Abstract
Introduction
Intracranial aneurysm rupture during embolization with detachable coils is reportedly among the gravest of intraprocedural complications. We present here our experiences with this outcome, and a potential intervention for managing this life-threatening complication.
Methods
From April 1998 to March 2005, 284 patients with cerebral aneurysms were treated with detachable coils. Intraprocedural aneurysm rupture occurred in ten patients with a history of a previously ruptured aneurysm. In the event of intraprocedural hemorrhage, we routinely performed heparin reversal with protamine sulfate.
Results
Of the 221 patients with a previously ruptured aneurysm, intraprocedural aneurysm rupture occurred in 10 (4.5%). These ruptures were caused by a microguidewire in one patient, a microcatheter in one, over-packing in two and a coil perforation in three. In the remaining three patients the ruptures were caused by both the microcatheter and the coils. Three patients died because of aneurysm re-rupture, yielding a mortality rate of 30%. One patient presented with a slight disability in the left leg and no neurological deficits were observed in the remaining six patients.
Conclusion
Intraprocedural aneurysm rupture during embolization is a rare, but unavoidable and life-threatening event. Proper measures should be taken to reduce and improve the outcome of this tragic occurrence. The majority of patients with an intraprocedural ruptured aneurysm can survive without severe sequelae if managed appropriately.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although direct endovascular occlusion of intracranial aneurysms with detachable platinum coils has gained increasing popularity as an alternative to surgical clipping because of its acceptable morbidity and mortality rates and an efficacy comparable with that of surgical treatment [1–9], intraprocedural aneurysm rupture continues to be among the most feared complications of endovascular treatment, and one that any interventional neuroradiologist treating aneurysms has to face. The rate of intraprocedural aneurysm rupture has been reported to be 2–7.6% for previously ruptured aneurysms [1, 4–7, 9–21]. Because of its relative infrequency, this event and its management and prevention have been poorly documented. We report here primarily on the outcomes, management and prevention of aneurysm rupture during detachable coil embolization in an unselected series of patients with ruptured intracranial aneurysms.
Materials and methods
Over the course of 7 years, from April 1998 to March 2005, we used detachable coils to treat 284 patients with an intracranial aneurysm in the Shanghai Sixth People’s Hospital. Among these, 221 had ruptured aneurysms. The treatments were performed, as soon as possible after patient admission, with loading and maintenance doses of heparin (2,000–4,000 U per hour and 1,000 U per hour, respectively). General or local anesthesia was administered, depending on the patient’s cooperation level and clinical condition. In patients with intraprocedural hemorrhaging, we performed heparin reversal with intravenous injections of 10 mg protamine sulfate per 1,000 U heparin.
For this study, we used Guglielmi detachable coils (GDC; Boston Scientific, Fremont, Calif.), EDC-10 series (eV3, Plymouth, Minn.) or DCS series (Cordis, Miami, Fl.). We used microcatheters from various sources, including the FasTracker series (Boston Scientific), Excel-10 series (Boston Scientific), Echelon series (eV3) or Prowler Plus series (Cordis). We used various microguidewires, including the soft-tip or platinum-tip Transcend series (Boston Scientific), SeekerLite-10 series (Boston Scientific), Agility-10 series (Cordis) or Silver Speed-10 series (eV3). All charts in our aneurysm database were reviewed, and ten patients in whom the aneurysm had ruptured during the coil embolization procedure were identified. Among these were eight females and two males with ages ranging from 38 to 67 years (mean 46.9 years). All patients were pretreated for acute aneurysmal subarachnoid hemorrhage (SAH).
The diagnoses of aneurysm rupture during embolization were ascertained based on visualization of various clinical parameters in all patients. These parameters included deteriorated neurological status in two patients, deteriorated neurological status and contrast material extravasation in one patient, contrast material extravasation in two patients, extruded microcatheter tip and extravasation of contrast material in one patient, and extruded coils in four patients. Control CT immediately following the embolization procedure showed more hemorrhaging in the subarachnoid space than on pre-embolization CT images. Table 1 presents the clinical data, as well as the causes and outcomes, of the ten patients with an aneurysm ruptured during embolization.
