Abstract
Introduction
Our purpose was to clarify the magnetic resonance (MR) imaging characteristics of the brachial and lumbar plexuses in patients with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) using various kinds of sequences, including diffusion-weighted images (DWI).
Methods
We evaluated the MR imaging findings for lumbar and/or brachial nerve plexuses in 13 CIDP patients and 11 normal volunteers. The nerve swelling was evaluated in comparison with normal controls by coronal short tau inversion recovery (STIR), and signal abnormalities were evaluated by coronal STIR, T1-weighted images, and DWIs. The degrees of contrast enhancement and apparent diffusion coefficient (ADC) values of the plexus were also assessed.
Results
In the patient group, diffuse enlargement and abnormally high signals were detected in 16 out of 24 plexuses (66.7%) on STIR, a slightly high signal was detected in 12 of 24 plexuses (50%) on T1-weighted images, and a high-intensity signal was detected in 10 of 18 plexuses (55.6%) on DWIs with high ADC values. Contrast enhancement of the plexuses was revealed in 6 of 19 plexuses (31.6%) and was mild in all cases. There were statistically significant differences between the ADC values of patients with either swelling or abnormal signals and those of both normal volunteers and patients without neither swelling nor abnormal signals. There were no relationships between MR imaging and any clinical findings.
Conclusion
STIR is sufficient to assist clinicians in diagnosing CIDP. T1-weighted images and DWIs seemed useful for speculating about the pathological changes in swollen plexuses in CIDP patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is an acquired peripheral neuropathy of presumed autoimmune etiology, which is either a chronically progressive or relapsing–remitting disorder [1]. The major symptoms are bilateral proximal and distal limb muscle weakness and sensory loss. Motor deficits are usually predominant. This disease commonly affects brachial or lumbar plexuses distributed in the muscles of the extremities. Cranial or diaphragmatic nerve involvement is rarely seen. The involved peripheral nerves in CIDP reveal a unique pathological finding. They are grossly enlarged due to proliferation of surrounding Schwann cells, causing an “onion bulb” appearance [1], although the axons are usually preserved.
A diagnosis of CIDP is generally based on clinical features and electrophysiological studies. As the disease has been studied in various clinical trials, several clinical definitions of this neuropathy have been proposed [2–4]. Cerebrospinal fluid examination, nerve biopsy, and magnetic resonance (MR) examinations provide supportive information but are not always required for diagnosis. However, demonstrable MR imaging findings have been reported, and they are helpful for diagnosis.
Several studies have described the MR imaging findings of CIDP patients. Swelling brachial or lumbar plexuses with increased signal intensity on T2-weighted images with or without contrast enhancement have been reported [5–9]. Coronal short tau inversion recovery (STIR) imaging is particularly helpful for depicting the signal abnormalities of brachial and lumbar plexuses [6]. However, no original reports have evaluated either the diffusion property in patients with CIDP or the T1-weighted images obtained before gadolinium administration, although the contrast enhancement pattern of the nerve plexus has been examined [5, 6].
In this study, we evaluated MR imaging findings of the brachial or lumbar plexus in patients with CIDP using STIR, T1-weighted images before and after contrast enhancement, and diffusion-weighted MRI (DWI), along with the calculation of apparent diffusion coefficient (ADC) values. The aim of this study was to demonstrate the MR imaging findings including some new sequences and to clarify the role of the MR examination in supporting the diagnosis of CIDP.
