Abstract
Introduction
The purpose of the study is to describe our experience in eight cases of horizontal stenting across the circle of Willis in patients with terminal aneurysms.
Methods
Eight patients were treated with horizontal stent placement and aneurysm coiling. All aneurysms had highly unfavourable dome to neck ratios. All patients were followed up with digital subtraction angiography at 3–12 months following treatment.
Results
The Enterprise stent was successfully deployed horizontally in vessels of less than 2-mm diameter with no stent occlusion. Neurological complications occurred in one patient. Immediate and follow-up angiographic results were encouraging with six stable occlusions at 6 months. There was one asymptomatic case of in-stent stenosis and one case of late organised in-stent thrombus.
Conclusions
Horizontal deployment of the Enterprise stent to assist coil embolisation of wide-necked terminal aneurysms is feasible. This device can be navigated via relatively small communicating arteries, in cases with favourable anatomy. Early angiographic results were favourable; however, longer-term follow-up will be required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction and purpose
The endovascular treatment of wide-necked terminal intracranial aneurysms remains technically challenging. Such aneurysms are prone to recur, and retreatment can often be difficult as the dome to neck ratio (D/N ratio) of such recurrences is often unfavourable. Treatment of such aneurysms often requires an adjunctive technique, i.e. balloon remodelling or stenting. Various novel stenting techniques have been described: Y stenting [1] and horizontal stenting via the circle of Willis [2–5]. We describe our clinical experience with early angiographic follow-up of eight cases of horizontal stenting across the circle of Willis in patients with terminal aneurysms, four of them recurrent after previous coil embolisation.
Methods
Horizontal stenting
This was defined as horizontal deployment of a stent across the neck of a terminal aneurysm achieved by navigating the stent through the circle of Willis, i.e. via a communicating artery. Basilar tip aneurysms were accessed from the carotid system via the most suitable posterior communicating artery (post. comm.) as determined by larger size or more favourable horizontal course; carotid tip aneurysms were accessed from the contralateral carotid artery via the anterior communicating artery (ant. comm.).
Patient selection
Between March 2007 and March 2008, we selected eight patients for horizontal stenting of terminal aneurysms out of a total of 180 aneurysms treated by an endovascular approach. Two patients with anterior communicating aneurysms were treated by stent placement from A1 to A2; these were excluded from this group as this was not considered true horizontal stenting.
Treatment
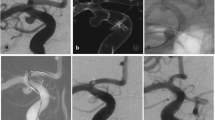
All procedures were performed on a Philips biplane flat panel angiographic system with facilities for 3D angiography and 3D road mapping (Philips Medical Systems, Best, Netherlands). Patients were pre-medicated with aspirin and clopidogrel for 3 days before the procedure. Dual anti-platelet therapy was continued for 90 days. Subsequently, clopidogrel was discontinued, and low-dose aspirin continued indefinitely. Bilateral femoral access was obtained. All patients were systemically heparinised. Typically, a 6-F guiding catheter (Envoy XB, Cordis Neurovascular, Miami Lakes, FL, USA) was placed in the target vessel for stent navigation. A second guider was placed in the vessel supplying the target aneurysm; this was used for road mapping and for microcatheter placement to coil the aneurysm. Three-dimensional angiograms were performed in all cases. When acquiring the 3D rotation of the target vessel for stent navigation, we increased the contrast volume from 5 ml/s to 6 or 7 ml/s, if necessary, to aid visualisation of the communicating artery. Virtual stent modelling was also performed using proprietary Philips software on the 3D workstation. This allowed selection of the appropriate stent length. The virtual stent image was laid over the 3D roadmaps to assist accurate placement of the stent (Fig. 1b). A Prowler Select Plus (Cordis Neurovascular) microcatheter with an inner diameter of 0.021 in. was used to traverse the communicating arteries and for subsequent stent delivery. The communicating arteries were selected using a variety of guidewires. In all but one case, the Enterprise stent (Cordis Neurovascular) was deployed before the aneurysm was catheterised across the stent. The aneurysms were coiled with an SL10 microcatheter (Boston Scientific, Fremont, CA, USA). In one case, a Courier 14 catheter (Micrus, San Jose, CA, USA) was used. Coil embolisation of the aneurysms was performed using PGA or PGLA enhanced coils (Micrus Cerecyte in five cases, ev3 Nexus in three cases). The femoral access sites were closed with a suture-mediated closure device (Perclose AT, Abbott Vascular). Two representative cases are described in Figs. 1 and 2.
