Abstract
Introduction
We sought to determine whether diffusion-tensor imaging (DTI) can detect in vivo axonal damage in the corticopontocerebellar pathway of patients with adult-onset ataxic neurodegenerative disease.
Methods
Conventional MRI and DTI were performed on 18 patients with adult-onset ataxic neurodegenerative disease and 28 age-matched control subjects. Fractional anisotropy (FA) and the mean diffusivity (MD) were measured in the ventral, central, and dorsal pons, middle cerebellar peduncle (MCP) and internal capsule to evaluate corticopontocerebellar projection. Changes in FA and MD values were compared between patients and controls. Clinical disability was assessed according to the International Cooperative Ataxia Rating Scale (ICARS). The relationship between DTI measurements and ICARS was studied. Follow-up MRI was performed in five patients approximately 1 year later.
Results
FA values were significantly lower in the ventral and central portions of the pons, MCP, and internal capsules than in these areas in control subjects (P < 0.05) with the lower FA values correlating with poorer ICARS (r > −0.57, P < 0.05). MD values were elevated in these areas, but the differences were smaller than for the FA values. No relationship was observed between the MD and ICARS. In the five patients who underwent the follow-up study, there were significant decreases between the initial study and the follow-up DTI study for FA in the MCP and internal capsule (P < 0.05).
Conclusion
DTI can demonstrate a degenerated corticopontocerebellar pathway in patients, and FA values can be correlated with ataxia severity. DTI may be a clinically useful tool as a quantitative surrogate marker for monitoring disease progression.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ataxic neurodegenerative diseases include some heterogeneous groups and have been classified by clinical and pathological observations. Recent advances in genetics have permitted hereditary spinocerebellar ataxia (SCA) to be divided into over 20 subtypes [1]. The majority of sporadic diseases are multiple system atrophy (MSA) and late-onset cerebellar cortical atrophy (LCCA). The dominant clinical feature of these diseases is progressive ataxia, and the primary lesion is considered to be a deterioration of the pontine nuclei, pontocerebellar tract, and cerebellum [1, 2].
In previous magnetic resonance (MR) imaging studies, the evaluation and assessment of neurodegenerative ataxic disorders has included morphological analysis [2–7], signal intensity changes [8, 9], proton MR spectroscopic imaging [10, 11], and diffusion-weighted imaging [12–19]. Diffusion-tensor imaging (DTI) is a recently developed MR technique that provides information regarding diffusion of water molecules in tissue [20]. Two scalar measurements, mean diffusivity (MD) and fractional anisotropy (FA) can be extracted from DTI and can be used to create quantitative isotropic and anisotropic maps, respectively. MD represents the magnitude of diffusion [21] and FA quantifies the preferential direction of water diffusion along white matter tracts [22]. The diffusion of water is faster along the longitudinal axis of a group of aligned fibers than along the perpendicular (transverse) axis [23, 24].
The pontocerebellar tract has been previously studied by DTI in patients with MSA (16, 17, 19), pure cerebellar syndrome [18], parkinsonian syndromes [19], cerebellar atrophy in phenytoin users [16], and crossed cerebellar diaschisis [25]. These studies have shown decreased FA values in the pons and/or middle cerebellar peduncle (MCP). In patients with MSA, a decrease FA in the pons has been debated. The area and size of the region of interest (ROI) were not defined in the previous studies [17, 18]. The purpose of this prospective study was to confirm the previous results and in particular to confirm which parameter (FA or MD) is more valuable in the determination of the loss of white matter integrity, to discover which area of the pons is most severely affected in patients with ataxic neurodegenerative disease, and to detect possible ongoing degeneration of the white matter tracts in patients who can be followed for a year. In addition, we also investigated the correlation between FA and clinical severity.