Results
Procedure-related aneurysm rupture occurred in ten patients during their endovascular treatment. In one patient (patient 1), the rupture was caused by a microguidewire perforation of the aneurysm wall. However, the inability to navigate the microcatheter subsequently within the aneurysm sac for coil packing resulted in intracranial re-hemorrhaging and patient death. In two patients (patients 2 and 9), the rupture was caused by over-packing when the last coil was delivered to occlude a neck remnant. The two patients died because we could not implement effective measures to stop the subsequent acute hemorrhage. In three patients (patients 3, 4 and 5), the rupture resulted from coil perforation. However, continuing to pack the aneurysms to achieve complete and dense embolization left the patients with no sequela at discharge. In patient 6, the rupture was microcatheter-related, but the patient recovered with a slight disability in her left lower extremity and remained fully ambulatory. In the remaining three patients (patients 7, 8 and 10), the rupture was related to both the microcatheter tip and the coils. In these situations, the tip of the microcatheter was in close contact with the aneurysm wall and delivery of the coils perforated the aneurysm wall. However, continued embolization rendered none of these patients paralyzed. Overall in our experience, three patients died from massive bleeding after the aneurysm ruptured during embolization (mortality rate of 30%). The intraprocedural aneurysm rupture rate for previously ruptured aneurysms was 4.5% (10/221), with a total intraprocedural rate of 3.5% (10/284). We present five representative cases (1, 2, 5, 6 and 7) individually below.
Illustrative cases
Case 1
A 67-year-old man presented with SAH and was classified clinically as Hunt and Hess (H&H) grade I. Digital subtraction angiography (DSA) showed a 3×3 mm aneurysm on the left anterior communicating artery (ACA) and endovascular management ensued with local anesthesia. During catheterization, we experienced great difficulty moving the microcatheter forward after it entered the A1 segment of the left anterior cerebral artery. At the orifice of the aneurysm, the tip of the microcatheter would not follow the guidewire to enter the aneurysm sac. Several attempts failed, and further advancing the microguidewire resulted in perforation of the aneurysm wall. The patient immediately convulsed and showed elevated blood pressure. Because we could not navigate the microcatheter tip into the aneurysm sac, further manipulation was not attempted and heparinization was reversed. The patient convulsed again 2 h later, gradually lost consciousness and died 2 days after the procedure.
Case 2
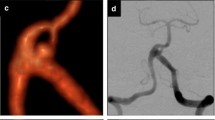
A 45-year-old woman was admitted to our hospital 24 h after SAH (H&H, grade II). Endovascular treatment was performed for a 5×8 mm aneurysm of the right posterior communicating artery (PCA) under local anesthesia. After a GDC-18 5×6 was placed within the aneurysm, another three coils of soft GDC-18 4×8, 3×6 and 2×4 were delivered into the aneurysm sac. When the last coil was introduced, a rupture occurred with a tear at the aneurysm neck, and massive extravasation of contrast material ensued (Fig. 1). The patient lost consciousness immediately. With reversal of heparinization, we immediately occluded the parent artery with coils. The patient remained in a coma after the procedure, and died 1 week later.
Patient 2: a 45-year-old woman with SAH for 2 days. Intraprocedural rupture was related to over-packing of coils. a DSA before embolization shows an aneurysm (5×8 mm) on the right PCA and a small dome (arrow) near the neck of the aneurysm. b DSA after the last coil was packed shows rupture of the aneurysm with massive extravasation of contrast medium (hollow arrow). An over-packed coil was demonstrated in the area of the small dome (arrow). c DSA after embolization shows nearly total occlusion of the aneurysm. This patient died 2 days after the procedure
Case 5
A 38-year-old woman presented with SAH of H&H grade II to III. After the patient was clinically stable, a DSA examination revealed a 4×8-mm aneurysm of the left PCA. Endovascular therapy was started immediately under general anesthesia. During delivery of the first coil (4×8), the aneurysm ruptured with immediate extravasation of contrast material (Fig. 2). Continued packing of the aneurysm with three additional coils (3×6, 3×4, 2×2) resulted in a near-complete occlusion. Angiography after packing showed total occlusion of the aneurysm with a small neck remnant, but no extravasation of contrast material. The patient recovered fully and was discharged from hospital 4 weeks later.