Materials and methods
Patients
The subjects included 13 consecutive patients (five males and eight females, ranging in age from 5 to 85 years old, mean age ± SD; 45.2 ± 25.0 years) who consulted our hospital from 2004 to 2007 and agreed to undergo an MR imaging examination. MR images of all 13 patients with CIDP were retrospectively reviewed. The patients all met the clinical and neurophysiological criteria for CIDP (Joint Task Force of the EFNS and PNS) [2]. No other cause of the neuropathy was found in clinical, laboratory, or histological investigations. None of the patients had a history of exposure to neurotoxic agents or a family history of neuropathy. Disease duration at the time of the MR studies ranged from 6 months to 20 years (mean ± SD; 5.0 ± 6.2 years). Eleven patients had the relapsing–remitting form of the disease and two had the progressive form (cases 3 and 7). In eight patients, no treatment was administered before the MR study. In two patients (cases 1 and 4), immunoglobulin was administered before the MR examination because Guillain–Barre syndrome had been one of their possible diagnoses. The remaining three patients (cases 5, 8, and 10) had long disease duration, and both immunoglobulin and corticosteroid therapy had already been administered before the MR studies. The F wave, which indicated motor conduction along the entire peripheral axon including the radicular segment, was examined as an electrodiagnostic study in all patients. The median nerve conduction was measured at the wrist, and the tibial nerve conduction was measured at the ankle. In one patient, a biopsy was performed at the sural nerve with pathological confirmation of CIDP.
MR studies were also performed in 11 normal volunteers (six males and five females) as a control group. Their ages ranged from 22 to 73 years (mean ± SD; 56.5 ± 16.7 years). This study had appropriate Ethics Committee approval.
Imaging
MR examinations were performed on a 1.0-T scanner (Harmony; Siemens, Erlangen, Germany) using a spine array coil. MR studies of both cervical and lumbar plexuses were acquired in all but two (cases 4 and 8) of the 13 patients. One of these two (case 8) underwent an MR examination of only the brachial plexuses, and the other underwent that (case 4) of only the lumbar plexus. In total, 24 nerve plexuses in thirteen patients were evaluated. Coronal STIR and fat-saturated T1-weighted images were acquired in all studies. Gadolinium was administered in all but three patients (cases 8, 10, and 11). One patient (case 4) underwent gadolinium administration only in the MR examination of the brachial plexus, not in the study of the lumbar plexus. The other patients underwent gadolinium administration in MR studies of both the brachial and lumbar plexuses. In total, gadolinium was administered in the examinations of 19 nerve plexuses in 10 patients. DWIs were not acquired in four patients (cases 4, 5, 6, and 8), and thus DWIs were obtained in examinations of 18 nerve plexuses in nine patients.
Follow-up MR studies were carried out in two patients. One patient (case 9) underwent MR studies three times over 8 months: before treatment, after treatment, and during the relapse phase. In another patient (case 1), a follow-up MR study was performed 1 year after the first study, although he had no clinical symptoms.
Among the 11 normal volunteers, MR studies of both the cervical and lumbar plexuses were carried out in two cases, of only the brachial plexus in five cases, and of only the lumbar plexus in four cases. In total, 13 plexuses were evaluated. Coronal STIR, fat-saturated T1-weighted images, and DWIs were obtained in all normal volunteers. Gadolinium administration was not performed.
The parameters of the coronal STIR sequence were as follows: repetition time/echo time/inversion time [TR/TE/TI], 4,210/85/150; 4-mm sections without gaps; fields of view [FOV], 260 × 260 (brachial plexus), 280 × 280 (lumbar plexus); imaging matrix, 512 × 512; number of excitations (NEX), 1. The parameters of the coronal fat-saturated T1-weighted image sequence before and after gadolinium administration were [TR/TE] = 1,330/16; 3-mm sections without gaps; FOV, 260 × 260 (brachial plexus), 280 × 280 (lumbar plexus); imaging matrix, 512 × 512, NEX, 2. The DWIs were obtained using an axial single-shot spin-echo echo-planar imaging sequence with the following parameters: [TR/TE/TI], 10,000.0/86.0/150; 4-mm sections without gaps; FOV, 400 × 400 (brachial and lumbar plexus); imaging matrix, 256 × 256; and NEX, 3. Motion-probing gradients were applied in six directions (xx, yy, zz, xy, xz, and yz) with b values of 0 and 1,000 s/mm2. The scan time was 8 min and 20 s in the brachial plexus and 7 min and 40 s in the lumbar plexus. We then performed maximum intensity projection (MIP) for a stack of isotropic DWIs to reconstruct the images rotated around the z-axis using software incorporated into the MR system. The isotropic DWIs were generated based on calculated isotropic ADC values.
