Abstract
Introduction
Protoplasmic astrocytomas are a poorly recognized and uncommon subtype of astrocytoma. While usually categorized with other low-grade gliomas, there is literature to suggest that protoplasmic astrocytomas have differences in biology compared to other gliomas in this group. This paper presents the MR imaging characteristics of a series of eight protoplasmic astrocytomas.
Methods
We retrospectively reviewed MR images and histopathology of eight consecutive cases of histologically proven protoplasmic astrocytomas.
Results
Patients ranged from 17 to 51 years of age with a 5:3 male to female ratio. The tumors were located in the frontal or temporal lobes and tended to be large, well defined, and had a very high signal on T2 (close to cerebrospinal fluid). Generally, a large proportion of the tumor showed substantial signal suppression on T2 fluid-attenuated inversion recovery (FLAIR). Six of the eight lesions also demonstrated a partial or complete rim of reduced apparent diffusion coefficient (ADC) around the T2 FLAIR suppressing portion.
Conclusions
The possibility that a primary cerebral neoplasm represents a protoplasmic astrocytoma should be considered in a patient with a large frontal or temporal tumor that has a very high signal on T2 with a large proportion of the tumor showing substantial T2 FLAIR suppression. A further clue is a partial or complete rim of reduced ADC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protoplasmic astrocytomas are a rare variety of astrocytomas consisting of process-poor astrocytes on a microcystic background [1]. They are classified as a grade II astrocytoma by the World Health Organization (WHO) [2], alongside fibrillary and gemistocytic astrocytomas. This grouping implies a similar behavior and prognosis of these subtypes; however, specific literature on protoplasmic astrocytomas is lacking. The available literature does suggest that the prognosis is relatively indolent, similar to the more common fibrillary astrocytoma, but with a tendency to present at a younger age and a greater propensity to involve the temporal lobe [1].
There are still considerable challenges in the recognition of this entity as there is a paucity of literature on this tumor, let alone in the radiology literature, which is restricted to a single angiographic case report [3]. Previous case reports of protoplasmic astrocytomas have shown that they can present as diagnostic dilemmas [3, 4]. With this in mind we sought to present the magnetic resonance imaging characteristics of eight histologically proven protoplasmic astrocytomas.
Methods
The institutional review board approval was obtained. Images of all eight patients (as determined by the department of pathology records) with histologically proven protoplasmic astrocytomas treated at our institution between January 2008 and December 2009 were retrospectively reviewed. Histopathology slides were available in all cases and reviewed by a neuropathologist. Patient demographics and clinical presentation were recorded.
Pre-operative MR images were available in all patients. Six of the patients were preoperatively imaged at our institution, four on a 1.5T EchoSpeed Plus LX 9 MRI (General Electric, Milwaukee, WI), and two on a 1.5T HDx platform (General Electric, Milwaukee, WI). One patient was imaged externally on a 1.5T Signa MRI (General Electric, Milwaukee, WI). The final patient was imaged overseas and information of the type of MR system was not available, but a partial study was performed at our institution to complete the pre-operative work-up, including T2 fluid-attenuated inversion recovery (FLAIR) images on a 3T Magnetom Trio (Siemens, Ehrlangen, Germany). Pre- and post-contrast T1 spin echo images were available in all patients (repetition time/echo time (TR/TE) = 400−600/6.4−9 ms), as were T2 fast spin echo (TR/TE = 3950−5800/102−140 ms), and T2 FLAIR images (inversion time (TI) = 2000−2200, TR/TE = 8000−9000/118−136 ms). A susceptibility sensitive sequence was also available in all patients, as was diffusion-weighted imaging. T2* perfusion was performed on two patients and magnetic resonance spectroscopy in three patients. Non-contrast and contrast-enhanced CT scans were available in three patients, though for one patient this was performed at an outside institution and only film sheets were available for review rendering Hounsfield unit measurements impossible.
All eight cases of protoplasmic astrocytomas were assessed in consensus by two neuroradiologists for location, size of lesion, signal characteristics, proportion and degree of T2 FLAIR signal suppression, contrast enhancement, surrounding vasogenic edema, apparent diffusion coefficient, susceptibility artifact, and adjacent calvarial scalloping. Where possible, presence of calcification, perfusion, and spectroscopic characteristics were also assessed.