Patient 5: a 38-year-old woman with SAH for 36 h. Intraprocedural rupture was related to coiling. a DSA before embolization shows an aneurysm (4×8 mm) on the left PCA. b Rupture of the aneurysm occurred on delivery of the first coil and DSA shows extravasation of contrast medium (arrow). c Nearly total occlusion was obtained after continued coil packing. The patient was discharged home without any sequela 4 weeks after the procedure
Case 6
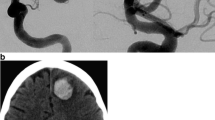
A 40-year-old woman presented with a recurrent headache over the previous 2 weeks. Her CT scan revealed SAH with H&H grade III to IV. Endovascular embolization was performed on a 4×8-mm aneurysm at the M1 and M2 junction of the right middle cerebral artery (MCA) under general anesthesia. One GDC-10 (4×8) and three coils of soft GDC-10 (3×6, 3×4 and 2×4) were used to embolize the aneurysm. During delivery of the third coil, the tip of the microcatheter perforated the aneurysm dome, and localized outside the aneurysm, as determined under fluoroscopy. The angiography, conducted through the guiding catheter, showed that the tip of the microcatheter had exited the aneurysm sac under the force of the high-pressure blood flow, and localized to the temporal lobe surface. The catheter tip was immediately extracted within the aneurysm, and the coil packing procedure was continued until complete occlusion was attained. Angiography after embolization showed no extravasation of contrast material (Fig. 3), and the CT scan revealed a hematoma in the right temporal lobe. Surgery was performed to evacuate the right temporal hematoma. The patient was discharged from hospital 1 month later with a slight disability in her left leg, which did not affect ambulation.
Patient 6: a 40-year-old woman with SAH for 2 weeks. Intraprocedural rupture was associated with the microcatheter during injection of contrast medium. a DSA before embolization shows an aneurysm (4×8 mm) in the shape of dumb-bell at the M1/2 bifurcation of the right MCA. b After delivery of the second coil, the microcatheter with the third coil inside escaped from the aneurysm sac and was then located at the surface of the right temporal lobe (arrow) during injection of contrast medium. c The microcatheter with the third coil inside was drawn back into the aneurysm sac (arrow), and then coil packing was continued. d DSA after embolization shows total occlusion of the aneurysm. This patient went home 1 month later with slight disability in her left leg after surgical removal of the hematoma in the right temporal lobe
Case 7
A 48-year-old man experiencing a H&H grade II SAH underwent a cerebral angiography, which revealed a 5×6-mm aneurysm on the left ACA. Endovascular therapy with general anesthesia was performed on the second day after SAH. After placement of the first coil (5×6), the coil was found to be located outside the limit of the aneurysm sac and to be in the subarachnoid space. Therefore, when placing the second of four additional coils of soft GDC-10 (4×6, 3×6, 3×4 and 2×4), we delivered a small portion of it outside the aneurysm, and the remainder inside the aneurysm sac, after withdrawing the microcatheter tip within the aneurysm. Thus, the cast of coils formed a dumb-bell shape to occlude the defect in the aneurysm wall. Subsequent packing of the aneurysm with more coils led to the dense occlusion. Angiography after embolization showed complete obliteration of the aneurysm, and no extravasation of contrast material (Fig. 4). However, the CT scan revealed denser material in the subarachnoid space than there was prior to embolization. Nevertheless, the patient recovered well, and was discharged 45 days later. A follow-up DSA at 6 months showed a good occlusion status of the aneurysm, with a stable coil cast.