Image analysis
Imaging assessment in the patient group was based on agreement between two neuroradiologists who reviewed the images in tandem and who were blinded to clinical information regarding neuropathies. Each neuroradiologist made initial evaluations independently, and any disagreements regarding the final conclusion were resolved by consensus.
Swelling of the plexuses was visually assessed on STIR in comparison to the controls. The signal intensity of each plexus was also evaluated on both STIR and T1-weighted images without contrast enhancement and compared to the signal intensity of the controls. We also visually assessed the signal intensity of the plexus on T1-weighted images after contrast enhancement and compared it to the intensity on images taken before contrast enhancement. We established a grading system for the degree of enhancement in the plexuses: moderate enhancement means that the plexuses have stronger enhancement than the ganglions, and mild enhancement means that the plexuses have milder enhancement than the ganglions. DWIs (b = 1000) with MIP reconstruction along the long axis of the spine were also assessed by comparison with the controls.
We measured the ADC values of the plexuses on ADC maps. All of the ADC measurements were made using an Aquarius Netstation Ver 1.4 (Tera Recon, San Mateo, CA, USA) by placing freehand circular regions of interest (ROIs) over the plexus on the ADC map. The average ROI was 4 ± 2 mm2, and the area varied depending on the plexus. ROIs were carefully placed by two trained operators within the plexus to avoid the partial volume effect at three points, and the ADC values were averaged in each lesion. The intra-class correlation coefficient for these measurements was 92.0%. Intra-class correlation coefficient values greater than 0.9 were regarded as excellent, and the results were thus considered reliable. The differences in the ADC values of the plexuses among healthy subjects, patients without neither swelling nor abnormal signals of the plexus, and patients with either swelling or abnormal signals of the plexus were evaluated by analysis of variance. P values less than 0.05 were considered significant.
Using the unpaired t test, we also evaluated the relationships between age at onset and each MR imaging finding, such as nerve swelling, high signal intensity on STIR, T1-weighted images, DWI, and degree of enhancement. Disease duration was also evaluated in the same way. Additionally, the relationships between each MR finding and the clinical findings (motor and sensory symptoms and F wave latency) were evaluated by Fisher's exact test.
Results
Clinical and imaging features
The clinical and MR imaging findings are summarized in Table 1. All patients presented with motor and/or sensory symptoms in both the upper and lower extremities. F wave latencies were prolonged in either the upper or lower extremities or both of all patients, except for two (cases 2 and 5), whose F waves were absent, probably due to severe nerve damage.
Swelling of the brachial and lumbar plexuses was observed in 16 of 24 plexuses (66.7%) on STIR in 9 out of 13 CIDP patients (Figs. 1 and 2). In the patients who underwent both brachial and lumbar MR examinations, either both plexuses were simultaneously swollen or neither was swollen.