Material and methods
Patients
This study was done between May 2004 and April 2006. A group of 25 patients with ataxic neurodegenerative disease were consecutively studied by DTI. The patients underwent neurological examinations, blood and cerebrospinal fluid analysis, and brain MR imaging. Paraneoplastic syndrome, metabolic encephalopathies, inflammatory diseases, tumors, and cerebellar vascular diseases were excluded. Clinical evaluation using the International Cooperative Ataxia Rating Scale (ICARS) was possible in 18 patients [26, 27]. This study focused on these 18 patients (10 women and 8 men with ages ranging from 35 to 77 years, mean ± SD 60.4 ± 12.4 years). The median duration of illness was 10 years (range 0.25 to 20 years). Based on the clinical profile and MR imaging, six patients were diagnosed with MSA. Two patients were diagnosed with LCCA and ten patients had a tentative clinical diagnosis of unclassified spinocerebellar degeneration (SCD). Nine of these ten patients had a family history of ataxia, six of the nine patients underwent gene analysis, which revealed SCA type 3 (SCA3) in two patients and SCA type 6 (SCA6) in one patient; the remaining seven patients had unclassified SCA. A control group comprising 28 age-matched healthy volunteers (14 women and 14 men with ages ranging from 41 to 80 years, mean ± SD 61.8 ± 12.0 years) with normal brain MR imaging were also examined.
Five patients agreed to have longitudinal imaging studies. Follow-up MR imaging was performed at approximately 1 year (mean 360.4 days) after the first MRI examination. The patients’ data are summarized in Table 1.
This prospective protocol was approved by the Institutional Review Board, and informed consent was obtained from both patients and controls.
MR imaging
All imaging was acquired using a 1.5-T scanner (Echo-speed Horizon LX; GE Medical Systems, Milwaukee, Wis.). Axial images were aligned perpendicular to the long axis of the brainstem. Axial T1-weighted, T2-weighted spin-echo images, FLAIR images, and sagittal T1-weighted images were obtained, with these sequences defined as conventional MR imaging. The parameters used for DTI were as follows: TR/TE 17,000/ 92 ms, flip angle 90°, section thickness 4 mm without gap, 40 sections, NEX 5, FOV 24×24 cm, matrix 128×128, and in-plane spatial resolution 1.9×1.9 mm. The sensitizing diffusion gradients were applied on six axes with two b values of 0 and 600 s/mm2 per axis. Although the b value of 1,000 s/mm2 is said to be suitable for the DTI in general, we chose to use the b value of 600 s/mm2 because of the dimensional distortion that occurs particularly in the posterior fossa structures with the higher value. The raw diffusion-tensor data were transferred to a dedicated workstation (Sun Microsystems, Santa Clara, Calif.), where the DTI data were postprocessed using image analysis software (FuncTool 3.1; GE Medical Systems). Maps of the FA and MD were calculated on a pixel-by-pixel basis as described by Basser and Pierpaoli [21].
Region of interest analysis
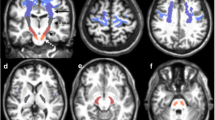
A ROI approach was used to measure FA and MD. A neuroradiologist (K.K.) with 6 years experience and blinded to the subject’s characteristics at the time of image analysis placed the 22-mm2 round ROI over the pons, the center of the bilateral MCP, and the posterior limb of the bilateral internal capsule on the FA and MD maps. The pons was divided into nine segments: ventral, central and dorsal rows in an AP direction, and the right, middle and left sides in the transverse direction (Fig. 1). Half-sized ROIs were used in patients with marked atrophy of the pons and MCP to avoid contamination by cerebrospinal fluid. The inferior and superior cerebellar peduncles were so small that no attempt was made to measure the FA and MD of these structures.
