Abstract
Introduction
Ganglioglioma is an uncommon neoplasm of the central nervous system, most frequently seen in the temporal lobe, and usually associated with medically refractory epilepsy in children and young adults. Few reports have considered ganglioglioma-associated epileptogenicity arising in the temporal lobe. The purpose of our study was to define the imaging features of ganglioglioma in the temporal lobe and their relation to the seizure foci revealed by electrocorticograms.
Materials and methods
We reviewed 24 patients with pathologically confirmed ganglioglioma in the temporal lobe.
Results
Computed tomography (CT) images showed gangliogliomas to be isodense (91.7%), and on T1-weighted images (T1-WI) most gangliogliomas (79.2%) were isointense to the gray matter. A cystic lesion was seen in 14 of 24 of the gangliogliomas (58.3%). Mass effects were not seen in any of the ten tumors without cystic components. One patient showed tumor recurrence. Dual pathology was seen in two cases (8.3%). In 23 cases, epileptogenicity was confirmed in the tumors by intraoperative electrocorticogram. The remaining case had no epileptogenicity.
Conclusion
A tumor presenting isointensity to gray matter on T1-WI without mass effects in the medial temporal lobe in a young patient with temporal lobe epilepsy (TLE) might be the characteristic imaging of temporal lobe ganglioglioma. However, such tumors are not always associated with epileptogenicity, even if a ganglioglioma is found in a patient with TLE. The seizure foci may be contralateral to the ganglioglioma. Therefore, we need to investigate the hippocampus, white matter abnormalities of the ipsilateral and contralateral anterior temporal lobe, and other focal lesions closely.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ganglioglioma is an uncommon neoplasm of the central nervous system, most frequently seen in the temporal lobe [1], and usually associated with medically refractory epilepsy in children and young adults, particularly with complex partial seizures. It is the most common cause (40%) of chronic temporal lobe epilepsy (TLE). There have been several studies investigating the imaging of ganglioglioma, but they do not focus on temporal lobe ganglioglioma. Temporal lobe ganglioglioma should be distinguished from gangliogliomas in other regions because of their clinical and radiological characteristics. Moreover, temporal lobe gangliogliomas share several clinical and radiological features with some cortically based temporal tumors. In TLE patients, gangliogliomas almost always have epileptogenicity. However, there are a few cases where epileptogenicity is not confined to ganglioglioma, but is observed by electroencephalogram (EEG), in other pathological changes, including hippocampal sclerosis (dual pathology). To our knowledge, few reports have considered ganglioglioma-associated epileptogenicity arising in the temporal lobe. The purpose of our study was to define the imaging features of ganglioglioma in the temporal lobe and their relation to the seizure foci revealed by EEG and intraoperative electrocorticograms (ECG).
Materials and methods
Twenty-four consecutive patients with pathologically confirmed ganglioglioma in the temporal lobe were admitted to our hospital from 1996 to 2006. The study group comprised 13 males and 11 females, ranging in age at operation from 2 to 41 years (mean age 18.8). All of the patients had suffered complex partial seizures and nine patients (37.5%) suffered partial seizures evolving to secondarily generalized seizure. The interval between onset of seizures and surgery ranged from 0.5 to 22 years (mean 5.4 years). Seizure frequency varied from several per day to one per month. All of the patients were retrospectively analyzed with regard to their computed tomography (CT) and magnetic resonance imaging findings. Each patient had a single tumor. Two neuroradiologists reviewed the CT and MR images, and the interpretation was obtained by consensus. The following findings were evaluated: location of the tumor, density on CT, signal intensity changes on T1- and T2-weighted images, the presence of a cystic component, calcification, contrast enhancement and mass effect. Density on CT and signal intensity changes on MR images were evaluated in the solid components. The existence of epileptogenicity, dual pathology, white matter abnormalities in the anterior temporal lobe and recurrence were also evaluated.
MR images were obtained with a 1.5T MR scanner (Signa-Horizon; GE Medical Systems, Milwaukee, WI, USA). Fast spin echo T2-weighted images (T2-WI), T1-weighted images (T1-WI) and fluid-attenuated inversion recovery (FLAIR) images were obtained in all of the patients. These studies were performed in a plane perpendicular to the long axis of the hippocampus. Moreover, axial T2-WI were obtained in all of the patients.