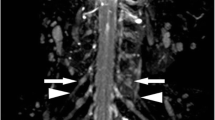
MR images of a 16-year-old female (case 2 in Table 1) with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) with symmetrical weakness of both legs and arms for 5 years. a Coronal STIR image of the lumbar plexus shows enlargement with a markedly high signal. b Lumbar plexus with hypertrophy shows slightly high signal intensity on T1-weighted images without contrast enhancement. c T1-weighted images following intravenous gadolinium administration shows an enhancement in the nerve ganglion without enhancement of the lumbar plexus. d DWI reconstructed by MIP on the lumbar plexus shows high intensity of the lumbar plexus. e ADC map of the lumbar plexus. The ADC value in the lumbar plexus with hypertrophy was 2.1 × 10−3 mm3/s. f, g Coronal STIR reveals hyperintensity and swelling of the bilateral brachial plexus (because this patient had torticollis, both plexuses do not appear in the same slice). h Brachial plexus with hypertrophy shows slightly high signal intensity on T1-weighted images. i T1-weighted images following intravenous gadolinium administration do not show contrast enhancement of the nerve plexus. j DWI demonstrates high signal intensity. k The mean ADC value in the brachial plexus was 1.7 × 10−3mm3/s
MR images from a 34-year-old man (case 9 in Table 1) with CIDP, with a 6-month history of weakness in both legs and arms as well as sensory loss in the arms. a Coronal STIR image reveals moderate swelling and hyperintensity of the lumbar plexus bilaterally. b Lumbar plexus with hypertrophy shows slightly high signal intensity on T1-weighted image. c T1-weighted image following intravenous gadolinium administration shows mild enhancement in the lumbar plexus. d DWI reconstructed by MIP on the lumbar plexus demonstrates high intensity. e ADC map of the lumbar plexus. The ADC value was 1.5 × 10−3 mm3/s. f T1-weighted images with gadolinium obtained 1 month after immunoglobulin therapy; the degree of enhancement is unchanged. g T1-weighted image with gadolinium obtained at the relapse phase, 6 months after immunoglobulin therapy; the degree of enhancement is unchanged. h DWI reconstructed by MIP on the lumbar plexus after treatment obtained at the same time as g shows no remarkable changes in the intensity of the lumbar plexus. i ADC map of the lumbar plexus. The ADC value was 1.5 × 10−3 mm3/s
High intensity was shown in 16 of 24 plexuses (66.7%) on STIR in 9 out of 13 CIDP patients, and all 16 plexuses were swollen (Fig. 1a). Slightly high intensity was found in 12 of 24 plexuses (50%) on T1-weighted images without contrast enhancement in six patients (Fig. 1b).
Contrast enhancement was shown in 6 of 19 plexuses (31.6%) in 3 out of 10 patients after gadolinium administration (Fig. 2c). All enhanced plexuses showed mild enhancement. In patients who underwent MR studies of both the brachial and lumbar plexuses, either both plexuses were simultaneously enhanced, or neither was enhanced. All enhanced plexuses showed swelling and hyperintensity on STIR.
On DWIs, high intensity was found in 10 of 18 plexuses in 5 out of 9 patients (55.6%); all 10 plexuses showed swelling and high intensity on STIR and slightly high intensity on T1-weighted images before gadolinium administration (Fig. 1a, b, e). The remaining eight plexuses that did not show high intensity on DWIs did not show swelling or high intensity on STIR or on T1-weighted images.
In one patient who was examined three times in total from before to after treatment (case 9), all MR findings such as swelling, signals in all sequences, and contrast enhancement remained unchanged throughout the examinations without interval changes even during the remission phase or relapse phase (Fig. 2c, f, g). Another follow-up study in case 1 also showed no interval changes.
Swollen plexuses were not observed in any of the 11 normal volunteers. All of their plexuses showed isointensity or faintly high intensity on both STIR and T1-weighted images (Fig. 3).
MR images of a 64-year-old normal male volunteer. a Coronal STIR image shows the lumbar plexus without swelling or abnormal high intensity. b T1-weighted image shows the lumbar plexus with isointensity except for the proximal portion. c DWI reconstructed by MIP depicts the lumbar plexus faintly at the proximal portion. d The ADC value of the lumbar plexus was 1.1 × 10−3 mm3/s
Among the patients, no significant relationships were observed between MR images and clinical findings.