ROIs used in the data analysis. a, c Color-coded FA maps on which regions of high anisotropy are shown in yellow and red, and regions of low anisotropy are shown in blue at the level of the pons (a) and the internal capsule (c). b, d Color-coded MD maps on which regions of high diffusivity are shown in yellow and red, and regions of low diffusivity are shown in blue at the level of the pons (b) and the internal capsule (d). The numbered circles show the regions of interest: 1 right, 2 middle, 3 left in the ventral row of the pons; 4 right, 5 middle, 6 left in the central row of the pons; 7 right, 8 middle, 9 left in the dorsal row of the pons; 10 right, 11 left in the MCP; 12 right, 13 left in the posterior limbs of the internal capsule
Imaging and data analysis
Conventional MR images were examined for signal intensity and morphological changes. T2-weighted axial images were visually inspected for the presence of hyperintensity of MCPs, cruciform hyperintensity in the pons [8, 9], and vertical slit-like hyperintensity in the pons [28] by two of the authors (K.K., K.N.). In the event of disagreement, a consensus was reached through discussion. For morphological changes, the distance between the anterior surface of the pons and the floor of the fourth ventricle (AP diameter) was measured in the T1-weighted midsagittal image displayed on the monitor by one of the authors (K.K.) (Fig. 2). Average FA and MD values in each ROI were compared with those of the control subjects. FA and MD values were compared between the group with hyperintensity in T2-weighted images and the group without hyperintensity. In five patients in whom follow-up MR imaging was performed, signal intensity and morphological changes were evaluated and FA and MD values were measured; these data were compared with those of the initial study.
Statistical analysis
Differences in the AP diameters of the pons and in DTI measurements between the patient group and the control subjects were evaluated using Student’s t-test for unpaired samples. The degree of correlation between the FA and MD values and ICARS scores were evaluated using Pearson correlation coefficients. The average FA and MD values calculated for each ROI were compared between the first and follow-up studies using Student’s t-test. P values less than 0.05 were considered to indicate a significant difference. The statistical software package StatView, version 5.0 (SAS Institute, Cary, N.C.) was used to perform all statistical analyses.
Results
On T2-weighted images, cruciform hyperintensity of the pons and hyperintensity of the MCP together were demonstrated in one patient and vertical hyperintensity of the pons alone was observed in another four patients. Hyperintensity of the MCP was observed in two of these five patients (Table 1). The mean AP diameters of the pons on T1-weighted midsagittal images were 21.1 ± 1.5 mm in the control group and 20.7 ± 1.5 mm in patients; the difference was with not statistically significant.
In most of the individuals, no side-related significant differences in the average FA and MD values were observed (Table 2). We found a statistically significant decrease in the FA values in the ventral and central rows of the pons, MCP, and internal capsule in patients compared with controls. No significant differences were noted in the dorsal row of the pons (Table 2). A clear relationship could not be found between the FA values and disease duration (r = 0.08, P = 0.77). A negative linear relationship was found between the FA values and the ICARS scores in the ventral and central rows of the pons, the internal capsule, and the MCP, with the relationship being particularly strong in the ventral pons (r = −0.82 to −0.85, P < 0.0001; Fig. 3), the central row of the pons (r = −0.73 to −0.77, P < 0.001) and the internal capsule (r = −0.74 to −0.82, P < 0.001). A good relationship was also found between the FA values and the ICARS scores in the MCP (r = −0.57, P = 0.01) but the relationship was not strong compared with that in the ventral and central row of the pons and the internal capsule (Table 2).
MD values in the patients were higher than in the controls. There was a significant difference in MD values of the ventral row of the pons (P < 0.05), but not of the internal capsule or the MCP (Table 2). The difference was less than that for the FA values. No relationship was noted between MD values and the ICARS score in any ROI.
The patients were divided into two groups: one group consisted of 5 patients who showed hyperintensity on T2-weighed images, and the other consisted of 13 patients who did not show T2 hyperintensity. An unpaired t-test showed no significant difference in FA/MD values between these two groups.
