Abstract
Introduction
Diffusion weighted imaging and diffusion tensor imaging (DTI) give information about the amount and directionality of water diffusion occurring in a given tissue. Here we study the role of diffusion tensor metrics including fractional anisotropy (FA) and spherical anisotropy (CS) in preoperative grading of diffusely infiltrating astrocytomas.
Methods
We performed DTI in 38 patients with pathologically proven diffusely infiltrating astrocytomas, who were classified into two groups, i.e., 15 patients with high-grade astrocytoma (HGAs, WHO grade III and IV) and 23 patients with low-grade astrocytoma (LGAs, WHO grade II). We measured maximum FA and minimum CS values in all cases from tumor. Histopathological diagnosis was established in all cases.
Results
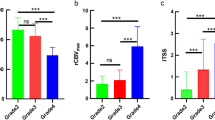
The mean maximum FA values were higher in HGA (0.583 ± 0.104) than LGA (0.295 ± 0.058), while mean minimum CS values were lower in HGA (0.42 ± 0.121) than LGA (0.722 ± 0.061). The difference in the diffusion tensor indices between HGA and LGA was found to be statistically significant with P value of <0.001. Keeping cutoff FA value of 0.4, all HGAs showed higher maximum FA values, and all LGAs showed lower maximum FA values. Also, all HGAs showed minimum CS values less than a cutoff value of 0.6, and all LGAs showed minimum CS values higher than 0.6.
Conclusion
Diffusion tensor metrics such as maximum FA and minimum CS can help to differentiate HGA from LGA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Astrocytomas are the most common primary neoplasms of the central nervous system [1]. It occurs in all age groups. Diffuse infiltrating astrocytomas have been classified into three grades by World Health Organization (WHO) [2]. Grade II are low-grade astrocytomas, and Grade III and IV are high-grade astrocytomas. Prognosis and management depends on the grade of astrocytoma [3, 4]. There are many conventional magnetic resonance imaging (MRI) features described to differentiate between high- and low-grade infiltrating astrocytomas [5–7]. Also, there are many advanced MRI techniques, like MR perfusion and MR spectroscopy, described to grade these tumors [8–13]. However, none of the imaging features or advanced imaging techniques is absolute, and there is always some overlap [10, 14]. Diffusion tensor imaging (DTI) is a new MR technique used to study the microstructure of brain lesions [15]. Various diffusion tensor metrics can give an idea about the cellularity and arrangement of cells within a neoplasm [16–21]. Here we study the role of intratumoral diffusion tensor metrics in preoperative grading of these gliomas.
Methods and materials
Patient population
Thirty-eight patients with post surgical pathologically proven diffusely infiltrating astrocytomas were included in this retrospective study. Astrocytomas were graded according to WHO grading. Patients were classified into two groups. The first group included 15 patients with high-grade astrocytomas (HGAs, WHO grade III (approximately 10%) and IV (approximately 90%)), and the second group included 23 patients with low-grade astrocytomas (LGAs, WHO grade II). None of the tumors showed oligodendroglial component. Mean age of patients was 42.13 years (range 22–68 years), 25 were males, 13 females. All tumors were located supratentorially. Institutional review board approval was waived for this retrospective study.
MR imaging
In all the patients, MR examination of the brain was performed on a 1.5 T clinical MR Scanner (Avanto TIM SQ engine; Siemens, Erlangen, Germany). Each patient underwent conventional MRI with T1, T2, FLAIR, and post-contrast T1 sequences. A spin echo-type echo planar imaging sequence with diffusion gradients applied in 30 non-collinear directions was used for the diffusion tensor imaging. The following imaging parameters were used: TR 3,500 ms, TE 105 ms, matrix 192 × 192, FOV 230 mm2, 5 mm slice thickness with 1.5 mm gap, averaged twice and with a b factor of 0 and 1,000 s/mm2.
Post-processing and analysis
The analysis and post-processing was performed on a separate workstation (Leonardo, Siemens, Erlangen, Germany). The DTI study analysis was jointly performed by two neuroradiologists (MJ and SP). Both the neuroradiologists were blinded to the histopathology results. Both DTI and post-contrast T1-weighted axial images (T1WI) were loaded to DTI software provided by the vendor. The regions of interest (ROI) were placed outlining the tumor in all post-contrast T1-weighted axial images in which tumor was seen. These ROIs were automatically interpolated on the respective fractional anisotropy (FA) and spherical anisotropy (CS) maps by the software. Then the maximum value of FA and minimum value of CS were calculated from all the ROIs of all the axial images showing tumor.
