Abstract
Introduction
The uncinate fasciculus (UF) consists of core fibers connecting the frontal and temporal lobes and is considered to be related to cognitive/behavioral function. Using diffusion tensor tractography, we quantitatively evaluated changes in fractional anisotropy (FA) and the apparent diffusion coefficient (ADC) of the UF by tract-specific analysis to evaluate the damage of the UF in patients with amyotrophic lateral sclerosis (ALS).
Methods
We obtained diffusion tensor images of 15 patients with ALS and 9 age-matched volunteers.
Results
Patients with ALS showed significantly lower mean FA (P = 0.029) compared with controls. No significant difference was seen in mean ADC.
Conclusion
The results suggest that damage of the UF in patients with ALS can be quantitatively evaluated with FA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a degenerative motor neuron disease that was thought to spare cognitive function. However, ALS with cognitive/behavioral impairment has recently been described and has attracted attention among researchers [1–3].
A large study revealed that 50% of sporadic ALS has some degree of cognitive impairment [2]. Another study reported that most patients with ALS have mild cognitive impairment, and 5% have a clinical subtype of frontotemporal lobar degeneration (FTLD) [3].
Changes in the frontal lobe, the temporal lobe, and the corticospinal tract (CST) have been described using various imaging techniques [4–6]. However, diffusion-tensor-derived parameters such as fractional anisotropy (FA) and apparent diffusion coefficient (ADC) are more useful for examining specific white matter changes. Several reports have shown lower FA values in the various neuron fiber tracts in patients with Alzheimer’s disease and schizophrenia compared with controls [7–9]. In patients with ALS, many reports have shown lower FA and ADC in the pyramidal tract [10–13], but few studies [14, 15] have investigated FA and ADC in the fiber tracts related to cognitive/behavioral function.
The uncinate fasciculus (UF) is the fiber tract that connects the frontal and temporal lobes. Frontotemporal changes have been shown in both aspects of radiographic findings and pathological findings in past studies in patients with ALS with dementia [16, 17]. The UF is involved in the naming deficits and impairment in retrieval of memories [18, 19] that are often seen in patients with ALS [2, 3, 20], and lower FA of the UF in patients with FTLD has been reported [21]. Therefore, the UF is likely to be an important fiber tract that is related to the cognitive/behavioral impairment in patients with ALS. The aim of this study was to investigate FA and ADC changes in the UF in patients with ALS and compare them with healthy controls. To our knowledge, this is the first report investigating FA and ADC changes in the UF in patients with ALS.
Materials and methods
Subjects
We studied 15 patients with ALS and 9 age-matched volunteers. The mean age of the patients was 60.2 ± 9.9 years and that of the volunteers was 62.0 ± 12.1 years. All patients met the revised EL Escorial criteria [22] as having definite, probable, or possible ALS. The study was approved by our institutional review board, and written informed consent was obtained from all patients and volunteers.
MRI acquisition
All scans were performed using a 1.5-T MRI unit (Signa Lx ver 9.0, General Electric) with a standard head coil. We obtained diffusion tensor images using echo planar imaging (TR/TE 6,000/78 ms, matrix size 128 × 128, slice thickness 5 mm, number of acquisitions 2, total acquisition time 5.5 min). Images were obtained with both 13-directional diffusion encoding (b = 1,000 s/mm2 for each direction) and no diffusion encoding (b = 0 s/mm2). We also acquired regular structural T1-weighted images and T2-weighted images.
DTI data postprocessing
Diffusion tensor data were transferred to an off-line workstation. Analysis was performed using dTV and VOLUME-ONE software (http://www.ut-radiology.umin.jp/people/masutani/dTV.htm) [23]. Interpolation along the Z-axis was performed to obtain isotropic data (voxel size 0.94 × 0.94 × 0.94 mm) [23] and to avoid voxel size-/shape-dependent change in FA values in regions with crossing fibers [24].
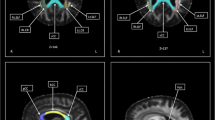
The seed regions of interest (ROI) were set in the frontal part of the UF in the coronal plane through the genu of the corpus callosum, just anterior to the anterior horn of the lateral ventricle. The target ROIs were manually set on the white matter in the coronal plane at the most anterior part of the temporal stem (Fig. 1). Color-coded FA maps were used to place these ROIs into the UF tracts precisely and objectively. A reconstructed sagittal section of the color-coded FA map was used to determine reconstructed coronal sections at the level of the genu of the corpus callosum. In the coronal slices of the color-coded maps, most of the UF tracts were displayed as green. The threshold of line tracking was set at FA = 0.18.