a Pre-treatment DSA shows residual/recurrent left carotid tip aneurysm. b Virtual stent modelling for estimation of stent length and position. c The stent positioning marker lies across the aneurysm neck. d Unsubtracted image shows proximal and distal stent markers of deployed stent. The proximal end of the stent ‘jumped back’ slightly into the ant. comm. during deployment. e Immediate post-coiling appearance showing complete occlusion of the aneurysm. f Six-month DSA demonstrates stable occlusion
a Three-dimensional DSA shows the unruptured basilar tip aneurysm. b Right internal carotid DSA shows the post. comm. artery. c AP view of Roadmap shows Prowler catheter and Enterprise stent just before deployment. The stent positioning marker is covering the aneurysm neck. d Lateral view of Roadmap shows Prowler catheter and Enterprise stent just before deployment. The stent positioning marker is covering the aneurysm neck. e The first coil is well supported by the stent. f Subtracted image shows immediate post-coiling appearance. g Unsubtracted images shows stent markers on immediate post-coiling appearance. h Six-month DSA showing continuing occlusion of the aneurysm
Aneurysm assessment and follow-up
Aneurysm, stented artery and communicating artery measurements were made from the 3D rotational angiograms with raw data reconstructed on a dedicated Philips 3D workstation. For the recurrent aneurysms, the filling portion of the aneurysm was measured for the purposes of calculating the D/N ratio [6]. All patients were followed up with digital subtraction angiography (DSA) at 3–12 months following treatment. Aneurysm occlusion was defined by the modified Raymond–Roy classification as complete, residual neck/dog-ear or residual aneurysm [7].
Results
Patients and aneurysms
Between March 2007 and February 2008, eight patients with eight terminal aneurysms were treated with horizontal stenting followed by coil embolisation. There was one male and seven female patients. The mean age of the patients was 50 years (range 27–69). There were six basilar tip and two carotid tip aneurysms. Four patients had recurrent aneurysms post-coiling, and one patient had recurrence post-clipping. One patient was treated in a staged manner after initial subtotal aneurysm occlusion at the time of subarachnoid haemorrhage. After gaining encouraging experience with this technique in aneurysm recurrences or after suboptimal primary treatment, we treated two patients with previously untreated unruptured aneurysms; both cases were basilar tip aneurysms with very wide necks (patients 6 and 7).
The majority of the aneurysms were very wide-necked, and all had unfavourable D/N ratio; the mean neck width was 6 mm (SD 2 mm, range 3 to 9.5), and the mean D/N ratio was 0.87 (SD 0.29, range 0.5 to 1.3). The mean calibre of the stented vessels at the site of proximal end of the stent was 1.58 mm (range 1.1 to 2.3 mm), and the mean calibre at the distal end of the stent was 1.58 mm (range 1.2 to 1.9 mm). The calibre of the traversed communicators (anterior or posterior) ranged from 0.8 to 1.5 mm. The patients and aneurysm characteristics are shown in Table 1.
Stent deployment and coil embolisation was technically successful in all cases. In patient 8, two procedures were required for stent placement, as navigation via the post. comm. was technically challenging; this may have been due to the presence of a fenestrated aneurysm clip, which partially obscured the right posterior communicating artery/P1 junction. After navigation beyond this point was achieved, it became apparent that the guider system was not providing sufficient support to allow microcatheter placement beyond the basilar aneurysm neck. The procedure was ended, and a second successful attempt was made the following day with better proximal support (7F Arrow sheath, Teleflex Medical, Stirrup Creek, NC, 6F Envoy XB, Cordis).
Complications
In patient 5, a brief <30-s period of asystole occurred during manipulation of the guidewire in the left posterior communicator/P1 junction. This was self-limiting although atropine was administered. It was not clear whether this event was related to wire manipulation or whether this was an incidental event related to anaesthesia in a patient with known multiple risk factors for ischaemic heart disease (obesity, smoking and non-insulin dependent diabetes mellitus).