Patient 7: a 48-year-old man with SAH for 2 days. Intraprocedural rupture was associated with both the microcatheter tip and coils. a, b DSA before embolization shows an aneurysm (5×6 mm) on the left ACA (arrow). c Plain film (lateral view) acquired after embolization shows a cast of coils in the aneurysm sac with another cast of coils outside the aneurysm sac (arrow). The two casts of coils form the shape of a dumbbell across the aneurysm wall occluding the aneurysm rupture. d, e DSA after embolization demonstrates total occlusion of the aneurysm. This patient had made a good recovery on discharge 45 days later
Discussion
Hemorrhage during endovascular therapy is a devastating complication [1, 5, 6, 9–18]. However, the management and prevention of this potentially fatal problem is still a challenge for experienced doctors. There are several mechanisms proposed for intraprocedural aneurysm bleeding, including blood pressure fluctuations, high-pressure contrast material injection, and perforation by microcatheter, microguidewire and/or coils [1, 5, 9–16]. Other postulated etiologies for intraprocedural rupture of aneurysms include diversion of blood flow by coils toward weaker portions of the aneurysm wall and a small dome size (≤3 mm). Small aneurysms (≤3 mm) are associated with an increased incidence of intraprocedural rupture because there is not enough space within the aneurysm cavity for safe maneuvering of the tips of both the microguidewire and the microcatheter, and the tips of these microdevices and the delivered coils frequently threaten the aneurysm wall. ACA aneurysms are more prone to perforation probably because it is more difficult to approach these aneurysms than those at other locations, and consequently there is more difficulty maneuvering the microguidewire and the microcatheter.
In our series, six out of the ten patients who experienced intraprocedural ruptures recovered well after the procedure. One patient (patient 6) had slight disability in one leg due to a concurrent intraparenchymal hematoma. Three patients (patients 1, 2 and 9) died giving a mortality rate of 30% (3/10), which conforms to the 20–50% risk of death in other reports [1, 5, 11, 12, 16–18, 22]. One intraprocedural rupture (patient 1), caused by microguidewire penetration, occurred in our early experience. The small size and upward orifice of the ACA aneurysm, the tip of the microcatheter being not properly shaped, and the use of a relatively stiff SeekerLite-10 microguidewire together resulted in difficult manipulation and re-rupture in this patient. Failure to send the microcatheter into the targeted aneurysm for timely coiling led to fatal bleeding. This is the only death caused by microguidewire perforation in the literature to the best of our knowledge [22]. The small size of the microguidewire tip (0.33 mm in diameter) creates a smaller hole on the aneurysm wall than that made by the microcatheter, which usually has a larger diameter (0.5–1.0 mm). This small puncture wound could close before excessive hemorrhaging occurs [5, 23]. It is likely that continued and careless maneuvering of the microcatheter and the microguidewire caused additional injury to the aneurysm wall and subsequent hemorrhage led to the patient’s death.
Tears in the aneurysm neck caused by over-packing resulted in another two deaths (patients 2 and 9). When the last coil was delivered to occlude the neck remnant in these two patients, the tip of the microcatheter was in the neck of the aneurysm. We believe that the coil’s mesh was forced through the fresh clot at the site of the original rupture of the aneurysm wall or the weaker portions of the aneurysm neck, as we were trying to achieve complete aneurysm obliteration. Unlike microcatheter or coil perforation, in which coil packing can be continued to stop the hemorrhage, there are no effective measures to prevent such an occurrence, and there is usually more bleeding in this situation. As a result, clinical prognosis is extremely poor (100% mortality in our study). Rupture caused by over-packing often happens during the last stage of embolization, which is quite different from other ruptures caused by microcatheters or coils in the later phase of embolization [1]. This is because there are no effective measures to prevent hemorrhage in the last stages of the procedure, except parent artery sacrifice. In some difficult-to-manage previously ruptured aneurysms, it may be safer to leave a small aneurysm neck remnant in the first embolization procedure than to attempt to achieve complete obliteration because of the increased risk of aneurysm re-rupture. The neck remnant may be occluded later by a second embolization procedure, or by surgical clipping when the patient presents with an improved brain condition and clinical status.