Statistical analysis
Statistical analysis was performed by a statistician. Mean maximum FA and minimum CS values with standard deviation were calculated for HGAs and LGAs. Differences in these metrics between HGAs and LGAs were evaluated using ANOVA test, and P values were calculated. P values less than 0.01 were considered to be statistically significant.
Results
Table 1 and Fig. 1 summarize the diffusion tensor metrics derived from HGAs and LGAs. Maximum FA and minimum CS values showed increased anisotropy in HGA (mean 0.583 ± 0.104 and 0.42 ± 0.121, respectively) compared to LGA (mean 0.295 ± 0.058 and 0.722 ± 0.061, respectively) (Figs. 2 and 3). The difference in both these diffusion tensor indices between HGAs and LGAs was found to be statistically significant with P value of <0.001. All HGAs showed maximum FA values higher than cutoff value of 0.4, and all LGAs showed maximum FA values <0.4 (sensitivity and specificity of 100%). Also keeping cutoff value of 0.6, all HGAs showed lower minimum CS values, and all LGAs showed higher minimum CS values.
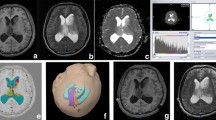
Right frontal lobe mass lesion in a 40-year-old woman. Solid anterior part of the lesion appears mildly hyperintense on T2-weighted image (a) and shows contrast enhancement on post-contrast T1-weighted image (b). FA map (c) and directionally encoded FA map (d) shows increased anisotropy in anterior solid part of tumor, while CS map (e) shows decreased isotropic diffusion. Histopathology slide (f) showing high-grade astrocytoma with increased cellularity causing increased FA
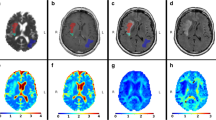
Thirty-two-year-old man presenting with tumor in left precentral gyrus, which appears hyperintense on T2 (a) and hypointense on T1-weighted image (b). It shows decreased anisotropy on FA map (c) and directionally encoded FA map (d) and shows increased isotropy on CS map (e). Histopathological slide (f) shows low-grade astrocytoma with predominantly microcystic changes causing decreased FA
Discussion
Diffusion weighted imaging displays the microscopic thermal Brownian motion of water molecules in the tissue [22]. Mean diffusivity values show whether the diffusion of water molecules is restricted or facilitated. Water diffusion may not occur equally in all directions (isotropic) but may occur greater in one direction than another (anisotropic). Such directionality can be quantified by diffusion tensor matrices like fractional anisotropy and spherical anisotropy (CS) [21, 23, 24]. The FA value is expressed as a numerical value between 0 and 1 without a unit. FA of more than 0.4 generally suggests omnidirectional structural organization [25]. CS is one of the three types of anisotropy described, i.e., linear, planar, and spherical [26, 27]. In linear anisotropy, the diffusion occurs along a line in the direction of the largest eigenvalue (λ1 ≫ λ2 and λ3); in planar anisotropy, the diffusion is restricted to a geometric plane formed by two largest eigenvalues (λ1 ~ λ2 ≫ λ3); whereas in spherical anisotropy, the diffusion occurs in all three directions and hence is isotropic (λ1 ~ λ2 ~ λ3). Thus CS gives the measure of isotropic diffusion within the tissue.
where λ1, λ2, and λ3 represent three eigenvalues of the diffusion ellipsoid [21, 26–28], and Dav is mean diffusivity.
In body tissues, water diffusion is not free but is abnormal due to hindrance by presence of complex microstructural barriers such as white matter tracts, cell membranes, and/or capillary vessels [29]. Diffusion weighted imaging and diffusion tensor imaging has been widely used to study the microstructure of brain tumors including the cellularity, arrangement of cells and/or vascularity [5, 16–21, 30–31].
FA and CS values in astrocytomas are thought to be affected by arrangement of cells within tumor. Tumors with higher cellularity and linear or planar arrangement will show increased anisotropy. So HGA will show increased FA and decreased CS, while LGA having lower cellularity with randomly arranged cells will show low FA and high CS [16–21].