In the first seven cases, no neurological complications were encountered. Patient 8, who had a large partially thrombosed basilar aneurysm recurrence (post-clipping) with a 9.3 mm neck, developed post-procedural complications, with a reduced conscious level following coil embolisation. Emergency DSA was performed, which demonstrated no evidence of in-stent thrombosis. Her conscious level improved following treatment with high dose steroids, at which time she was noted to have bilateral third nerve palsies causing bilateral ptosis (on a background of a long-standing residual right third nerve palsy post-clipping). Her problems were initially thought to be due to mass effect; however, a delayed brain CT later confirmed a small right thalamic infarct. This patient subsequently improved but has persistent right third nerve dysfunction (partially pre-existing post-clipping) and gait ataxia. The same patient suffered a femoral pseudoaneurysm that required surgical repair.
Angiographic follow-up
Immediate and follow-up angiographic results are shown in Table 1. Two aneurysms were completely occluded at initial treatment (grade 1): One remained occluded, and the other showed recurrence (grade 3) at 6 months. Six aneurysms showed initial residual neck or dog-ear filling (grade 2). At follow-up, four had progressed to complete occlusion, one remained stable and one showed significant aneurysm recurrence.
In patient 5, there was an asymptomatic in-stent stenosis (ISS) in the left P1, revealed by a first follow-up angiogram performed at 10 months post-embolisation (Fig. 3a–c). Pre-stenting this vessel had measured 1.2 mm. In patient 6, check angiography was delayed as the patient did not attend for follow-up. However, he presented at 12 months with a TIA-like episode despite regular low-dose aspirin. MRI showed no diffusion abnormality; DSA demonstrated complete aneurysm occlusion but a new filling defect in the mid-portion of the stent, presumed to represent organised in-stent thrombus (Fig. 3d–e).
a Patient 5 pre-stenting showing broad-based aneurysm recurrence. b Immediate result post-horizontal stenting and coiling. c Ten-month DSA shows asymptomatic delayed in-stent stenosis of left P1. d Patient 6 immediate post-embolisation angiogram showing some dog-ear aneurysm filling (Raymond 2). e Twelve-month follow-up angiogram showing remodelling of basilar termination due to presumed organised in-stent thrombus and complete aneurysm occlusion
Discussion
We describe our experience of the horizontal stent technique in a select group of patients with technically challenging terminal aneurysms with highly unfavourable D/N ratios. In our experience of such aneurysms, a single balloon or stent may be insufficient to bridge the aneurysm neck. The use of novel balloon techniques is another option in such situations such as retrograde balloon placement [8] or the use of two remodelling balloons. Placement of a remodelling balloon across a communicator is more difficult than placement of the more flexible Prowler microcatheter, so this technique would not have been suitable for most of our cases due to the small size of the communicator. The use of two balloons relies on good proximal access, which was not available in three cases. It also requires simultaneous placement of three catheters, which can be problematic in small vessels. We did not choose this technique in the remainder due to the unfavourable D/N ratios. In patient 3, a previous attempt at embolisation with both balloon techniques had failed. Y stenting is another option for treatment in these cases; however, two stents are required for this technique, delivering twice the amount of metal work to the proximal parent vessel and increasing costs.
The successful implementation of the horizontal technique relies on careful case selection. In five of our cases, prior endovascular treatment had failed or been suboptimal. In two of these, the first aneurysm coiling session had led to incomplete aneurysm occlusion (patients 1 and 4). In another, access to a recurrent basilar aneurysm from the vertebral arteries was limited, making basilar-to-P1 or Y stenting impossible; this procedure was therefore converted to a horizontal stenting during the same session (patient 2). In other two cases, a previous attempt at balloon remodelling had failed to support further coil embolisation of the aneurysm recurrence (patients 3 and 5). In the final three cases, we opted for this technique as we felt other adjunctive techniques would provide suboptimal neck protection. In the six basilar cases, safe access through the post. comm. was possible with the Prowler even though the vessels were small (mean size 1 mm). However, it is important to note that the arteries had a relatively horizontal and straight course, making catheterisation relatively safe. Despite the small size of the communicators, we did not encounter significant problems with catheterisation. There was one episode of self-limiting transient asystole during wire manipulation in the left post. comm./P1 junction. It is unclear whether this was related to anaesthesia or precipitated by wire manipulation. It did not recur after atropine administration.