Follow-up examination
Approximately 1 year after the first examination, a follow-up MRI study was performed in five patients. These patients had moderately severe ataxia but no abnormal hyperintensity was noted on conventional T2-weighted images: three patients (patients 2, 3 and 4) showed a small decrease in the AP diameter of the pons from the initial examination. No objective interval change was found on neurological examination. On follow-up conventional MRI, no hyperintensity had developed between the first examination and the follow-up study. The AP diameter of the pons on midsagittal images showed no interval change. On the follow-up DTI study, a paired t-test showed significant differences in FA in the MCP and the internal capsule. FA values in the MCP were 0.53 ± 0.05 at the initial examination and 0.50 ± 0.06 at the follow-up examination (P = 0.01). FA values in the internal capsule were 0.66 ± 0.02 at the initial examination and 0.63 ± 0.01 at the follow-up examination (P = 0.02). MD values were slightly elevated, but not significantly (Table 3).
Discussion
The dominant clinical feature of ataxic neurodegenerative diseases is progressive ataxia with the primary lesion considered to be a deterioration of the pontine nuclei, pontocerebellar tract, and cerebellum [1, 2]. Histopathologically, affected areas are characterized by a loss of neurons and variable astrocytosis. Pallor or loss of myelin is seen in the white matter tracts and can be related to the severity of the loss of neurons in affected areas [1]. The pontocerebellar tract sends impulses from the pontine nuclei to the entire cerebellar cortex except for the flocculonodular lobe via the MCP. The cerebellar cortical neurons project to the cerebellar nuclei, particularly to the dentate nuclei. Fibers of cerebellar nuclei which form the superior cerebellar peduncle decussate completely at the level of the inferior colliculus and ascend to the ventrolateral nucleus of the thalamus. The major projection of the ventral nuclei of the thalamus is to area 4 of the cerebral cortex. Neurons of the motor cortex project into the pontine nuclei, from where the fibers project to the contralateral cerebellar cortex via the MCP.
Conventional MR imaging is a useful tool for assessing the integrity of neural tissues in patients with ataxic neurodegenerative disease: white matter hyperintensity on T2-weighted images and atrophy of the brainstem and cerebellum are common findings [2–9]. This study revealed signal intensity changes in five patients (28%) and pontine atrophy in three patients (17%). However, abnormal findings such as these usually appear on MRI images only in advanced phases of the disease.
DTI has been demonstrated to show white matter integrity. Diffusion anisotropy in white matter originates approximately from the specific organization of this tissue, as bundles of more or less myelinated axonal fibers running in parallel; diffusion is in the direction of the fibers [29]. Intact fiber tracts show restricted diffusion and bundles of myelinated axonal fibers running in parallel would show high FA values. In disease processes that disrupt the integrity of the myelin sheath and/or nerve axons, such as Wallerian degeneration, the restriction of water diffusion across the fiber tract is reduced, so FA values would be decreased.
In patients with ataxic neurodegenerative disease, DTI was able to show decreased FA and increased MD in the corticopontocerebellar pathway (ventral and central rows of the pons, MCP and internal capsule). Comparing the FA and MD values between the patients and the controls, FA was a more valuable indicator than MD for demonstrating degenerative changes of the corticopontocerebellar pathway in patients (Table 2), although both values showed significant differences between patients and controls.
In the patients, the most significant decrease in FA was observed in the ventral row of the pons and the MCP followed by the central row of the pons and the internal capsule (Table 2). The significant decrease in FA in the ventral row of the pons may be explained by a loss of pontine nuclei and astrocytosis and pallor or loss of myelin in the descending longitudinal fiber tracts and transverse fibers. The decrease in FA in the central row of the pons was slightly less significant. This result may be because there are fewer neuronal fibers in the central row than in the ventral row of the pons. In contrast, the dorsal row of the pons did not show any significant FA changes in patients. This could be because the dorsal row of the pons has many ascending fiber tracts as described above which appeared to be less deteriorated than those of the corticopontocerebellar pathway. A significant decrease in FA in the basis pontis has been reported in patients with MSA [17, 19]. In those studies, however, FA was measured in a single ROI in the central portion of the basis pontis and the size and placement of the ROI was not clearly defined. In this study, a ROI was placed in the ventral, central, and dorsal row of the basis pontis, including the lateral and central portion of each row. Detailed analysis of the FA values in the patients suggested that in the pons the ventral row would better reflect the degenerative change than the central or dorsal row.