Measurements of ADC values
The mean ADC values in the plexuses of patients and normal volunteers were 1.27 ± 0.43 × 10−3 mm2/s (n = 18) and 0.92 ± 0.11 × 10−3 mm2/s (n = 13), respectively. The mean ADC values in patients with and without both swelling and high intensity of the brachial/lumbar plexus on STIR were 1.56 ± 0.34 × 10−3 mm2/s (n = 10) and 0.89 ± 0.12 × 10−3 mm2/s (n = 8), respectively. There were statistically significant differences between the ADC values of the patients with either swelling or abnormal signals and both normal volunteers and patients without neither swelling nor abnormal signals. However, there was no significant difference in ADC values between patients without neither swelling nor abnormal signals and normal volunteers.
Discussion
The diagnosis of CIDP is based mainly on the clinical presentation and on nerve conduction findings that are consistent with demyelination. However, in clinical practice, CIDP is often difficult to diagnose. MR examination of the brachial and lumbar plexuses will be very helpful in diagnosing CIDP.
In the present study, the MR imaging findings for nerve plexuses in CIDP patients were examined using various kinds of sequences, including T1-weighted images and DWIs, which had not previously been evaluated in a large number of CIDP patients. Although the most sensitive sequence for detecting signal abnormalities was STIR, T1-weighted images and DWI sequences also detected approximately 50% of the abnormalities. Moreover, high intensities on both DWI and the ADC map, which are indicative of T2 shine-through, may reflect a pathological condition in which the nerve plexus is shaped like an onion bulb.
There have been several MR studies of CIDP, some of which have shown diffuse brachial and lumbar plexus swelling and high signal intensity on T2-weighted images or STIR [5–8]. In one study, hypertrophy on MR images of plexuses in CIDP was observed in 57.1% of patients; this result is slightly lower than, but relatively consistent with the frequency observed in our study. If swelling and increased signal intensity can be detected on STIR in the plexuses of patients with clinically suspected CIDP, these findings will be of diagnostic value [6, 8].
DWIs have been investigated in several previous attempts to visualize extraspinal neural structures [10–12]. In one recent study, the quality of depiction of the brachial plexus on DWIs was evaluated [12]. The study involved five volunteers, three patients with cervical schwannoma, and two with traumatic lesions of the brachial plexus. Their study showed that DWI reconstructed by MIP provided an overview image of the brachial plexus. However, no ADC values were calculated, and the lumbar plexus was not included. Tsuchiya et al. have described the nerve roots and peripheral nerves in patients with various diseases, including three cases with CIDP, six with multiple sclerosis, and four with neurogenic tumors, using DWI [11]. They showed the affected lesion in the cord and proximal nerve roots clearly on DWI, which suggested that the DW sequence would have the potential to visualize the intramedullary and nerve root lesions, and thus facilitating a differential diagnosis. However, these studies examined several kinds of peripheral nerve diseases and included only a few CIDP cases. In addition, the ADC values were not obtained in these studies. Thus far, there have been no original MR studies with DWIs focusing on CIDP patients, nor have any studies evaluated normal brachial or lumbar plexuses on DWIs with ADC values. However, several studies have examined DWIs of normal peripheral nerves [13, 14] and have shown the normal ADC value of the median nerve to be 1.01 ± 0.13 × 10−3 mm2/s. Our study also demonstrated similar ADC values of the brachial and lumbar plexuses (mean 0.92 ± 0.11 × 10−3 mm2/s) in normal volunteers.
In this study, the DWIs showed a high signal in 55.6% of the plexuses of CIDP patients; thus, DWIs would be helpful for detecting abnormal nerve plexuses. Furthermore, patients with plexus hypertrophy had significantly higher mean ADC values than patients without hypertrophy or in the normal volunteers. Thus, the high intensity of swollen plexuses on DWIs was attributed to the T2 shine-through effect. In a study by Crino et al., the swelling of the nerve plexuses of CIDP patients was found to be caused by several proliferating layers of Schwann cells around the axon, which increased endoneurial collagen [15]. These pathological changes would explain the increased ADC values within the hypertrophic nerves. The ADC values of Schwann cell cytoplasm and endoneurial collagen surrounding bare axons in CIDP patients may be higher than those for normal myelin with tight junctions.
