlusion. The effectiveness of this approach is currently under debate since three major randomized trials (Interventional management of stroke (IMS) III, SYNTHESIS expansion and Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE)) were simultaneously published [1–3]. These trials compared intravenous thrombolysis (IVT) or standard stroke treatment with endovascular recanalization but failed to demonstrate improved clinical outcome in the endovascular arm. On the other hand, all three trials suffered from profound limitations: low recruitment rates and long study duration, inappropriate imaging methods as well as very heterogeneous and mostly outdated endovascular techniques were the most important targets for criticism [4]. Especially endovascular technique progressed significantly since the initiation of these trials and became more effective with the introduction of stent retriever technology. The Solitaire with the intention for thrombectomy (SWIFT) and Trevo2 trials proved stent retrievers to be superior compared to the Merci device [5, 6]. In IMS III, SYNTHESIS expansion and MR RESCUE stent retriever were only used in a negligible small proportion of patients. Randomized trials testing standard treatment versus stent retriever thrombectomy are already under way.
The obvious success of this new technical concept yielded several devices representing refinements and modifications of the stent retriever design. One of the new-generation stent retrievers is the pREset 4/20 (phenox GmbH, Bochum, Germany), which was approved in Europe in August 2011. A larger version—pREset 6/30—followed in May 2012. Device approval is mainly based on bench and animal experiments with limited clinical data. Therefore, careful evaluation after approval is mandatory. The purpose of the present study was to analyze the performance of the pREset stent retriever in routine practice focusing on recanalization results and safety issues.
Materials and methods
Ethical adherence
Data for this analysis were extracted from a prospectively maintained single-center database of endovascular stroke treatments. The local ethics committee approved data collection and analysis.
The pREset stent retriever
pREset (phenox GmbH, Bochum, Germany) is a laser-cut nitinol stent retriever which is eccentrically connected to a 180-cm pusher wire (Fig. 1). The device is not detachable. One unique feature is a helical slit within the nitinol tube that allows adaption to varying vessel diameters without deformation of the cell configuration. The proximal cells are connected by a nitinol-ring to stabilize the device and to reduce tapering in narrow curves. Two distal and one proximal marker guarantee visibility during deployment and retraction. The total device length of pREset 4/20 is 30 mm, with a “usable length” of 20 mm. “Usable length” is defined as the part of the retriever interacting with the thrombus between the distal end of the stabilizing ring and the distal marker. The maximum expanded diameter is 4 mm. pREset 6/30 has a maximum diameter of 6 mm; total device length is 45 mm, with a “usable length” of 30 mm. pREset 4/20 is approved for vessels larger than 2 mm, with pREset 6/30 for vessels larger than 3 mm. A 0.021-in. inner lumen micro-catheter is sufficient to introduce pREset 4/20 as well as 6/30.
a The pREset 4/20 thrombectomy device has two distal (black arrows) and one proximal maker. b A helical slit along the lenght of the retriever (arrowheads) allows for adaption to different vessel diameters without change of cell size. c The proximal cells are connected with a helical ring which stabilizes the device in curves (arrowheads)
Patient selection and characterization
From an institutional database containing all endovascular stroke cases performed since January 2007, we selected patients treated with pREset between August 2011 and June 2013. In our analysis, we included patients with an embolic intracranial vessel occlusion, excluding cases with an underlying intracranial stenosis or stent thrombosis. Patients with extracranial arterial stenoses or occlusions in the access site were not excluded. pREset had to be the first-choice device and at least three recanalization attempts had to be performed with pREset before the treatment strategy was changed.
The patient population was characterized by gender, age, duration of symptoms, stroke etiology, NIHSS at presentation, and intracranial target vessels. The modified Rankin score (mRS) at 90 days was used to assess the clinical outcome, defining mRS 0–2 as favorable outcome.
Treatment protocol
Patients referred for mechanical recanalization had an acute onset of clinical symptoms caused by cerebral ischemia and a relevant neurological deficit (NIHSS ≥ 4). In case of fluctuating symptoms, we also treated patients with minor stroke severity at presentation (NIHSS < 4). Treatment was performed within 8 h from symptom onset. Exceptions were made for patients beyond this time window in case of small infarct size and severe or fluctuating symptoms, suggesting a relevant proportion of salvageable brain tissue and good collaterals. Patients with unknown time window were also selected based on this concept of clinical mismatch.
CT or MRI was used as baseline imaging according to the local standards of the referring hospital. CT or DWI Alberta Stroke Program Early CT Score (ASPECTS) was determined for anterior circulation stroke. Since DWI lesions may be reversible after reperfusion, patients with low DWI ASPECTS but no T2 signal abnormalities were also considered for endovascular treatment [7]. Large vessel occlusion was confirmed by CT or MR angiography.