Placement of the seed and target ROIs. a Coronal plane diffusion tensor image color-coded FA map through the genu of the corpus callosum. We set the seed ROI in the frontal part of the UF at this level. b Coronal plane diffusion tensor image color-coded FA map at the most anterior part of the temporal stem. We set the target ROI at this level
The tracts of both sides of the UF of all patients and volunteers were then visualized, and voxelization was performed by the function of dTV. Next, we divided each of them into five sections with exactly the same distances between the plane of the seed and target ROIs. We limited the size of each ROI within 20–30 voxels to prevent variety of size. We calculated mean FA and ADC of tracts on both sides of the brain by tract-specific analysis and statistically analyzed them using the Student’s t test.
Results
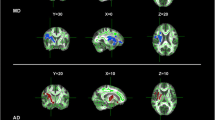
Tractographies of the UF were obtained as semicircular tracts in all patients with ALS and controls (Fig. 2). The UF coursed from the anterior part of the temporal lobe lateral to the amygdala to the lower region of the frontal lobe at the level of the anterior commissure. The mean FA was 0.410 ± 0.045 for patients with ALS and 0.459 ± 0.056 for the controls (Fig. 3a), and the difference was statistically significant (P = 0.029). The mean ADC were 0.820 ± 0.039 × 10−3 mm2/s for patients with ALS and 0.801 ± 0.033 × 10−3 mm2/s for the controls (Fig. 3b), which were not significantly different.
Diffusion tensor parameters of the UF. a FA values of the UF. Patients with ALS demonstrated significantly lower FA values than healthy volunteers. (*P = 0.029). b Apparent diffusion coefficient (ADC) values of the UF. There were no significant differences in ADC values (×10−3 mm2/s) between patients with ALS and healthy volunteers
Discussion
The UF is one tract in the temporal stem. The UF makes up the core of the anterior temporal stem [25] and is critical for frontal–temporal interactions. When frontal–temporal interactions are disrupted, amnesia is more likely to occur than when only the frontal lobe or temporal lobe is involved [26]. In addition, lesions in the UF often result in the naming deficits and impairment in retrieval of memories [18, 19, 27] that are often seen in patients with ALS [2, 3, 20].
Fibers in the UF course from the temporal lobe laterally to the amygdala and hook around the sylvian fissure. The UF then curves upward through the anterior temporal stem, and the fibers fan out into the frontal lobe. The UF connects the anterior temporal lobe and the orbital and inferior frontal gyri of the frontal lobe. At its most superior level, it is below the level of the frontal horns of the lateral ventricles [25, 28]. The tracts we visualized in our study are consistent with this reported anatomy.
Characteristic changes seen in patients with ALS are behavioral variant frontotemporal dementia [2], the behavioral and personality changes [4, 29], impairments in executive functions, and impairments in verbal fluency [1–3, 20]. These impairments are difficult to detect, and patients with ALS with these changes have relatively normal scores on the Mini-Mental State Examination which is often used for evaluation of dementia [1, 30, 31]. Useful measures for assessment of cognitive or behavioral impairment in patients with ALS include executive measures, memory/learning measures, attention/concentration measures, language measures, and visual–spatial measures [1]. We think objective evaluation of neurodegeneration using imaging is very helpful to support these measures because carrying them out takes much time, and sometimes assessment can be misinterpreted under factors such as delirium.
One of the limitations of our study is that the FA was not compared with these measures. However, it can be said that most relevant reasons for lower FA of the UF in patients with ALS is their cognitive/behavioral impairment. Because other possible causes for lower FA such as motor neuron degeneration or aging are hard to think in this presented study. Motor neuron tracts do not run within the UF, and the control subjects were age-matched.
Second limitation is that education level of two groups were not matched, and the number of control subjects was small. We will recruit larger healthy controls and improve these in the future.