The parameters for T2-WI were 4,000/99/1 [repeat time (TR)/echo time (TE)/number of excitation pulses, a 220 mm field of view (FOV)], with a slice thickness of 3 mm and a slice gap of 0.7 mm. The FLAIR image parameters were 10,002/152/1/220 [TR/TE/number of excitation pulses/inversion time (TI)], slice thickness at 5 mm and slice gap at 1 mm. T1-WI parameters were 300/9/1 (TR/TE/number of excitation pulses), slice thickness at 3 mm and slice gap at 0.7 mm. The CT examinations were performed using a four detector-row CT (Asteion; Toshiba Medical System, Tokyo, Japan) with the parameters at 120 kV, 250 mA s, 240 mm FOV, 4 mm image thickness, and 4 mm image interval.
Results
Location
Gangliogliomas were located in the uncal part of the temporal lobe (n = 7), parahippocampal gyrus (n = 5), amygdala (n = 5), hippocampus (n = 1), superior temporal gyrus (n = 2), fusiform gyrus (n = 1), and middle temporal gyrus (n = 3). Collectively, 19 gangliogliomas were located in the medial temporal lobes and five tumors in the lateral temporal lobes. Tumor dimensions varied from 0.5 to 3.5 cm, with a mean size of 1.8 ± 0.6 cm.
CT findings
CT images showed that the tumors were isodense to cortical gray matter in 22 patients (91.7%). The remaining tumors appeared low density on CT.
Calcifications were detected in 12 gangliogliomas (50%). The tumors with calcification were located in the uncal part (n = 4), amygdala (n = 3), hippocampus (n = 1), parahippocampal gyrus (n = 1), middle temporal gyrus (n = 2), and superior temporal gyrus (n = 1). Calcifications were nodular (n = 7; Fig. 1), linear (n = 3), or punctuate (n = 2).
Two-year-old girl. a Axial CT image shows a dense calcification in the uncal part of the left temporal lobe (white arrow). b Coronal T1-WI shows a hyperintense lesion, indicating the calcification (white arrow), and small cystic lesions adjacent to the calcification (arrowhead). No mass effects were observed
MR imaging findings
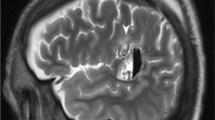
On T1-WI, 19 gangliogliomas were isointense to gray matter (Fig. 2), four tumors were hypointense, and the remaining one was hyperintense. On T2-WI and FLAIR images, 23 gangliogliomas showed high signal intensity changes and the remaining one appeared isointense to gray matter.
Seven-year-old girl. a Axial CT image shows a round area with low density, consistent with cystic component (white arrow). b Coronal T1-WI shows a cystic component (white arrow) and a solid component with isointensity to the gray matter (black arrow) in the uncal part of the left temporal lobe. There were no mass effects. c Coronal T1-WI after an injection of contrast medium showed intense enhancement (black arrow). Cystic components (white arrows) were also observed
Contrast material was administered to all the patients. Contrast enhancement revealed five of 24 gangliogliomas (Fig. 2). Enhancing tumors were nodular (n = 2) or punctuate (n = 3).
Cystic components
A cystic lesion was seen in 14 of 24 of the gangliogliomas (58.3%; Figs. 1 and 2). The cystic components were solitary (n = 8) or multiple (n = 6). Cyst dimensions varied from 0.2 to 3.5 cm.
Of the 14 patients presenting cystic components, 11 (78.6%) were younger than 20 years old. Cystic components were observed in the gangliogliomas of three patients who were aged over 20 years old (3/14, 21.4%). The mean age of patients with cystic components was 13.6 years, which was younger than those without cystic components (25.8 years).
Mass effects
Mass effects were not seen in any of the ten tumors without cystic components.
Dual pathology
In this study, we defined dual pathology as patients who had both ganglioglioma and pathologically confirmed hippocampal sclerosis. Dual pathology was seen in two cases (8.3%), and hippocampal sclerosis and ganglioglioma were ipsilateral in these cases (Fig. 3). Dual pathology was considered as an indication for surgical intervention. Epileptogenicity was confirmed by ECG and both the hippocampus and ganglioglioma underwent surgical resection.
Six-year-old boy dual pathology. a Axial CT image shows a small curved calcification (white arrow) adjacent to a cyst (arrowhead) in the uncal part of the right temporal lobe. b Coronal T2-WI shows a solid component with mild hyperintensity (white arrow) and a cyst with marked hyperintensity (arrowhead). c Coronal FLAIR image shows high signal intensity changes and atrophy in the right hippocampus (white arrow), indicating hippocampal sclerosis. Pathologic examinations confirmed ganglioglioma in the uncal part and hippocampal sclerosis
Epileptogenicity
In 23 cases, epileptogenicity was confirmed in the tumors by intraoperative ECG. The remaining patient had no epileptogenicity in the tumor. In this case [2], the epileptogenicity was defined by microdysgenesis in the contralateral temporal lobe by intraoperative ECG, and was not detected on MR images. Both the microdysgenesis and the ganglioglioma were resected.