Results
The mean age of the patients was 32 (range, 17 to 51) with five males and three females. Six of the patients presented with new onset seizures, one with headache and one with a new neurological deficit.
Imaging characteristics are presented in Table 1 and examples are shown in Figs. 1 and 2.
None of the three tumors with preoperative CT imaging demonstrated calcification or hemorrhage. All three tumors were of low density, with measurements of six and 15 Hounsfield units for the lesions where measurement was possible.
In the two lesions where perfusion imaging was available, cerebral blood flow and volume was clearly below that of the white matter. One of these tumors had a small line of cerebral blood flow/volume similar to the cortex, which possibly represented an area of preserved cortex.
Magnetic resonance spectroscopy was performed on three patients. Choline to creatine ratio was moderately elevated in all three tumors, ranging from approximately 1.6 to 2.3. N-Acetylaspartate (NAA) was substantially depressed leading to an elevated choline to NAA ratio, measuring 1.3 in one case and even higher in the other two cases that were hard to accurately quantify as the very small NAA peak was difficult to confidently distinguish from baseline noise. A myo-inositol peak was demonstrated in all three tumors, and there was a possible small doublet at 1.3 parts per million in two of the tumors suggesting the presence of a lactate peak.
An example of the perfusion and spectroscopic findings is shown in Fig. 3.
26-year-old female patient presenting with seizures. a T2, b cerebral blood flow, c cerebral blood volume of a left frontal lobe lesion demonstrating cerebral blood flow and volume below that of the white matter d single voxel point-resolved spectroscopy MRS with a TE of 30 ms showing an elevated choline/creatine ratio and a substantially elevated choline/NAA ratio
When the histology of the resected specimens was reviewed, six of the eight tumors demonstrated increased microcystic change in the central portions of the lesion relative to the peripheral portions of the lesions (Fig. 4). The remaining two tumors demonstrated regions with a greater portion of microcysts, but retrospective orientation of the specimens to the central versus peripheral portion of the lesion was not possible.
Discussion
In this retrospective series, the protoplasmic astrocytomas tended to be large, well-defined lesions in young adults with a predilection for the frontal and temporal lobes, which is consistent with the findings from a previous pathological case series by Prayson of 16 such tumors [1]. As in that paper, the most common presenting symptom was seizures. The mean age of patients at presentation in our series of 32.2 years was higher than in Prayson's paper, where the mean age was 20.7. This could be accounted for by the absence of pediatric cases at our institution.
Most of the imaging features demonstrated were consistent with those of low-grade astrocytomas. The lesions were generally well defined, of reasonably homogenous high T2 signal, without vasogenic edema, and without susceptibility artifact to suggest hemorrhage or calcification.
The cerebral blood volume was also very low, a feature consistent with the low-grade nature of the lesion, though the cerebral blood volume observed was noticeably lower than the white matter, which is less than typically reported for low-grade astrocytomas [5]. Macroscopically, protoplasmic astrocytomas demonstrate replacement of white matter by gelatinous tissue with a semi-translucent, homogenous appearance [6], which presumably is not very vascular and may account for the cerebral blood volume result.
Four of eight lesions did not demonstrate enhancement while three of the lesions demonstrated faint enhancement. Though enhancement raises the possibility of a higher grade lesion, enhancement has been described in low-grade astrocytomas [7], and the enhancement in these three cases was very mild. One of the cases did demonstrate moderate enhancement of a nodule. While it was not possible to definitively correlate the location of this nodule on the resected specimen no area of higher grade dedifferentiation was noted on histology. Meningeal enhancement was not demonstrated in any of the cases, but has been described in a previous case report [4].
In our series, however, we have also documented features in protoplasmic astrocytomas that are not typically ascribed to low-grade astrocytomas. Notably, while the lesions were generally high signal on T2, all of the lesions demonstrated suppression of portions of the lesions on T2 FLAIR. For five of the eight tumors this signal suppression involved greater than 50% of the lesion (on a typical axial slice) to white-matter signal or darker. The remaining cases showed lesser degrees of signal suppression or this suppression involved a smaller proportion of the tumor. Presumably this implies that these portions of the tumor have similar T1 times to CSF.