The MCP consists of densely packed fibers running in parallel. The most significant decrease in FA values in the MCP in the patients seems to reflect myelin pallor and loss of myelin of the pontocerebellar fibers, almost all of which seem to be involved in the disease process. This result is consistent with those of prior studies in patients with MSA [16, 17, 19]. The decrease in FA in the internal capsule was an expected result, since degenerative change of the pyramidal tract has been pathologically described in patients with SCD [1, 30, 31]. In this study, the internal capsule showed a significant decrease in FA but to a lesser degree than the ventral row of the pons and the MCP (Table 2). It is speculated that this result can be explained by the fact that the internal capsule not only contains the pyramidal tract, which also has compact axonal fibers running in parallel, but contains many fibers from the cerebral cortex, some of which would not be affected by ataxic neurodegenerative disease.
Reduced FA values of the internal capsule have been reported in patients with amyotrophic lateral sclerosis (ALS) as well as in those with ataxic neurodegenerative disease [32–35]. The ALS patients showed a reduction in FA values in the posterior limb of the internal capsule in comparison with volunteers. Sach et al. concluded that DTI could be useful for assessing upper motor neuron involvement in ALS patients before clinical symptoms of the corticospinal tract lesion become apparent [33].
In this study, the severity of ataxia was evaluated with ICARS. There was a negative correlation between the ICARS score and the FA value in the ventral and central rows of the pons, internal capsule, and MCP. A strong correlation was noted in the ventral row of the pons and internal capsule. There have been few studies investigating FA/MD values in patients with neurodegenerative ataxia using brain MR-DTI [17–19]. Shiga et al. studied 11 MSA patients by DTI and found that FA values decreased in the MCP, basis pontis, and internal capsule. They also found that ataxia was negatively correlated with FA values in the MCP but not in the internal capsule, and suggested that the degree of ataxia in MSA patients may be parallel the degenerative process in the MCP [17]. This study’s results suggest that clinical severity is correlated with the degenerative changes of the pontocerebellar tract, as Shiga et al. reported, and may correlate to some extent with those of the internal capsule. It is unclear why the correlation between the FA values of the ventral and central rows of the pons and the internal capsule and clinical severity was stronger than that between the FA value of the MCP and clinical severity. Yoon et al. studied five patients with sporadic cerebellar ataxia and found decreased FA values in the bilateral MCP and deep cerebellar hemisphere, but normal FA values in the basis pontis [18]. They found no clear relationship between FA and ICARS score. No significant difference in FA change in the basis pontis in their patients was probably due to the size and placement of the ROI. In their study, FA was measured by a single large ROI placed in the central portion of the basis pontis, which appears to contain the central and dorsal row of the pons and may not contain a sufficient amount of pontine nuclei and descending longitudinal tracts. Therefore, FA values of the pons in their study do not seem to represent the corticopontocerebellar tract. Another group studied 17 patients with MSA, 17 patients with progressive supranuclear palsy, and 12 patients with Parkinson disease with DTI [19]. In the patients with MSA, FA was markedly reduced in the MCP, and MD was increased in the pons and MCP. Unfortunately, these authors did not study FA values in the pons because they thought that FA changes in the pons would be difficult to interpret since the pons includes both white matter tracts and pontine nuclei.
Follow-up conventional MR imaging on five patients revealed no significant interval change in signal intensity or AP diameter in the pons. The follow-up DTI study, however, revealed a significant decrease in FA in the MCP, the ventral and central rows of the pons, and the internal capsule. These results suggest that there are ongoing axonal degenerative changes that are detectable only by DTI as a significant decrease in FA, but could not be visualized even by modern conventional MR imaging. Since the number of patients studied by follow-up DTI was limited, further studies are recommended.