Recurrence and outcome
Serial postoperative MR images revealed no recurrence in 22 patients. In one patient, a small residual tumor was revealed on MR images postoperatively, but was stable on follow-up MR studies. In another patient, epilepsy did not improve and a residual tumor was found on MR images postoperatively. Although repeated operations were performed, recurrence was persistent. In the latest MR images, the tumor was seen to have increased in size with a large mass effect and dissemination anterior to the medulla oblongata (Fig. 4). However, pathological examinations did not detect any malignant features.
White matter abnormalities in the anterior temporal lobe
White matter abnormalities in the anterior temporal lobe (loss of gray-white matter demarcation and increased signal intensity in the white matter on T2-WI) are sometimes observed on patients with TLE. These findings were observed in ten (41.7%) of the 24 patients.
Discussion
Temporal lobe epilepsy is characterized by seizures originating in or primarily involving temporal lobe structures. Most clinicians distinguish medial temporal epilepsy from neocortical or lateral temporal epilepsy. The medial structures are important for the generation of seizures [3]. Our study revealed that 19 of 24 gangliogliomas (79.2%) were located in the medial temporal lobes. The most common location for a ganglioglioma is the temporal lobe. Additionally, we think that gangliogliomas especially had a predilection for the medial temporal lobe. This finding corresponds to clinical findings that the foci of TLE are generally found in the medial temporal lobes [4].
On CT images, the solid components of tumors were isodense to gray matter in 22 of 24 cases (91.7%) and were difficult to distinguish. In a previous report, CT was apparently normal in 36.8% of gangliogliomas, but abnormal in TLE patients with other temporal tumors such as: pilocytic astrocytoma, dysembryoplastic neuroepithelial tumor (DNT), pleomorphic xanthoastrocytoma (PXA), and oligodendroglioma [5]. CT examinations were insufficient for ruling out ganglioglioma.
In previous studies, on T1-WI, gangliogliomas appeared isointense or hypointense [6, 7]. In this study, T1-WI showed that most gangliogliomas (79.2%) were isointense to gray matter. This finding was compatible with a previous report of temporal lobe ganglioglioma [3]. Therefore, we suggest temporal lobe ganglioglioma appear isointense on T1-WI and isodense on CT. In contrast, DNTs and astrocytomas in the temporal lobe appear hypointense on T1-WI and hypodense on CT [8]. T2-WI tended to show high signal intensity in all kinds of tumor including gangliogliomas, and this finding was considered to be nonspecific.
In agreement with a previous report [1], more than half of the tumors (58.3%), in the current study, had cystic components. Zhang et al. described that the size of pure cystic lesions was significantly smaller than that having solid components. In another previous study [9], cystic tumors were more common in early childhood (<10 years old), and the average percentage of cysts was much higher in the younger group. Moreover, in younger groups, tumors were associated with edema more frequently than in older groups. In this study, temporal lobe ganglioglioma tended to be smaller than in other regions described by previous reports [7, 8], and cystic appearance was more common in younger patients, particularly in those aged less than 20 years old. Cysts in patients over 20 years old were solitary and small (<5 mm). No mass effects or peritumoral edema was observed in gangliogliomas without cystic components. We think that temporal lobe ganglioglioma might present clinical symptoms at an earlier stage in spite of the small mass effect. One possible explanation might be that the mass effects of the cystic components cause seizures, leading to detection of such tumors at a younger age.
Calcification has been previously reported as a common finding in gangliogliomas [1, 8], with various reported patterns (nodular, patchy, circular, puncture, or linear), and the solid portion of gangliogliomas showed variable enhancement. In the present study, calcification and contrast enhancement showed various patterns. These findings were considered to be nonspecific.