There are several counter measures that we can use to manage intraprocedural rupture. Some general measures include medically induced hypotension, immediate reversal of heparin anticoagulation with protamine sulfate, emergent external ventricular drainage and emergent surgical clipping of the perforated aneurysm if the patient’s clinical condition allows [1, 5, 10–14, 24]. More specific management techniques can be undertaken in the event of perforation caused by specific devices such as microguidewires, microcatheters and/or coils. Should the rupture occur in this manner, basic rules to follow include leaving the microguidewire in place, advancing the microcatheter over the microguidewire into the aneurysm sac as quickly and as gently as possible, and then performing the coil packing after removing the microguidewire. However, if the microcatheter cannot be navigated subsequently into the aneurysm, further manipulation of both the microcatheter and the microguidewire should be ceased. This is because the opening created by the microguidewire on the aneurysm wall is usually small, and is not life-threatening. Further manipulation may cause greater damage to the aneurysm wall and cause excessive hemorrhaging. If hemorrhaging cannot be stopped, then it is imperative to attempt a temporary or permanent occlusion of the parent artery, in addition to providing some general stabilization measures, such as induced hypotension and immediate heparin reversal. If the rupture occurs because of coil perforation (Fig. 2), and the tip of the microcatheter still resides in the aneurysm, withdrawal of the coil must be avoided. Further delivery of this and additional coils to pack the aneurysm, striving for complete embolization, usually stops aneurysm hemorrhaging.
There may be several approaches to managing the graver complications caused by microcatheter perforation, because microcatheter-induced defects are relatively larger than those induced by a coil or a microguidewire. Once aneurysm penetration has occurred, the perforating microcatheter should be left in place. Then, one could place a part of a coil outside the aneurysm and deliver the rest of the coil into the aneurysm after withdrawing the microcatheter tip from the aneurysm sac. In this way, the delivered coil would form a dumbbell shape (Fig. 4), which would block the hemorrhage. Following this, the aneurysm can be packed with the coils until it is completely obliterated. In patient 6 (Fig. 3), the microcatheter tip that had extruded into the subarachnoid space of the right temporal lobe should not have been retracted immediately. Rather, it should have been left in place, and we should have continued delivering the third coil partially, in order to form a dumbbell across the aneurysm wall, thus stopping the hemorrhage. Immediate retraction of the microcatheter tip might cause the formation of an intracerebral hematoma and a poor outcome. A second approach could be to place a coil entirely in the subarachnoid space to occlude the aneurysm defect and then coil the remainder of the aneurysm after withdrawing the microcatheter tip into the aneurysm sac. A third method is to introduce a coil to the tip of the microcatheter before pulling back the microcatheter tip into the aneurysm to place the coil, and then place additional coils until they are densely packed [5].
Another important solution to microcatheter perforation is to use two microcatheters in a technique described by Willinsky and terBrugge [23]. With the perforating microcatheter in place, a second microcatheter is introduced to pack the aneurysm. This allows the aneurysm defect to remain occluded while the coils are detached through the second microcatheter. This method leads to a good clinical outcome. In perforations associated with both the microcatheter and the coil, the tip of the microcatheter is usually in close contact with the aneurysm wall. When one coil perforates the sac and extrudes through the aneurysm, the microcatheter should not be withdrawn, and coil delivery should be continued. Only after a portion of the next coil is introduced outside the aneurysm should the microcatheter tip be retracted away from the aneurysm wall to the center of the aneurysm sac and the rest of the coil delivered. In this way, the coil can occlude the defect by forming a dumbbell across the aneurysm wall (Fig. 4).