This signal suppression can result in a very heterogeneous appearance on T2 FLAIR and could be misinterpreted as cyst formation or necrosis, features more typically associated with higher grade astrocytomas [8]. Mild enhancement, if present, may add to the impression of a higher grade lesion. This has potential management consequences as it could affect the treatment options offered to the patient or alter the scope of an attempted resection with implications for post-treatment morbidity.
The other imaging feature that may wrongly indicate a higher grade astrocytoma is the spectroscopy. The choline to creatine ratio was sufficiently elevated to suggest a higher grade lesion, and the NAA was substantially attenuated resulting in a markedly elevated choline to NAA ratio, further implying a high-grade lesion [9, 10]. Review of the three cases demonstrated that voxel placement included T2 FLAIR suppressing portions of the lesions. These regions were hard to avoid in the tumors in our series and may be a confounding factor in these spectra as studies on spectroscopy in gliomas have generally avoided areas of apparent necrosis or cyst formation [9, 10]. Inclusion of these “cystic” regions where there is displacement or replacement of neurons could conceivably explain the reduction of NAA and creatine relative to choline.
It is also of note that the spectra, including these “cystic” regions did not demonstrate the markedly elevated lipid/lactate and depletion of the other metabolites typically seen in frank necrosis[11], which is consistent with the histological finding that these regions contain tumor cells on a background of microcystic change rather than necrosis.
Based on the imaging features in this series, protoplasmic astrocytomas could also be confused with dysembryoplastic neuroepithelial tumors (DNTs), in which suppression of internal T2 signal on T2 FLAIR imaging has been described [12]. Like protoplasmic astrocytomas DNTs tend to occur in young patients, presenting with seizure. They are generally located in the frontal or temporal lobes, do not demonstrate surrounding vasogenic edema and may demonstrate calvarial scalloping [13]. To complicate matters DNTs can appear similar to protoplasmic astrocytomas on histology, and it has been suggested by some that protoplasmic astrocytomas represent a variant of DNTs [14]. There are, however, histological features, such as the multinodular/multifocal architecture, participation of glial and neural elements and associated cortical dysplasia in DNTs that may allow histological distinction [15].
From an imaging differential perspective, protoplasmic astrocytomas in our series tended to be larger (mean diameter, 54 mm) than reported for DNTs (30 mm) [13]. Like DNTs, the protoplasmic astrocytomas also involved the cortex, but subcortical white matter was involved in all cases (versus 12 out of 53 cases in a series for DNTs) [13], and in three of the cases, the tumor appeared to be centered here. Features such as a triangular appearance, tapering towards the ventricle, and internal septations described for DNTs [16] were generally not a feature of protoplasmic astrocytomas in our series.
With regard to the T2 FLAIR suppression, the paper on DNTs [12] described this more as a thin rim of high T2 FLAIR signal, suggesting that the bulk of the tumor signal suppressed, with the ring having a mean width of 1.3 mm and a range of 0.8 to 3.1 mm. In our case series, while substantial portions of the tumor demonstrated T2 FLAIR suppression, there were generally larger areas that demonstrated residual high T2 FLAIR signal. Two of the eight cases did demonstrate only a thin rim of residual high T2 FLAIR signal, which would fall into the range described for DNTs, but the remainder showed thicker (even >1 cm) residual areas of high T2 FLAIR signal. Regardless, this would suggest that protoplasmic astrocytomas are a differential for lesions that demonstrate internal T2 FLAIR signal suppression.
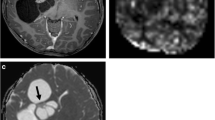
The other distinct imaging feature in most of the protoplasmic astrocytoma cases was the presence of a ring (complete or incomplete) of reduced apparent diffusion coefficient (ADC) (Fig. 5). This was convincingly present in six of the eight cases. The rings were generally thin and around the area of T2 FLAIR signal suppression, rather than involving the whole non-T2 FLAIR suppressing lesion. The ADC was not reduced below that of the cerebral cortex, but was reduced compared to the surrounding lesion, which demonstrated elevated ADC.
ADC in gliomas is thought to relate to cellularity [17, 18], so it could be hypothesized that the rim of reduced ADC could reflect a more cellular portion of the tumor. This was supported by histological findings in our series, which generally showed the lesions to have more microcystic change in the central portions of the lesions and a more solid, cellular appearance in the periphery of the lesion.