Cortically based temporal tumors include ganglioglioma, pilocytic astrocytoma, DNT, PXA, astrocytoma, and oligodendroglioma. Differential diagnosis of ganglioglioma is necessary. All of the tumors are well demarcated except for astrocytoma and oligodendroglioma, which are less distinct. Pilocytic astrocytoma is a discrete, solid mass with a cystic component. It shows intense, but heterogeneous enhancement and mass effect. DNT shows low density on CT and hypointensity on T1-WI but no enhancement. PXA shows a solid mass with a solitary cyst. CT images show a mass with mixed density. Calcification occurs in 35.8% of PXA [10]. MR images usually reveal well-delineated contrast enhancement. Contrast enhancement of adjacent meninges (dural tail) is common (70%) [11]. Oligodendroglioma is a hemispheric mass that extends to the cortex with mixed density on CT. Cerebral edema and mass effects are common [12, 13].
In previous reports [14, 15], dual pathology (hippocampal sclerosis with a focal lesion) was observed in 5–30% of the temporal lobes operated on because of medically refractory TLE. Focal lesions included heterotopia, cortical dysplasia, low grade tumors (gangliogliomas, DNTs, PXAs and low grade astrocytoma), cavernous angiomas, contusion, and infarction in childhood [14]. Dual pathology was almost exclusively seen in patients whose focal lesions were congenital, or occurred early in life, suggesting that in early childhood the hippocampus is more vulnerable and more likely to develop hippocampal sclerosis [14]. Dual pathology was evident in two of the 24 (8.3%) patients in this study, and it is less frequent than in other congenital epileptic lesions [14]. No correlation with patient’ age or disease duration was found. The resection of both the hippocampal and extra-hippocampal lesions has been suggested as the optimal surgical management of dual pathology. The seizure outcome after epilepsy surgery in cases with dual pathology was less favorable when only one of the lesions was removed [15]. Therefore, when we read MR images of TLE patients, we should always carefully examine the hippocampus (dual pathology).
Completeness of a tumor resection is still considered to be the main factor affecting seizure outcome, although subtotal resections do not necessarily result in a deterioration of the epilepsy [16]. In this study, although a small residual tumor was revealed postoperatively on MR images of one patient, the patient had a prognosis of fewer seizures.
In another patient, the seizure foci on EEG were contralateral to the tumor. Additionally, loss of gray-white matter demarcation and increased signal intensity changes were observed ipsilateral to the seizure foci. Adachi et al. described that white matter abnormalities in the anterior temporal lobe, which were sometimes observed on MR images of patients with TLE, were ipsilateral to the seizure foci revealed by EEG or ECG. White matter abnormalities in the anterior temporal lobe are clinically useful because they indicate the side of the seizure foci. Even if gangliogliomas were observed on MR images of patients with TLE, the seizure foci on EEG were not confined to the tumor. When interpreting MR images of TLE, we should pay careful attention to the anterior temporal lobe.
Histologically, gangliogliomas are grade I or II according to the World Health Organization (WHO) criteria; however, some gangliogliomas show anaplastic features in their glial component and are considered to be grade III (anaplastic gangliogliomas) according to WHO criteria [13]. Some previous studies showed anaplastic ganglioglioma in the temporal lobe with spinal metastasis [14, 17]. Malignant degeneration of gangliogliomas is rare, with an estimated occurrence of 6% [1]. Radiological findings could not differentiate gangliogliomas of grades I, II, or III, nor prognose malignant degeneration. Luyken et al. reported that five of 184 patients (3%) experienced tumor recurrence, which resulted in malignant progression in three patients and death in two patients [18]. Pathologically, anaplastic features or malignant transformation was not always observed in recurrent tumors. Frontal tumor location, grade II or III according to the WHO criteria, or a residual tumor (as identified by postoperative MR images) indicated an increased risk of recurrence. On MR spectroscopy (MRS), gangliogliomas tend to show reduced Cho/Cr and NAA/Cr ratios and increased Cho/NAA [19]. Moreover, the Cho/Cr ratio tends to be lower in gangliogliomas than in gliomas and NAA/Ca ratios are higher [7]. However, even though ganglioglioma is a slowly growing benign tumor, which could be demonstrated by MRS, there is a chance of malignant transformation, especially in cases of incomplete tumor resection [19]. In our study, one patient showed tumor recurrence, with a recurrence rate similar to that in previous reports [17, 20]. A residual tumor was demonstrated in this patient by MR images 1 month after surgery. Pathologically, the tumor was diagnosed as grade I, and malignant transformation has not been observed in 4 years of follow-up.