Aneurysm re-rupture caused by over-packing at the end of the procedure is the most disastrous event in endovascular aneurysm correction procedures. This is because there are no quick or effective measures for managing the injury except temporary occlusion or permanent sacrifice of the parent artery. If proven safe enough and done immediately, parent vessel occlusion may be an effective way to safely control aneurysm hemorrhage during endovascular embolization, especially when subsequent coiling following intraprocedural rupture is difficult or impossible. Levy et al. [15] reported successful sacrifice of the right vertebral artery to control intraprocedural aneurysm hemorrhages with good outcomes. But permanent occlusion of the parent vessel requires an occlusion test, which is time consuming and potentially positive. Permanent sacrifice of the parent artery cannot be adopted if the occlusion test is positive, and if the temporary occlusion plays a major role in hemostasis. However, if a major rupture from a microcatheter or a coil occurs, especially early on in the procedure, temporary occlusion of the parent vessel can be performed to control the hemorrhage so that aneurysm coiling can be safely continued [25]. The reason for the death of the patient 2, presented here, despite sacrifice of the parent artery, was likely the excessive amount of subarachnoid bleeding before sacrifice. Therefore, we should perform parent artery occlusion as soon as the decision is made. However, one should remember that it is the patient’s vascular anatomy and aneurysm location that dictates whether vessel sacrifice is safe or not.
In conclusion, all endovascular devices placed into an aneurysm lumen can cause perforation. If this occurs, the perforating device should not be retracted until proper measures have been taken to control or prevent hemorrhage as the device may be partially occluding the perforation, and removing the device could cause further injury to the aneurysm wall and cause increased hemorrhage.
Because of the life-threatening and difficult-to-manage nature of intraprocedural aneurysm rupture, prevention is of vital importance. A few measures to reduce this possibility include:
-
1.
Try to use general anesthesia rather than local anesthesia or neuroleptic analgesia in all patients regardless of the severity of the clinical situation. General anesthesia can provide stable conditions for careful endovascular intervention particularly when intraprocedural rupture occurs. Intraprocedural rupture occurring in patients with neuroleptic analgesia may result in the patient’s restlessness which may go against careful management of the re-rupture neck.
-
2.
Injection of contrast material through the microcatheter, if necessary, should be carried out very carefully after the microcatheter has reached the center of the aneurysm sac.
-
3.
The pressure of contrast material injection through the guiding catheter should be controlled because of the close location of the guiding catheter tip to the intracranial aneurysm.
-
4.
In the presence of vessel tortuosity, in which the aneurysm may be very difficult to selectively catheterize, the microguidewire should not be introduced by force or advanced too far. Moreover, the tip of the microguidewire should be shaped like a small coil or a small tight C curve, so that it will reside within the aneurysm sac naturally without threatening the aneurysm wall.
-
5.
When the microcatheter reaches the aneurysm sac, its tension should be appropriately relieved before withdrawal of the microguidewire or placement of coils, in order to prevent the microcatheter tip from leaping forward and possibly perforating the aneurysm wall.
-
6.
The positioning of the microcatheter tip, after placing each coil, should be carefully adjusted so as to avoid inadvertently pushing the tip against the original rupture site.
-
7.
Before packing the aneurysm neck remnant for complete aneurysm obliteration, a precise evaluation of the diameter and volume of the neck remnant is necessary in order to select coils of appropriate size(s) to avoid over-packing, which may cause laceration of the aneurysm.
References
Kwon BJ, Han MH, Kim KH, Change KH (2003) Procedure-related haemorrhage in embolization of intracranial aneurysms with Guglielmi detachable coils. Neuroradiology 45:562–569
Johnston SC, Zhao S, Dudley RA, Berman MF, Gress DR (2001) Treatment of unruptured cerebral aneurysms in California. Stroke 32:597–605