The exact pathological mechanism of changes of FA and ADC are not clear. Based on the perspective of diffusion tensor principles, FA represents the degree of diffusion anisotropy and decreases as irregularity in alignment of cellular structures. Thus, FA is somewhat linked to the quality and density of fibers. ADC represents the directionally averaged magnitude of diffusion. It may be related to the integrity of the local brain tissue [32–35]. We consider that FA reflects damages specific to the neuron fibers better than ADC based on these principles. A reduction in FA has been reported in some tracts in neuodegenerative diseases such as Alzheimer’s disease and schizophrenia [7–9]. FA can be an objective parameter for measuring degenerative changes including changes related to cognitive/behavioral impairment.
Especially, the UF is considered to be deeply involved in cognitive/behavioral impairment and intelligence from several reports. One study reported a reduction in FA of the UF in asymptomatic progranulin gene mutation carriers which causes FTLD followed by aphasia onset [36]. The greatest decrease in mean FA of the UF was seen in advanced frontotemporal dementia among the corpus callosum (CC), bilateral arcuate fasciculi, inferior longitudinal fasciculi, and UF [21]. Another study reported the difference of FA between healthy adults with normal intelligence and high intelligence only in the UF among the CC, cingulum, UF, optic radiation, and CST. Thus, the UF is likely to be an important neural basis of human intelligence [37].
The FA of the UF may reflect higher cognitive function in humans, and it may be an objective and sensitive parameter for determining cognitive/behavioral impairment in patients with ALS. Our findings showed a decrease in FA of the UF in patients with ALS. These results suggest that the UF is damaged in patients with ALS, and FA can be used for objective evaluation of the damage of the UF which may be related to the cognitive/behavioral functional impairment.
Further studies, including those with a larger number subjects that have undergone clinical evaluation with consensus, are needed.
References
Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH et al (2009) Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 10(3):131–146
Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE (2005) Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 65:586–590
Phukan J, Pender NP, Hardiman O (2007) Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol 6:994–1003
Gibbons ZC, Richardson A, Neary D, Snowden JS (2008) Behaviour in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 9:67–74
Sarac H, Zagar M, Vranjes D, Henigsberg N, Billic E, Pavlisa G (2008) Magnetic resonance imaging and magnetic resonance spectroscopy in a patient with amyotrophic lateral sclerosis and frontotemporal dementia. Coll Antropol 32(Supple 1):205–210
Ishikawa T, Morita M, Nakano I (2007) Brain perfusion imaging in amyotrophic lateral sclerosis with dementia. Brain Nerve 59:1093–1098, Japanese
Yasmin H, Nakata Y, Aoki S, Abe O, Sato N, Nemoto K et al (2008) Diffusion abnormalities of the uncinate fasciculus in Alzheimer's disease: diffusion tensor tract-specific analysis using a new method to measure the core of the tract. Neuroradiology 50:293–299
Taoka T, Iwasaki S, Sakamoto M, Nakagawa H, Fukusumi A, Myochin K et al (2006) Diffusion anisotropy and diffusivity of white matter tracts within the temporal stem in Alzheimer disease: evaluation of the “tract of interest” by diffusion tensor tractography. AJNR Am J Neuroradiol 27:1040–1045
Price G, Cercignani M, Parker GJM, Altmann DR, Barnes TRE, Barker GJ et al (2008) White matter tracts in first-episode psychosis: a DTI tractography study of the uncinate fasciculus. NeuroImage 39:949–955
Iwata NK, Aoki S, Okabe S, Arai N, Terao Y, Kwak S et al (2008) Evaluation of corticospinal tracts in ALS with diffusion tensor MRI and brainstem stimulation. Neurology 70:528–532
Aoki S, Iwata NK, Masutani Y, Yoshida M, Abe O, Ugawa Y et al (2005) Quantitative evaluation of the pyramidal tract segment by diffusion tensor tractography: feasibility study in patients with amyotrophic lateral sclerosis. Radiat Med 23:195–199
Abe O, Yamada H, Masutani Y, Aoki S, Kunimatsu A, Yamasue H et al (2004) Amyotrophic lateral sclerosis: diffusion tensor tractography and voxel-based analysis. NMR Biomed 17:411–416