There were some limitations to this study. First, the patients with ataxic neurodegenerative disease consisted of a heterogeneous group of patients with inherited and sporadic disease sharing the clinical features of progressive ataxia. Underlying pathological changes, however, are not the same but are similar between these groups. Therefore, we believe that the results of this study are reliable and reflect the underlying pathological processes. Second, the ROI methodology applied might not be sufficiently sensitive to detect a potential underlying abnormality in a proportion of the tracts since so many different fiber tracts are comingled, particularly in the pons and internal capsule. ROIs were manually placed, not automatically. Determining the boundaries of the structure of interest and avoiding partial volume contamination from unrelated fiber tracts are problems with this method. Third, the circumference of the ROIs was held constant for the sampled regions in each patient, and a ROI of 22 mm2 might be too large to reflect changes of a microscopic order for neuronal fibers.
In conclusion, this study confirmed that DTI provides a powerful tool for detecting degenerated corticopontocerebellar pathways in patients with ataxic neurodegenerative disease and may provide a useful quantitative tool for monitoring disease progression. The most reliable sites for measuring FA values seem to be the ventral aspect of the pons, the MCP, and the posterior limb of the internal capsule.
References
Graham DI, Lantos P (eds) (2002) Greenfield’s neuropathology, 7th edn. Arnold, London, pp 343–346, 359–372
Iwabuchi K, Tsuchiya K, Uchihara T, Yagishita S (1999) Autosomal dominant spinocerebellar degenerations. Clinical, pathological, and genetic correlations. Rev Neurol (Paris) 155(4):255–270
Nabatame H, Fukuyama H, Akiguchi I, Kameyama M, Nishimura K, Nakano Y (1988) Spinocerebellar degeneration: qualitative and quantitative MR analysis of atrophy. J Comput Assist Tomogr 12:298–303
Klockgether T, Schroth G, Diener H-C, Dichgans J (1990) Idiopathic cerebellar ataxia of late onset: natural history and MRI morphology. J Neurol Neurosurg Psychiatry 53:297–305
Gallucci M, Splendiani A, Bozzao A et al (1990) MR imaging of degenerative disorders of brain stem and cerebellum. Magn Reson Imaging 8:117–122
Ormerod IE, Harding AE, Miller DH et al (1994) Magnetic resonance imaging in degenerative ataxic disorders. J Neurol Neurosurg Psychiatry 57:51–57
Nicoletti G, Fera F, Condino F et al (2006) MR imaging of middle cerebellar peduncle width: differentiation of multiple system atrophy from Parkinson disease. Radiology 239:825–830
Savoiard M, Strada L, Girotti F et al (1990) Olivopontocerebellar atrophy: MR diagnosis and relationship to multisystem atrophy. Radiology 174:693–696
Bürk K, Bühring U, Schulz JB, Zühlke C, Hellenbroich Y, Dichgans J (2005) Clinical and magnetic resonance imaging characteristics of sporadic cerebellar ataxia. Arch Neurol 62:981–985
Mascalchi M, Cosottini M, Lolli F et al (2002) Proton MR spectroscopy of the cerebellum and pons in patients with degenerative ataxia. Radiology 223:371–378
Tedeschi G, Bertolino A, Massaquoi SG et al (1996) Proton magnetic resonance spectroscopic imaging in patients with cerebellar degeneration. Ann Neurol 39:71–78
Nave RD, Foresti S, Tessa C et al (2004) ADC mapping of neurodegeneration in the brainstem and cerebellum of patients with progressive ataxias. Neuroimage 22:698–705
Guerrini L, Lolli F, Ginestroni A et al (2004) Brainstem neurodegeneration correlates with clinical dysfunction in SCA1 but not in SCA2. A quantitative volumetric, diffusion and proton spectroscopy MR study. Brain 127:1785–1795
Adachi M, Hosoya T, Yamaguchi K, Kawakami T, Kato T (2000) Diffusion- and T2-weighted MRI of the transverse pontine fibers in spinocerebellar degeneration. Neuroradiology 42:803–809