Johnston SC, Dudley RA, Gress DR, Ono L (1999) Surgical and endovascular treatment of unruptured cerebral aneurysms at university hospitals. Neurology 52:1799–1805
Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group (2002) International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet 360:1267–1274
Doerfler A, Wanke I, Egelhof T, Dietrich U, Asgari S, Stolke D, Foristing M (2001) Aneurysmal rupture during embolization with Guglielmi detachable coils: causes, management and outcome. AJNR Am J Neuroradiol 22:1825–1832
Viñuela F, Duckwiler G, Mawad M (1997) Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 86:475–482
Raymond J, Roy D (1997) Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery 41:1235–1246
Cognard C, Weill A, Castaings L, Rey A, Moret J (1998) Intracranial berry aneurysms: angiographic and clinical results after endovascular treatment. Radiology 206:499–510
Brilstra EH, Rinkel GJ, van der Graaf Y, van Rooij WJ, Algra A (1999) Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke 30:470–476
McDougall C, Halbach VV, Dowd CF, Higashida RT, Larsen DW, Hieshima GB (1998) Causes and management of aneurysmal hemorrhage occurring during embolization with Guglielmi detachable coils. J Neurosurg 89:87–92
Ricolfi F, Guerinel CL, Blustajn J, Combes C, Brugieres P, Melon E, Gaston A (1998) Rupture during treatment of recently ruptured aneurysms with Guglielmi electrodetachable coils. AJNR Am J Neuroradiol 19:1653–1658
Tummala RP, Chu RM, Madison MT, Myers M, Tubman D, Nussbaum ES (2001) Outcomes after aneurysm rupture during endovascular coil embolization. Neurosurgery 49:1059–1066
Valavanis A, Machado E, Chen JJ (1996) Aneurysm rupture during GDC treatment: incidence, management and outcome. Neuroradiology 38 [Suppl 2]:45
Henkes H, Fischer S, Weber W, Miloslavski E, Felber S, Brew S, Kuehne D (2004) Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery 54:268–285
Levy E, Koebbe CJ, Horowitz MB, Jungreis CA, Pride GL, Dutton K, Kassam A, Purdy PD (2001) Rupture of intracranial aneurysms during endovascular coiling: management and outcomes. Neurosurgery 49:807–813
Park HK, Horowitz M, Jungreis C, Genevro J, Koebbe C, Levy E, Kassam A (2005) Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol 26:506–514
Kole MK, Pelz DM, Kalapos P, Lee DH, Gulka IB, Lownie SP (2005) Endovascular coil embolization of intracranial aneurysms: important factors related to rates and outcomes of incomplete occlusion. J Neurosurg 102:607–615
Tateshima S, Murayama Y, Gobin YP, Duckwiler GR, Guglielmi G, Vinula F (2000) Endovascular treatment of basilar tip aneurysms using Guglielmi detachable coils: anatomic and clinical outcomes in 73 patients from a single institution. Neurosurgery 47:1332–1342
Baltsavias GS, Byrne JV, Halsey J, Coley SC, Sohn MJ, Molyneux AJ (2000) Effects of timing of coil embolization after aneurysmal subarachnoid hemorrhage on procedural morbidity and outcomes. Neurosurgery 47:1320–1331
Lozier AP, Connolly ES Jr, Lavine SD, Solomon H, Le Gars D (2002) Guglielmi detachable coil embolization of posterior circulation aneurysms: a systematic review of the literature. Stroke 33:2509–2518
Sedat J, Dib M, Lonjon M, Litrico S, Von Langsdorf D, Fontaine D, Paquis P (2002) Endovascular treatment of ruptured intracranial aneurysms in patients aged 65 years and older: follow-up of 52 patients after 1 year. Stroke 33:2620–2625
Cloft HJ, Kallmes DF (2002) Cerebral aneurysm perforations complicating therapy with Guglielmi detachable coils: a meta-analysis. AJNR Am J Neuroradiol 23:1706–1709
Willinsky R, terBrugge K (2000) Use of a second microcatheter in the management of a perforation during endovascular treatment of a cerebral aneurysm. AJNR Am J Neuroradiol 21:1537–1539
Short JG, Marx WF, Lanzino G, Ellegala DB, Kassell NF (2002) Surgical salvage of microcatheter-induced aneurysm perforation during coil embolization. AJNR Am J Neuroradiol 23:682–685
Bendok BR, Hanel RA, Hopkins LN (2003) Coil embolization of intracranial aneurysms. Neurosurgery 52:1125–1130
Acknowledgements
We thank Tina Kiss and Zhi-Shing Zee, MD, from the Department of Radiology, USC University Hospital (Los Angeles, Calif.) for their kindness in revising the language during preparation of the manuscript.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, MH., Gao, BL., Fang, C. et al. Prevention and management of intraprocedural rupture of intracranial aneurysm with detachable coils during embolization. Neuroradiology 48, 907–915 (2006). https://doi.org/10.1007/s00234-006-0147-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-006-0147-3