Intravenous rtPA was given prior to the endovascular procedure in a small subset of patients based on generally accepted inclusion criteria. The referring neurologist was the decision-maker regarding the use of systemic fibrinolysis. Patients with a suspected or confirmed cervical artery stenosis or dissection received a loading dose of 500 mg acetylsalicylic acid and 600 mg clopidogrel but no rtPA to reduce the risk of hemorrhage. If a patient did not receive a loading dose but stenting had to be performed, 500 mg acetylsalicylic acid was applied intravenously followed by 600 mg of clopidogrel via a nasogastric tube.
Procedures were routinely performed under general anesthesia by six experienced interventional neuroradiologists as a single-operator procedure. In the anterior circulation, an 8 French guide catheter was used mainly in combination with an intermediate catheter. A balloon guide catheter was rarely chosen. Vascular access in the posterior circulation was gained with a 6 F guide catheter or 8 F with intermediate catheter if the vessel diameter was sufficient. Cervical access vessel stenoses or occlusions were treated by stent angioplasty prior to the intracranial recanalization procedure.
The occluded target vessel was catheterized with a 0.021-in. inner lumen micro-catheter using a 0.014-in. guide wire. Under pulsed fluoroscopy, the device was deployed beyond the assumed occlusion site. After 5 min of incubation and intraarterial injection of 1–2 mg glyceroltrinitrate, the device was slowly withdrawn under continuous manual aspiration. Arterial hypotension was pharmacologically compensated by intravenous injection of cafedrinhydrochloride/theodrenalinhydrochloride. In case of persistent occlusion or incomplete recanalization, thrombectomy was repeated with the same or another device. If recanalization could not be achieved after several mechanical thrombectomy (mTE) maneuvers, the procedure was either aborted or continued using angioplasty and/or stent deployment.
After mTE, the patient was kept sedated and ventilated until the next day to allow for precise management of blood pressure tolerating a maximum peak systolic value of 130 mm Hg. Follow-up imaging was done within 24 to 48 h using either CT or MRI.
Device evaluation
The modified thrombolysis in cerebral infarction (TICI) score was used to evaluate recanalization results, defining TICI ≥2b within three passes as successful recanalization [8]. For comparability with the SWIFT and TREVO2 trials, we separately analyzed recanalization results after three pREset passes, more than three pREset passes, and combined treatments with rescue devices. Procedure time was defined as the interval between placement of the guide catheter and the final angiographic result. All procedure-related adverse events and association with the study device were reported.
Post-treatment imaging was assessed for parenchymal hemorrhages and hemorrhagic transformation type I and II (PH I, PH II, HT I, HT II) according to the European Cooperative Acute Stroke Study (ECASS) [9]. We reported the rate of subarachnoid hemorrhage (SAH) discriminating focal (confined to the site of treatment) and diffuse (spreading to the contralateral hemisphere and/or transtentorial) distribution.
Statistical analysis
Categorical data of different treatment groups were compared using Fisher’s exact test. Mann–Whitney U-test was applied for continuous variables. p-values ≤0.05 were considered as statistically significant. All analyses were performed with STATA/IC 11.2 for Windows software (StataCorp, College Station, TX, USA).
Results
Inclusion criteria were met by 271 of 513 stroke patients treated endovascularly during the defined study period. Mean time from symptom onset to treatment was 265 min (range 85–639). For 83 (30.6 %) patients, the time of onset was unknown or symptoms were fluctuating or progressive. Detailed patient characteristics and target vessels are summarized in Table 1.
TICI 2b or 3 was achieved in 207 (76.4 %) patients within three passes, and an additional 23 (8.5 %) targets were successfully recanalized with pREset alone but more than three mTE-maneuvers. No statistically significant difference existed in the probability to achieve TICI 2 or 3 between these two groups (p = 0.362). Additional devices or procedures were used in 32 (11.8 %) patients. The probability for a TICI 2b or 3 result in the latter group was lower compared to treatments with three pREset passes (p = 0.000). Overall, a TICI 2b or 3 score at the end of procedure was achieved in 257 (94.8 %) patients. The average procedure time was 67 min (8–738 min) for all treatments. Procedure time rose from 44 min (8–192 min) in procedures with up to three passes to 114 min (51–274 min) with more than three passes (p = 0.000) and 185 min (17–738 min) if rescue devices had to be used (p = 0.000) (see Table 2 for details).