Conclusion
A tumor presenting isointensity to gray matter on T1-WI without mass effects in the medial temporal lobe in a young patient with TLE might be the characteristic imaging of temporal lobe ganglioglioma. However, such tumors are not always associated with epileptogenicity, even if a ganglioglioma is found in a patient with TLE. The seizure foci on EEG may be contralateral to the ganglioglioma. Therefore, we need to investigate the hippocampus, white matter abnormalities of the ipsilateral and contralateral anterior temporal lobe, and other focal lesions. Although recurrence is rare, careful follow-up may be required.
References
Koeller KK, Henry JA (2001) Superficial gliomas: radiologic–pathologic correlation. Radiographics 21:1533–1556
Adachi Y, Yagishita A, Arai N (2006) White matter abnormalities in the anterior temporal lobe suggests the side of the seizure foci in temporal lobe epilepsy. Neuroradiology 48(7):460–464 Jul
Kuzniecky RI, Jackson GD (eds) (2005) Magnetic resonance in epilepsy, 2nd ed. Elsevier, Philadelphia, pp 99–160
Trescher WH, Lesser RP (1996) The epilepsies. In: Bradley WG, Daroff RB, Fenichel GM, Marsden CD (eds) Neurology in clinical practice: the neurological disorders vol. 2. 2nd edn. Butterworth-Heinemann, Boston, pp 1625–1654
Brainer-Lima PT, Brainer-Lima AM, Azevedo- HR (2006) Ganglioglioma: comparison with other low-grade brain tumors. Arq Neuropsiquiatr 64(3A):613–618
Zentner J, Wolg HK, Ostertun B (1994) Gangliogliomas: clinical, radiological, and histopathological findings in 51 patients. J Neurol Neurosurg Psychiatry 57(12):1497–502
Zhang D, Henning TD, Zou LG, Hu LB, Wen L et al (2008) Intracranial ganglioglioma: clinicopathological and MRI findings in 16 patients. Clin Radiol 63:80–91
Fernandez C, Girard N, Paredes AP et al (2003) The usefulness of MR imaging in the diagnosis of dysembryoplastic neuroepithelial tumor in children: a study of 14 cases. AJNR Am J Neuroradiol 24:829–834
Provenzale J, Ali U, Barboriak DP et al (2000) Comparison of patient age with MR imaging features of gangliogliomas. AJR Am J Roentgenol 174:859–862
Crespo-Rodrigues AM, Smirniotopoulos JG, Rushing EJ (2007) MR and CT imaging of 24 pleomorphic xanthoastrocytomas (PXA) and a review of the literature. Neuroradiology 49:304–315
Osborn AG, Blaser SI, Salzman KL et al (2004) Diagnostic imaging: brain. Amirsys, Salt Lake City, Utah pp I(6):34–37
Grossman RI, Yousem DM (eds) (2003) Neuroradiology: the requisites, 2nd ed. Elsevier, Philadelphia, pp 141–146
Kleihues P, Cavenee WK (2000) Pathology and genetics of tumors of the nervous system. In: Nelson JS, Bruner JM, Wiestler OD, VandenBerg SR (eds) Ganglioglioma and gangliocytoma. IARC (International Agency for Research on Cancer), Lyon, pp 56–61
Salanova V, Markand O, Worth R (2004) Temporal lobe epilepsy: analysis of patients with dual pathology. Acta Neurol Scand 109:126–131
Cendes F, Li LM, Andermann F et al (1999) Dual pathology and its clinical relevance. Adv Neurol 81:153–164
Guilinoni M, Galassi E, Zucchelli M, Volpi L (2005) Seizure outcome of lesionectomy in glioneuronal tumors associated with epilepsy in children. J Neurosurg 102:283–293
Nakajima M, Kidooka M, Nakasu S (1998) Anaplastic ganglioglioma with dissemination to the spinal cord: a case report. Surg Neurol 49(4):445–448
Luyken C, Blumcke I, Fimmers R (2004) Supratentorial gangliogliomas: histopathologic grading and tumor recurrence in 184 patients with a median follow-up of 8 years. Cancer 101:146–155
Im SH, Chung CK, Wang KC et al (2002) Intracranial ganglioglioma: preoperative characteristics and oncologic outcome after surgery. J Neurooncol 59(2):173–83
Hukin J, Siffert J, Cohen H et al (2003) Leptomeningeal dissemination at diagnosis of pediatric low-grade neuroepithelial tumors. Neuro-oncology 5:188–196
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adachi, Y., Yagishita, A. Gangliogliomas: characteristic imaging findings and role in the temporal lobe epilepsy. Neuroradiology 50, 829–834 (2008). https://doi.org/10.1007/s00234-008-0410-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-008-0410-x