Currently, the prognosis and treatment of protoplasmic astrocytomas is assumed to be consistent with other WHO grade 2 astrocytomas, though there is very little literature on this and it has been previously suggested that protoplasmic astrocytomas may potentially have a better prognosis than other grade 2 astrocytomas [15]. It is of note that there is significant heterogeneity among the WHO grade 2 astrocytomas with gemistocytic astrocytomas having an inferior prognosis to other WHO grade 2 astrocytomas [19]. The molecular significance of the different subtypes of CNS tumors is not clear, but likely relates to differences in genetics and molecular biology. Identification of imaging biomarkers that may correlate with these subtypes may improve our understanding of these tumors
Conclusion
The possibility that a lesion represents a protoplasmic astrocytoma should be considered in a large cortical or subcortical frontal or temporal lobe lesion especially if a substantial portion of the tumor demonstrates T2 FLAIR suppression and the lesion demonstrates a partial or complete rim of reduced apparent diffusion coefficient around the T2 FLAIR suppressing portion. Better identification of this lesion may facilitate further research into the biology of this tumor versus other astrocytomas.
References
Prayson RA, Estes ML (1995) Protoplasmic astrocytoma. A clinicopathological study of 16 tumors. Am J Clin Pathol 103:705–709
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) (2007) WHO classification of tumours of the central nervous system, 4th edn. Lyon, IARC
Gnekow W, Prolo D (1971) Protoplasmic astrocytoma: an angiographic case report. Radiology 98:619–622
Manley S, Crooks D, Artingstall L et al (2010) Diffuse central nervous system protoplasmic astrocytoma. Pediatr Blood Cancer 54:768–769
Knopp EA, Cha S, Johnson G et al (1999) Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 211:791–798
Hedges TR (2004) Tumors of neuroectodermal origin. In: Miller NR, Newman NJ, Biousse V, Kerrison JB (eds) Walsh & Hoyt's clinical neuro-ophthalmology, 6th edn. Lippincott Williams & Wilkins, Philadelphia, pp 1413–1482
Piepmeier J, Christopher S, Spencer D et al (1996) Variations in the natural history and survival of patients with supratentorial low-grade astrocytomas. Neurosurgery 38:872–878
Dean BL, Drayer BP, Bird CR et al (1990) Gliomas: classification with MR imaging. Radiology 174:411–415
Law M, Yang S, Wang H et al (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24:1989–1998
Kim JH, Chang KH, Na DG et al (2006) 3 T 1H-MR spectroscopy in grading of cerebral gliomas: comparison of short and intermediate echo time sequences. AJNR Am J Neuroradiol 27:1412–1418
Ricci PE, Pitt A, Keller PJ, Coons SW, Heiserman JE (2000) Effect of voxel position on single-voxel MR spectroscopy findings. AJNR Am J Neuroradiol 21:367–374
Parmar HA, Hawkins C, Ozelame R, Chuang S, Rutka J, Blaser S (2007) Fluid-attenuated inversion recovery ring sign as a marker of dysembryoplastic neuroepithelial tumors. J Comput Assist Tomogr 31:348–353
Stanescu Cosson R, Varlet P, Beuvon F et al (2001) Dysembryoplastic neuroepithelial tumors: CT, MR findings and imaging follow-up. A Study of 53 cases. J Neuroradiol 28:230–240
Daumas-Duport C (1993) Dysembryoplastic neuroepithelial tumors. Brain Pathol 3:283–295
Prayson RA, Cohen ML (2000) Practical differential diagnosis in surgical neuropathology. Humana Press, Totowa, pp 17–20
Fernandez C, Girard N, Paz Paredes A, Bouvier-Labit C, Lena G, Figarella-Branger D (2003) The usefulness of MR imaging in the diagnosis of dysembryoplastic neuroepithelial tumor in children: a study of 14 cases. AJNR Am J Neuroradiol 24:829–834
Sugahara T, Korogi Y, Kochi M et al (1999) Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 9:53–60
Kono K, Inoue Y, Nakayama K et al (2001) The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 22:1081–1088
Krouwer HG, Davis RL, Silver P, Prados M (1991) Gemistocytic astrocytomas: a reappraisal. J Neurosurg 74:399–406
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tay, K.L., Tsui, A., Phal, P.M. et al. MR imaging characteristics of protoplasmic astrocytomas. Neuroradiology 53, 405–411 (2011). https://doi.org/10.1007/s00234-010-0741-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-010-0741-2