In terms of rescue therapy, stent retrievers of a different manufacturer were used in 24 (8.9 %) target vessels. Other rescue strategies included thrombectomy with other devices (n = 2 (0.7 %)), balloon angioplasty (n = 6 (2.2 %)), permanent implantation of a stent (n = 15 (5.5 %)), distal aspiration without a retriever (n = 1 (0.4 %)), and intraarterial fibrinolysis (n = 2 (0.7 %)).
Procedural adverse events occurred in 54 (19.9 %) patients. Four (1.5 %) patients had reperfusion hemorrhages unrelated to the study device. In five (1.8 %) patients, vessel perforation occurred. Since wire- and device-associated perforation were indistinguishable, all were classified as possibly device-related. Two (0.7 %) were clinically significant. Emboli to previously unaffected vessels occurred in 16 (5.9 %) patients and 14 (5.2 %) were attributable to pREset. Three (1.1 %) new infarcts resulted from these emboli. Intracranial vessel dissections in four (1.5 %) patients were device-related and clinically relevant in one occasion. Inadvertent detachment of two (0.7 %) devices was either due to entangling in an already implanted stent or in a dissection. The first retriever was attached to the wall with a second stent. The second was extracted with another retriever without clinical sequelae. Other procedure-related adverse events were access site vessel dissection in 13 (4.5 %) patients, inguinal pseudoaneurysms in eight (3 %), and retroperitoneal hematoma in one (0.4 %). One surgical revision of the carotid artery after direct puncture (0.4 %) was performed. In summary, 25 (9.2 %) possibly or definitely pREset-related adverse events occurred, of which six (2.2 %) were clinically significant.
Follow-up imaging revealed HT I in 27 (10.0 %), HT II in 25 (9.2 %), PH I in 14 (5.2 %), and PH II in 13 (4.8 %) patients. Focal SAH was detected in 33 (12.2 %) and diffuse in six (2.2 %) individuals. There was an overlap between focal SAH and PH/HT in 13 (4.8 %) patients and between diffuse SAH and PH/HT in four (1.5 %) patients. The rate of any hemorrhage detected on follow-up imaging was 37.3 % (n = 101). The rate of HHI, HHII, PH II, focal, and diffuse SAH as well as any hemorrhage on imaging was not significantly increased in patients treated with > 3 thrombectomy maneuvers or rescue therapy as compared to patients treated with up to three passes. The rate of PH I was increased in patients treated with more than three passes (p = 0.019) but not with rescue treatments (Table 2).
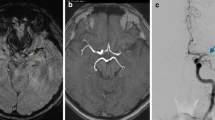
After 90 days, 107 (39.5 %) patients were functionally independent and 67 (24.7 %) were deceased (Fig. 2). The chance to gain functional independence was significantly decreased in procedures with more than three pREset passes (p = 0.003) or rescue treatment (p = 0.02) as compared to procedures with up to three passes.
Discussion
Well-documented clinical cases are a prerequisite for device approval in Europe, but the number of treatments to meet the requirements is limited. Approval studies are rarely published in scientific journals and physicians have to make choices between devices based on limited data or informal exchange of personal experience. Reviewing device performance after approval in a clinical setting adds valuable information regarding safety and efficacy.
Technical modifications of pREset compared to other stent retrievers include a constant cell configuration in variable vessel diameters, a homogeneous distribution of radial forces along the working length, and reduced tapering in curves. Preclinical testing was performed in bench experiments and clinical assessment was carried out in a swine animal model. pREset showed recanalization rates as high as 93.8 % in vivo [10]. Clinical comparative studies with other devices and data from everyday practice are not yet available.
Recanalization results were categorized according to the TICI score. Considering all TCI ≥2, 78.6 % of patients were successfully treated within three passes, closely matching the results of the SWIFT and Trevo2 trial (60.7 and 86.4 %). The rate of TICI ≥2b was 76.4 %, coming close to the 79.2 % reported in the prospective multicenter evaluation of the Solitaire FR device [11]. Obviously, there are no major differences in recanalization rates between the most common stent retriever devices.