Kelly et al. [4] described five aneurysms treated with the Neuroform stent using the horizontal technique; this series included two anterior communicating aneurysms stented from A1 to A2. Our two such cases were excluded from this series as we did not consider this to be true ‘horizontal’ stenting. In all of their cases, the communicator measured more than 2 mm in diameter; this was necessary to allow placement of the Neuroform stent delivery catheter. We have demonstrated that use of the Enterprise stent allows access across the circle of Willis via much smaller calibre communicating arteries in five cases via arteries of less than 1 mm. However, vessel size is not the only consideration when making a decision on catheterisation of the communicator; a relatively horizontal course and minimal tortuosity are also desirable. In contrast to the cases performed with Neuroform, it was not necessary to use an exchange procedure in any of our cases for successful placement of the stent delivery catheter. In our view, this reduces the risk of guidewire perforation during stent delivery system placement. Benndorf et al. [3] reported a single case of a terminal carotid aneurysm treated via this approach using the Enterprise stent. Wanke et al. [5] reported a single case of horizontal stenting using Enterprise for treatment of a basilar tip aneurysm, where vertebral access precluded successful Y stenting. Both cases were successful; however, no angiographic follow-up is available in these cases.
The closed cell design of the Enterprise stent [9] offers some advantages over the Neuroform, and in our opinion, it provides better support for coil delivery. Its low profile and flexibility allow the stent to be delivered via tortuous anatomy. The Enterprise stent has the added advantage over the Neuroform of being retrievable during deployment, a feature that aids accurate deployment. There was accurate placement of the Enterprise stent in all eight cases.
In patient 8, a small right thalamic infarct developed post-treatment. This was located in the dorsomedial aspect of the thalamus. Perforator supply to the thalamus varies but we feel that this was most likely related to occlusion of a paramedian perforator (superior ramus) arising from the right P1, rather than relating to occlusion of a tubero-thalamic perforator from the post. comm., which would typically supply the anterior aspect of the thalamus [10]. In this case, the traversed right post. comm. was larger than most in the series, at 1.5 mm diameter, so it seems unlikely that catheterisation of this vessel would lead to perforator occlusion, when it did not in the earlier seven cases with smaller communicators (see Table 1). We feel that the complications encountered in this case, i.e. mass effect, and perforator occlusion were a significant risk using any endovascular technique, given the aneurysm's very large size (20 mm) and very wide neck (9.3 mm). Because of the previous clipping, further surgery was also considered very high risk.
During the time period March 2007–February 2008, the manufacturer recommended the use of Enterprise in 3- to 4-mm-diameter vessels only; we demonstrate that it can be deployed successfully in vessels of smaller calibre. The longer-term effect of the presence of a stent in small vessels is not yet known. The risk of in-stent stenosis may be higher when stenting small arteries. Our early angiographic follow-up demonstrated one case of in-stent stenosis in the left P1 at 10 month follow-up. This was asymptomatic and may relate to a flow effect rather than true in-stent stenosis (Fig. 3c). In patient 6, late follow-up angiography demonstrated a filling defect within the mid-portion of the stent, presumed to represent organised in-stent thrombus. This had led to remodelling of the basilar termination (Fig. 3e). As a precaution, this patient was prescribed clopidogrel, in addition to aspirin, and will be followed closely with DSA. Both cases illustrate the importance of close angiographic and clinical follow-up of patients who are treated by this technique. It is important to note that good quality 3D time of flight MR angiography (MRA) was performed in patient 6 at the time of DSA; this study failed to demonstrate the abnormality within the stent. We therefore strongly recommend that DSA, and not MRA, is used to follow such patients.
In view of the small size of the stented arteries, all of our patients continue life-long aspirin. It is imperative that patients clearly understand the risk of discontinuing anti-platelet therapy. We recommend giving written information to patients that clearly explains the anti-platelet drug treatment strategy.