Nicoletti G, Lodi R, Condino F et al (2006) Apparent diffusion coefficient measures of middle cerebellar peduncle differentiate the Parkinson variant of MSA from Parkinson’s disease and progressive supranuclear palsy. Brain 129:2679–2687
Lee SK, Mori S, Kim DJ et al (2003) Diffusion tensor MRI and fiber tractography of cerebellar atrophy in phenytoin users. Epilepsia 44:1536–1540
Shiga K, Yamada K, Yoshikawa K, Mizuno T, Nishimura T, Nakagawa M (2005) Local tissue anisotropy decreases in cerebellopetal fibers and pyramidal tract in multiple system atrophy. J Neurol 252:589–596
Yoon B, Kim SJ, Lee SK, Kim BS, Chung SR, Kim YI (2006) Early pathological changes in the cerebellum of patients with pure cerebellar syndrome demonstrated by diffusion-tensor imaging. Eur Neurol 56:166–171
Blain CRV, Barker GJ, Jarosz JM et al (2006) Measuring brain stem and cerebellar damage in parkinsonian syndromes using diffusion tensor MRI. Neurology 67:2199–2205
Henkelman R, Stanisz G, Kim J et al (1994) Anisotropy of NMR properties of tissues. Magn Reson Med 32:592–601
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111:209–219
Pierpaoli C, Basser PJ (1996) Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 36:893–906
Beaulieu C, Allen PS (1994) Determinants of anisotropic water diffusion in nerves. Magn Reson Med 31:394–400
Shimony JS, McKinstry RC, Akbudak E et al (1994) Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology 212:770–784
Kim J, Lee SK, Lee JD, Kim YW, Kim DI (2005) Decreased fractional anisotropy of middle cerebellar peduncle in crossed cerebellar diaschisis: diffusion-tensor imaging-positron-emission tomography correlation study. AJNR Am J Neuroradiol 26:2224–2228
Trouillas P, Takayanagi T, Hallett M et al (1997) International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. J Neurol Sci 145:205–211
Storey E, Tuck K, Hester R et al (2004) Inter-rater reliability of the International Cooperative Ataxia Rating Scale (ICARS). Mov Disord 19:190–192
Yagishita A (1999) MRI of spinocerebellar degeneration (in Japanese). J Clin Radiol 44:1295–1303
le Bihan D (2006) Looking into the functional architecture of the brain with diffusion MRI: functional and molecular imaging of stroke and dementia – updates in diagnosis, treatment, and monitoring. In: Nishimura T, Sorensen AG (eds) Proceedings of the International Symposium on Functional and Molecular Imaging of Stroke and Dementia, Kyoto, Japan, 14 and 15 October 2005. International Congress Series, vol. 1290 (Radiology), pp 1–24. Elsevier, Amsterdam
Papp MI, Lantos PL (1994) The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain 117:235–243
Watanabe H, Saito Y, Terao S et al (2002) Progression and prognosis in multiple system atrophy. An analysis of 230 Japanese patients. Brain 125:1075–1083
Ellis CM, Simmons A, Jones DK et al (1999) Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 53:1051–1058
Sach M, Winkler G, Glauche V et al (2003) Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 127:340–350
Graham JM, Papadakis N, Evans J et al (2004) Diffusion tensor imaging for the assessment of upper motor neuron integrity in ALS. Neurology 63:2111–2119
Toosy AT, Werring DJ, Orrell RW et al (2003) Diffusion tensor imaging detects corticospinal tract involvement at multiple levels in amytrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 74:1250–1257
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kitamura, K., Nakayama, K., Kosaka, S. et al. Diffusion tensor imaging of the cortico-ponto-cerebellar pathway in patients with adult-onset ataxic neurodegenerative disease. Neuroradiology 50, 285–292 (2008). https://doi.org/10.1007/s00234-007-0351-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-007-0351-9