Ellis CM, Simmons A, Jones DK, Bland J, Dawson JM, Horsfield MA et al (1999) Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 53:1051–1058
Senda J, Ito M, Watanabe H, Atsuya N, Kawai Y, Katsuno M et al (2009) Correlation between pyramidal tract degeneration and widespread white matter involvement in amyotrophic lateral sclerosis: a study with tractography and diffusion-tensor imaging. Amyotroph Lateral Scler 29:1–8
Sach M, Winkler G, Glauche V, Liepert J, Heimbach B, Koch MA et al (2004) Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 127:340–350
Mori H, Yagishita A, Takeda T, Mizutani T (2007) Symmetric temporal abnormalities on MR imaging in amyotrophic lateral sclerosis with dementia. AJNR Am J Neuroradiol 28:1511–1516
Yoshida M (2004) Amyotrophic lateral sclerosis with dementia: the clinicopathological spectrum. Neuropathology 24:87–102
Lu LH, Crosson B, Nadeau SE, Heilman KM, Gonzalez-Rothi LJ, Raymer A et al (2002) Category-specific naming deficits for objects and actions: semantic attribute and grammatical role hypotheses. Neuropsychologia 40:1608–1621
Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, Luders HO (2008) Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia 49:1409–1418
Abrahams S, Goldstein LH, Simmons A, Brammer M, Williams SCR, Giampietro V et al (2004) Word retrieval in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Brain 127:1507–1517
Matsuo K, Mizuno T, Yamada K, Akazawa K, Kasai T, Kondo M et al (2008) Cerebral white matter damage in frontotemporal dementia assessed by diffusion tensor tractography. Neuroradiology 50:605–611
Books BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Masutani Y, Aoki S, Abe O, Hayashi N, Otomo K (2003) MR diffusion tensor imaging: recent advance and new techniques for diffusion tensor visualization. Eur J Radiol 46:53–66
Oouchi H, Yamada K, Sakai K, Kizu O, Kubota T, Ito H et al (2007) Diffusion anisotropy measurement of brain white matter is affected by voxel size: underestimation occurs in areas with crossing fibers. AJNR Am J Neuroradiol 28:1102–1106
Wang F, Sun T, Li XG, Liu NJ (2008) Diffusion tensor tractography of the temporal stem on the inferior limiting sulcus. Laboratory investigation. J Neurosurg 108:775–781
Levine B, Black SE, Cabeza R, Sinden M, Mcintosh AR, Toth JP et al (1998) Episodic memory and the self in a case of isolated retrograde amnesia. Brain 121:1951–1973
Nestor PG, Kubicki M, Gurrera RJ, Sinden M, Mcintosh AR, Toth JP et al (2004) Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology 18:629–637
Kier EL, Staib LH, Davis LM, Bronen RA (2004) MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. AJNR Am J Neuroradiol 25:677–691
Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D (2001) Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry 70:323–332
Rakowicz WP, Hodges JR (1998) Dementia and aphasia in motor neuron disease: an underrecognised association? J Neurol Neurosurg Psychiatry 65:881–889
Heidler-Gary J, Hillis AE (2007) Distinctions between the dementia in amyotrophic lateral sclerosis with frontotemporal dementia and the dementia of Alzheimer’s disease. Amyotroph Lateral Scler 8:276–282
Wang S, Melhem ER (2005) Amyotrophic lateral sclerosis and primary lateral sclerosis: the role of diffusion tensor imaging and other advanced MR-based techniques as objective upper motor neuron markers. Ann N Y Acad Sci 1064:61–77
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B111:209–219
Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267
Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di CG (1996) Diffusion tensor MR imaging of the human brain. Radiology 201:637–648
Borroni R, Alberici A, Premi E, Archetti S, Garibotto V, Agosti C et al (2008) Brain magnetic resonance imaging structural changes in a pedigree of asymptomatic progranulin mutation carriers. Rejuvenation Res 11:585–595
Yu C, Li J, Liu Y, Qin W, Li Y, Shu N et al (2008) White matter tract integrity and intelligence in patients with mental retardation and healthy adults. NeuroImage 40:1533–1541
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sato, K., Aoki, S., Iwata, N.K. et al. Diffusion tensor tract-specific analysis of the uncinate fasciculus in patients with amyotrophic lateral sclerosis. Neuroradiology 52, 729–733 (2010). https://doi.org/10.1007/s00234-010-0653-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-010-0653-1