Within SWIFT, Trevo2, and STAR, the cutoff for successful recanalization per study device was set after three retrievals. Our series shows that with additional attempts, another 8.5 % of patients can be recanalized with the same device but limits clinical efficacy and increases rates of PH I. With other rescue treatments, the chance for successful recanalization as well as favorable outcome was diminished without excess bleeding rates. Not only reperfusion but timely reperfusion is of major importance for clinical outcome after endovascular stroke treatment [12]. Procedure time significantly increased with any type of rescue therapy after three passes and most likely contributed significantly to the decreased chance of good clinical outcome in these patients. Given the increased rate of futile recanalization and potential additional harm caused by escalation of therapy, the decision to extend the procedure after three unsuccessful retrieval attempts should only be taken after careful consideration of individual factors defining the likelihood for a clinical success. In general, our procedure times were longer than reported in other studies. This may be attributed to the fact that thrombectomy was carried out by a single operator, which causes a time delay for material preparation. Device incubation time was at least 5 min and sums up significantly in multiple pass procedures. Additionally, we included patients with cervical artery stenoses and occlusions. Stent angioplasty was regularly performed before thrombectomy and adds to the procedure time.
In terms of device-related complications, we found 9.2 % adverse events that were possibly or definitely related to the pREset retriever, but only 2.2 % were clinically significant. A recent review of stent retriever thrombectomy reports device-related complications ranging between 0 and 24 %, with an average of 5 and 6 % [13]. The most frequently encountered device-related adverse event in our series was spread of thrombus material to previously unaffected territories. Distal aspiration was used as a protective tool but could not prevent this side effect in every case. Some investigators advocate proximal balloon occlusion, but also, with flow interruption, thrombus fragmentation cannot be avoided completely [14]. An interesting question is whether a combination of both approaches could lead to a significant improvement. Vessel perforation was encountered in five cases, of which only two were clinically relevant. Vessel laceration may be either due to blind probing of the occluded vessel with the wire and microcatheter or by the device itself. Since the etiology was not clearly distinguishable, we counted all perforations as possibly device-related, hence applying a conservative interpretation. Improved road map technologies with simultaneous visualization of the vessel stump and retrograde filling of the distal occlusion site by collateral vessels could make probing safer in the future. Currently available stent retrievers are approved for a minimum vessel diameter of 2 mm, but proximal thrombi often extend into M2 segments measuring less than the given lower limit in most cases. To achieve successful recanalization, device deployment in smaller vessels is often necessary and may cause dissection or even perforation, especially after repeated thrombectomy maneuvers. New devices with less radial force allowing for safe distal deployment are warranted and may help to avoid dissections.
In the population that we treated, 39.5 % had favorable outcome, virtually the same proportion compared to the SWIFT and TREVO2 trials as well as the North American Solitaire Stent retriever registry [1, 2, 15]. Within the European Endostroke registry, 90 days favorable outcome for anterior circulation stroke (carotid-t and proximal middle cerebral artery occlusion) was reported in 41 % of the treated subjects [16]. The retrospective and prospective multicenter studies to evaluate the Solitaire FR for revascularization in acute ischemic stroke reported higher rates of favorable outcome of 55 and 57.9 % [11, 8]. This obvious difference may be explained to a large extent by patient selection issues. The prospective multicenter STAR trial included only patients with a known time window, anterior circulation stroke, and smaller infarcts (CT ASPECTS >6, MRI ASPECTS >4). Cervical carotid artery dissections, occlusion or stenosis, age >85 years, recent myocardial infarction, and endocarditis were an exclusion criterion. For the patients we treated, we set no age limit and we also considered patients with larger infarcts, endocarditis, recent myocardial infarction, unknown time window as well as posterior circulation large vessel occlusion and cervical vessel occlusions or stenoses for endovascular recanalization. Within the retrospective multicenter Solitaire FR evaluation, no predefined criteria existed, but patient selection was done according to the “local institutional standards” of the participating hospitals. These various standards may well have been more restrictive than in our institution, although this remains speculative.
IMS III and SYNTHESIS expansion compared IVT and endovascular therapy. In the IVT arm, the rate of mRS 0–2 was as high as 38.7 and 46.4 %, with no significant difference compared to endovascular treatment (40.8 and 42.0 %). Numerically, there is also no difference compared to the results that we achieved using exclusively a new-generation stent retriever for recanalization. On the other hand, these two randomized trials were confined to patients fulfilling the inclusion criteria for IVT. This again excludes patients >80 years, presenting later than 3–4.5 h or unknown time window, extended infarction in imaging studies, recent surgery or endocarditis as well as any other condition interfering with the thrombolytic medication. Most of these exclusion criteria are known predictors of poor outcome. In addition, the pretreatment vessel status was only known in a subset of patients in IMS III and completely unknown in SYNTHESIS expansion. MR RESCUE, on the other hand, was confined to patients not eligible for IVT (63 %) and IVT failures (37 %). With this modified pre-selection, the rate of good outcome was as low as 20.4 % with standard treatment and 18.6 % after embolectomy with the MERCI retriever. Given the fact that we treated patients with a very broad spectrum of preconditions, the good clinical outcome in 39.5 % cannot generally be regarded as a poor result. Only randomized trials with equal inclusion criteria for endovascular and standard treatment will be able to provide a conclusive answer as to whether stent retriever-based thrombectomy is superior.