Diffuse infiltrating astrocytomas are well known to contain different grades within same tumor [1]. So if the diffusion tensor metrics (DTMs) are calculated from whole tumor, there is a theoretical chance to miss the small foci of high-grade tumor within otherwise low-grade tumor. Also in HGAs with necrosis, calculating DTMs from whole tumor will not give real value of the cellularity and arrangement of cells within the solid component of the tumor. Here, we propose a new method of calculating diffusion tensor metrics. We placed ROIs in whole tumor and calculated the maximum FA and minimum CS values. These are the FA and CS values from the voxel having maximum FA and minimum CS, i.e., having most regularly and compactly arranged cells. So even a small foci (or voxel) with regularly compactly arranged cells can be detected using this method.
In our study, we found significant difference in maximum FA and minimum CS values of HGAs and LGAs (P value <0.001). We tried to propose the cutoff values of maximum FA and minimum CS in differentiating HGA from LGA. Keeping the cutoff value of maximum FA of 0.4, we found that all HGAs showed higher maximum FA values, and all LGAs showed lower maximum FA values. Similarly keeping the cutoff value of minimum CS of 0.6, we found that all HGAs showed lower minimum CS values, and all LGAs showed higher minimum CS values.
FA and CS values in astrocytomas is determined by balance between the factors decreasing the degree of the directionality of water diffusion, such as fiber destruction or displacement, and factors increasing it, such as high cell density with linear or planar arranged cells and/or vascularity [17–20]. So in HGA, though there is destruction of white matter fibers causing decrease in FA compared to normal appearing white matter, the FA does not decrease much. The increase in cell density with symmetric histological organization and vascularity gives some directionality to the water diffusing in extracellular space resulting in relatively high FA [17–20]. In LGA, cells are loosely and randomly arranged in fibrillary matrix, allowing water to diffuse freely in all direction and thereby decreasing FA significantly [17–20].
In a study by Beppu et al. [17], the authors were not able to differentiate between anaplastic and low-grade astrocytomas using FA values. However, in our study we were able to differentiate between these tumors. This may be due to the difference in method of ROI placing within the tumor. Beppu et al. had placed the ROI on a slice showing maximal tumor size in post-contrast T1-weighted or T2-weighted image and on the ring-enhancing region of a necrotic tumor and calculated the mean FA value. Also, in a study by Inoue et al. [18], authors have not taken into account the heterogenicity of the astrocytomas, which is well documented in literature [1]. We placed ROI in tumor on post-contrast T1-weighted images in all the axial cuts in which tumor was seen and calculated the maximum FA and minimum CS values. By doing this, we were able to detect the areas of high-grade within otherwise low-grade tumors; and because of this technique, the mean maximum FA values in HGAs and LGAs reported in our study are much higher than the mean FA values that are reported in literature [17–20].
Our study possesses some limitations regarding interpretation of the FA values. Though we hypothesize that high FA corresponds histologically to compactly and regularly arranged tumor cells, we do not have direct pathological correlation.
Conclusion
Based on the results of this study, the values of diffusion tensor metrics like maximum FA and minimum CS can help to differentiate diffuse infiltrating HGAs from LGAs. Keeping maximum FA cutoff value of 0.4 and minimum CS cutoff value of 0.6, we were able to differentiate high- from low-grade diffuse infiltrating astrocytomas. Further longitudinal studies with more number of patients are needed to evaluate whether these measurements can help in detecting foci of malignant change in a low-grade tumor on follow-up imaging.