T1-weighted images before contrast enhancement have rarely been evaluated in CIDP patients. This study showed slightly high signal intensity in the brachial and lumbar plexuses on T1-weighted images in 50% of patients with CIDP. During the process of re-myelination of peripheral nerves in CIDP patients, the plexus gradually becomes brighter on T1-weighted images, probably reflecting the development of the onion bulb and increased endoneurial collagen [16]. Additionally, in the course of demyelination and re-myelination, phagocytic macrophages of myelin debris are increased. Some of the lipids taken up by Schwann cells may be used to supply cholesterol for rapid membrane biogenesis by macrophages, in order to prepare for the formation of neuritis [17]. We speculate that these conditions shorten the T1 of the plexus in CIDP patients.
In the peripheral nerves of CIDP patients, increased permeability of the blood–nerve barrier appears to be the cause of spinal roots and plexus enhancement. Gadolinium enhancement has been reported in some, but not all cases of CIDP [5, 8, 10]. Midroni et al. detected the enhancement of the nerve root and the extraforaminal segment in 28.6% of cases, which was slightly lower than the 31.6% frequency observed in the present study. There has been no agreement regarding whether the enhancement of peripheral nerves indicates disease activity [7, 9]. In one of our patients who showed clinical improvement after treatment, the mild enhancement remained unchanged from before to after the treatment.
The differential diagnoses of CIDP are Charcot–Marie–Tooth (CMT) disease, distal demyelinating polyneuropathy associated with monoclonal gammopathy, and multifocal motor neuropathy (MMN). Swelling and increasing intensity on STIR with or without enhancement in nerve plexuses have also been noted in patients with distal demyelinating polyneuropathy associated with monoclonal gammopathy [5]. CMT disease, which is a hereditary peripheral neuropathy, also mimics MR findings of CIDP [18]. Because recurrent demyelination and re-myelination occur in CMT disease, the peripheral nerves often show an onion bulb as in CIDP [16]. MMN is characterized by asymmetric weakness without sensory loss. Usually, MR abnormalities are detected asymmetrically in MMN but symmetrically in CMT disease, CIDP, and distal demyelinating polyneuropathy associated with monoclonal gammopathy [19]. In particular, the affected nerve areas in CMT disease are more diffuse than in the other conditions.
Our study has several limitations. First, 12 of the 13 patients met the clinical and neurophysiological criteria for CIDP without nerve biopsy. Furthermore, in the pathologically confirmed patient, a biopsy was performed at the sural nerve and not at the plexus itself, because a biopsy at the lumbar nerve plexus carries some risks and is not usually done for the purpose of diagnosis. Although the obtained DWIs and ADC values were useful for speculating about the pathological changes in nerve plexuses in CIDP, they were not conclusive. Second, we had one follow-up study of gadolinium administration (case 9), and two follow-up studies on DWIs (cases 1 and 9). Both studies revealed no interval changes, even though one patient showed improved clinical symptoms (case 1). We think it is important for clinicians to know that there is no definite relationship between the response to treatment and any MR findings. The lack of interval changes suggests that once an onion bulb has formed, it is rarely resorbed by treatment. However, follow-up was carried out in only two patients, and a longer-term follow-up study on more patients needs to be performed.
Conclusions
Although the standard method for diagnosing CIDP includes the evaluation of clinical features and electrophysiological examinations, characteristic MR findings on STIR in CIDP patients are found to be helpful in making a diagnosis. In particular, DWIs and T1-weighted images without contrast enhancement seem useful for speculating about the pathological changes in swollen plexuses in CIDP patients.
No definite relationship was observed between MR imaging findings and clinical findings.