Study limitations
Data collection was performed prospectively but the selection criteria and endpoints for this analysis were defined in retrospect. Therefore, the investigation has all potential drawbacks of a retrospective analysis. Since this is a single-center case series, imaging and clinical data were not independently assessed.
Conclusion
In terms of safety and efficacy, pREset performed similarly to other commercially available stent retrievers. Prospective comparative studies are needed to detect minor differences within this family of devices. To avoid futile recanalization and potential additional harm, escalation of therapy beyond three thrombectomy passes should only be performed after careful individual consideration of each case.
References
Broderick J, Palesch Y, Demchuk M et al (2013) Endovascular therapy after intravenous t-PTA versus t-PA alone for stroke. N Engl J Med 368:893–903
Ciccone A, Valvassori L, Nichelatti M et al (2013) Endovascular treatment for acute ischemic stroke. N Engl J Med 368:904–913
Kidwell C, Jahan R, Gornbein J et al (2013) A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 368:914–923
Pierot L, Södermann M, Bendszus M et al (2013) Statement of ESMINT and ESNR regarding recent trials evaluating the endovascular treatment at the acute stage of ischemic stroke. Neuroradiology 55:1313–1318
Saver J, Jahan R, Levy E et al (2012) Solitaire flow restoration device versus the MERCI retriever in patient with acute ischemic stroke (SWIFT): a randomized, parallel-group, non-inferiority trial. Lancet 380:1241–1249
Nogueira RG, Lutsep HL, Gupta R et al (2012) Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet 380:1231–1240
Kranz P, Eastwood J (2009) Does diffusion-weighted imaging represent the ischemic core? An evidence-bases systematic review. Am J Neuroradiol 30:1206–1212
Tomsick T, Broderick J, Carrozella J (2008) et a. (2008) Interventional Management of Stroke II investigators. Revascularization results in the Interventional Management of Stroke II trial. Am J Neuroradiol 29:582–587
Hacke W, Kaste M, Fieschi C et al (1998) Randomized double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischemic stroke (ECASSII). Lancet 352:1245–1251
Mordasini P, Brekenfeld C, Byrne J et al (2012) Experimental evaluation of immediate recanalization effect and recanalization efficacy of a new thrombus retriever for acute stroke treatment in vivo. Am J Neuroradiol. doi:10.3174/ajnr.A3275
Pereira V, Gralla J, Davalos A et al (2013) Prospective, multicenter, single-arm study of mechanical thrombectomy using Solitaire flow restoration in acute ischemic stroke. Stroke 44:2802–2807
Mazigi M, Chaudry S, Ribo M et al (2013) Impact of onset-to-reperfusion time on stroke mortality. Circulation 127:1980–1985
Walcott B, Boehm K, Stapleton C et al (2013) Retrievable stent thrombectomy in the treatment of acute ischemic stroke: analysis of a revolutionizing treatment technique. J Clin Neurosci. doi:10.1016/j.jocn.2013.03.015
Davalos A, Mendes Pereira V, Chapot R et al (2012) Retrospective multicenter study of Solitaire FR for revascularization in the treatment of acute ischemic stroke. Stroke 43:2699–2705
Zaidat O, Castonguay A, Gupta R et al. (2013) North American Solitaire stent retriever acute stroke registry: post marketing revascularization and clinical outcome results. J Neurointervent Surg 0:1-5 doi: 10.1136/neurointersurg-2013-010895
Singer O, Haring H, Trenkler J (2013) Age-dependency of successful recanalization in anterior circulation stroke: the ENDOSTROKE study. Cerebrovasc Dis 365:437–445
Acknowledgements
We thank James Lago for language revision of the manuscript and Hiltrud Niggemann for statistical support.
Conflict of interest
WK has a consulting agreement with phenox. MA-P has proctoring contracts with Covidien/ev3 and phenox. HH is co-inventor of Solitaire, has consulting and proctoring contracts with Codman, Covidien/ev3 and Balt and is co-founder and shareholder of phenox.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurre, W., Aguilar-Pérez, M., Schmid, E. et al. Clinical experience with the pREset stent retriever for the treatment of acute ischemic stroke—a review of 271 consecutive cases. Neuroradiology 56, 397–403 (2014). https://doi.org/10.1007/s00234-014-1346-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-014-1346-y