References
Lantos PL, Louis DN, Rosenblum MK, Kleihues P (2002) Tumors of nervous system. In: Graham DI, Lantos PL (eds) Greenfield’s neuropathology, vol II, 7th edn. Arnold, London, pp 767–1052
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) (2007) WHO classification of tumours of the central nervous system. IARC, Lyon
Kayama T, Kumabe T, Tominaga T, Yoshimoto T (1996) Prognostic value of complete response after the initial treatment for malignant astrocytoma. Neurol Res 18:321–324
Black PM (1991) Brain tumors. Part 1. N Engl J Med 324:1471–1476
Sugahara T, Korogi Y, Kochi M et al (1999) Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 9:53–60
Dean BL, Drayer BP, Bird CR et al (1990) Gliomas: classification with MR imaging. Radiology 174:411–415
Watanabe M, Tanaka R, Takeda N (1992) Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 34:463–469
Law M, Yang S, Wang H et al (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24:1989–1998
Law M, Yang S, Babb JS et al (2004) Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 25:746–755
Tamiya T, Kinoshita K, Ono Y, Matsumoto K, Furuta T, Ohmoto T (2000) Proton magnetic resonance spectroscopy reflects cellular proliferative activity in astrocytomas. Neuroradiology 42:333–338
Shimizu H, Kumabe T, Shirane R, Yoshimoto T (2000) Correlation between choline level measured by proton MR spectroscopy and Ki-67 labeling index in gliomas. AJNR Am J Neuroradiol 21:659–665
Castillo M, Smith JK, Kwock L (2000) Correlation of myo-inositol levels and grading of cerebral astrocytomas. AJNR Am J Neuroradiol 21:1645–1649
Kim J, Chang K, Na DG et al (2006) 3T 1H-MR spectroscopy in grading of cerebral gliomas: comparison of short and intermediate echo time sequences. AJNR Am J Neuroradiol 27:1412–1418
Kondziolka D, Lunsford LD, Martinez AJ (1993) Unreliability of contemporary neurodiagnostic imaging in evaluating suspected adult supratentorial (low-grade) astrocytoma. J Neurosurg 79:533–536
Sundgren PC, Dong Q, Gómez-Hassan D, Mukherji SK, Maly P, Welsh R (2004) Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology 46:339–350
Toh CH, Castillo M, Wong AMC et al (2008) Differentiation between classic and atypical meningiomas with use of diffusion tensor imaging. Am J Neuroradiol 29:1630–1635
Beppu T, Inoue T, Shibata Y et al (2003) Measurement of fractional anisotropy using diffusion tensor MRI in supratentorial astrocytic tumors. J Neurooncol 63:109–116
Inoue T, Ogasawara K, Beppu T, Oqawa A, Kabasawa H (2005) Diffusion tensor imaging for preoperative evaluation of tumor grade in gliomas. Clin Neurol Neurosurg 107:174–180
Beppu T, Inoue T, Shibata Y, PhDb YN et al (2005) Fractional anisotropy value by diffusion tensor magnetic resonance imaging as a predictor of cell density and proliferation activity of glioblastomas. Surgical Neurology 63:56–61
Sinha S, Bastin ME, Whittle IR, Wardlaw JM (2002) Diffusion tensor MR imaging of high-grade cerebral gliomas. AJNR Am J Neuroradiol 23(4):520–527
Jolapara M, Kesavadas C, Radhakrishnan VV et al (2009) Diffusion tensor mode in imaging of intracranial epidermoid cysts: one step ahead of fractional anisotropy. Neuroradiology 51(2):123–129
Hagmann P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, Meuli R (2006) Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. RadioGraphics 26:S205–S223
Le Bihan D, Mangain JF, Poupon C et al (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13(4):534–546
Thomas B, Sunaert S (2005) Diffusion tensor imaging: technique, clinical and research applications. Rivista de Neuroradiologia 18:419–435
Chenevert TL, Sundgren PC, Ross BD (2006) Diffusion imaging: insight to cell status and cytoarchitecture. Neuroimag Clin N Am 16:619–632
Bahn MM (1999) Invariant and orthonormal scalar measures derived from magnetic resonance diffusion tensor imaging. J Magn Reson 141:68–77
Ennis DB, Kindlmann G (2006) Orthogonal tensor invariants and the analysis of diffusion tensor magnetic resonance images. Magnetic Resonance in Medicine 55:136–146
Peled S, Gudbjartsson H, Westin CF, Kikinis R, Jolesz FA (1998) Magnetic resonance imaging shows orientation and asymmetry of white matter fiber tracts. Brain Res 780:27–33
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111(3):209–219
Kono K, Inoue Y, Nakayama K et al (2001) The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 22:1081–1088
Castillo M, Smith JK, Kwock L, Wilber K (2001) Apparent diffusion coefficients in the evaluation of high-grade cerebral gliomas. AJNR Am J Neuroradiol 22(1):60–64
Acknowledgements
We thank Dr. Sankara Sarma, Additional Professor of Biostatistics, for his advice on statistics.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jolapara, M., Patro, S.N., Kesavadas, C. et al. Can diffusion tensor metrics help in preoperative grading of diffusely infiltrating astrocytomas? A retrospective study of 36 cases. Neuroradiology 53, 63–68 (2011). https://doi.org/10.1007/s00234-010-0761-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-010-0761-y