References
Hahn A, Hartung H, Dick P (2005) Chronic inflammatory demyelinating polyradiculoneuropathy. In: Dick P (ed) Peripheral neuropathy, vol 2, 4th ed. Elsevier, Philadelphia, pp 2221–2253
Joint Task Force of the EFNS and the PNS (2005) European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. J Peripher Nerv Syst 10:220–228
Latov N (2002) Diagnosis of CIDP. Neurology 59(Suppl 6):S2–S6
Koller H, Kieseier BC, Jander S, Hartung HP (2005) Chronic inflammatory demyelinating polyneuropathy. N Engl J Med 352:1343–1356
Eurelings M, Notermans NC, Franssen H, Van Es H, Ramos H, Wokke J, Van Den Berg H (2001) MRI of the brachial plexus in polyneuropathy associated with monoclonal gammopathy. Muscle Nerve 24:1312–1318
Bradley L, Wilhelm T, King RH, Ginsberg L, Orrell RW (2006) Brachial plexus hypertrophy in chronic inflammatory demyelinating polyradiculoneuropathy. Neuromuscul Disord 16:126–131
Midroni G, Tilly TN, Gray B, Vajsar J (1999) MRI of the cauda equine in CIDP: clinical correlations. J Neurol Sci 170:36–44
Duggins A, McLeod J, Pollard J, Davies L, Yang F, Thompson EO, Soper JR (1999) Spinal root and plexus hypertrophy in chronic inflammatory demyelinating polyneuropathy. Brain 122:1383–1390
Kuwabara S, Nakajima M, Matsuda S, Hattori T (1997) Magnetic resonance imaging at the demyelinative foci in chronic inflammatory demyelinating polyneuropathy. Neurology 48:874–877
Tsuchiya K, Imai M, Tateishi H, Nitatori T, Fujikawa A, Takemoto S (2007) Neurography of the spinal nerve roots by diffusion tensor scanning applying motion-probing gradients in six directions. Magn Reson Med Sci 6:1–5
Tsuchiya K, Honya K, Yoshida M, Nitatori T (2008) Demonstration of spinal cord and nerve root abnormalities by diffusion neurography. J Comput Assist Tomogr 32:286–290
Takahara T, Hendrikse J, Yamashita T, Mali W, Kwee T, Imai Y, Luijten P (2008) Diffusion-weighted MR neurography of the brachial plexus: feasibility study. Radiology 249(2):653–660
Bendszus M, Stoll G (2005) Technology insight: visualizing peripheral nerve injury using MRI. Nat Clin Pract Neurol 1:45–53
Kababci N, Gurses B, Firat Z. Bayram A, Ulug A, Kovanlikaya A (2007) Diffusion tensor imaging and tractography of median nerve: Normative diffusion values. AJR 189:923–927
Crino B, Grossman I, Rostami A (1993) Magnetic resonance imaging of the cauda equine in chronic inflammatory demyelinating polyneuropathy. Ann Neurol 33:311–313
Graham I, Lantos L (2002) Greenfield’s neuropathology, 7th edition, Vol. 2. New York Oxford University Press, pp 617–620
Naba I, Yoshikawa H, Sakoda S, Itabe H, Suzuki H, Kodama T, Yanagihara T (2000) Onion-bulb formation after a single compression injury in the macrophage scavenger receptor knockout mice. Exp Neurol 166:83–89
Sureka J, Charian RA, Alexander M, Thomas BP (2009) MRI of brachial plexopathies. Clin Radiol 64:208–218
Van Es H, Van den Berg L, Franssen H et al (1997) Magnetic resonance imaging of the brachial plexus in patients with multifocal motor neuropathy. Neurology 48(5):1218–1224
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adachi, Y., Sato, N., Okamoto, T. et al. Brachial and lumbar plexuses in chronic inflammatory demyelinating polyradiculoneuropathy: MRI assessment including apparent diffusion coefficient. Neuroradiology 53, 3–11 (2011). https://doi.org/10.1007/s00234-010-0684-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-010-0684-7