Ours is a select group of very wide-necked and mostly recurrent aneurysms with most unfavourable D/N ratios, making comparisons with published occlusion rates inappropriate. The immediate post-treatment occlusion grades were favourable; all Raymond grade 1 or 2. At follow-up, six aneurysms showed stable or improved occlusion. Two patients had recanalisation of the aneurysm: One changed from complete occlusion to residual aneurysm, and another progressed from residual neck to residual aneurysm. Both cases had been previously recurring: one post-clipping and the other post-coiling. Given the difficult nature of the aneurysms, the early follow-up occlusion rates are good. Longer-term follow-up is required.
Conclusions
This case series demonstrates that horizontal deployment of the Enterprise stent to assist coil embolisation of wide-necked terminal aneurysms is feasible. The use of this device allows access across the circle of Willis via relatively small communicating arteries in patients with favourable anatomy. The stents were successfully and accurately deployed horizontally in vessels of less than 2 mm in diameter with no occurrences of stent occlusion. The closed cell design of the stent supports coil delivery well. Early angiographic follow-up results indicate that in-stent stenosis may be an issue, and therefore, horizontal stenting is recommended only when other adjunctive techniques have been considered.
References
Thorell WE, Chow MM, Woo HH et al (2005) Y-configured dual intracranial stent-assisted coil embolisation for the treatment of wide-necked basilar tip aneurysms. Neurosurgery 56(5):1035–1040
Cross DT, Moran CJ, Derdeyn CP et al (2005) Neuroform Stent Deployment for Treatment of a Basilar Tip Aneurysm via a Posterior Communicating Artery Route. AJNR Am J Neuroradiol 26:2578–2581
Benndorf G, Klucznik RP, Meyer D et al (2006) ‘Cross-over’ technique for horizontal stenting of an internal carotid bifurcation aneurysm using a new self-expandable stent: technical case report. Neurosurgery 58(1 Suppl):ONS-E172
Kelly ME, Turner R, Gonugunta V (2007) Stent reconstruction of wide-necked aneurysms across the circle of Willis. Neurosurgery 61(5(Suppl 2)):249–254
Wanke I, Gizewski E, Forsting M (2006) Horizontal stent placement plus coiling in a broad-based basilar tip aneurysm: an alternative to the Y-stent technique. Neuroradiology 48(11):817–820. doi:10.1007/s00234-006-0128-6
Debrun GM, Aletich VA, Kehrli P et al (1998) Selection of cerebral aneurysms for treatment using Guglielmi detachable coils: the preliminary University of Illinois at Chicago experience. Neurosurgery 43:1281–1295. doi:10.1097/00006123-199812000-00011
Roy D, Milot G, Raymond J (2001) Endovascular treatment of unruptured aneurysms. Stroke 32:1998–2004. doi:10.1161/hs0901.095600
Moret J, Ross IB, Weill A et al (2000) The retrograde approach: a consideration for the endovascular treatment of aneurysms. AJNR Am J Neuroradiol 21(2):262–268
Higashida RT, Halbach VV, Dowd CF et al (2005) Initial clinical experience with a new self-expanding nitinol stent for the treatment of intracranial cerebral aneurysms: the Cordis Enterprise stent. AJNR Am J Neuroradiol 26:1751–1756
Schmahmann JD (2003) Vascular syndromes of the thalamus. Stroke 34:2264–2278. doi:10.1161/01.STR.0000087786.38997.9E
Conflict of interest statement
Dr Siddiqui has received sponsorship/funding to attend national and international conferences and courses from Boston Scientific Inc, Cordis Neurovascular, ev3 Inc. and Micrus Corporation over the last 5 years.
Dr Bhattacharya has received sponsorship/funding to attend national and international conferences and courses from Boston Scientific Inc, Cordis Neurovascular, ev3 Inc. and Micrus Corporation over the last 5 years.
Dr Jenkins has received sponsorship/funding to attend national and international conferences and courses from Boston Scientific Inc, Cordis Neurovascular, ev3 Inc., Micrus Corporation and Pyramed Limited over the last 5 years.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siddiqui, M.A., J. Bhattacharya, J., W. Lindsay, K. et al. Horizontal stent-assisted coil embolisation of wide-necked intracranial aneurysms with the Enterprise stent—a case series with early angiographic follow-up. Neuroradiology 51, 411–418 (2009). https://doi.org/10.1007/s00234-009-0517-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-009-0517-8