Abstract
We report the follow-up of a previously published case (Forlodou et al. Neuroradiology 38:595–597, 1996) of carotido-cavernous fistulas (CCFs) in a patient presenting with type IV Ehlers–Danlos syndrome (EDS 4) that were successfully treated twice by an endovascular approach. Initial treatment with a detachable balloon was in 1994 for a right CCF, and, 8 years later, a left CCF was treated by selective transarterial occlusion of the cavernous sinus with coils. Unfortunately, the patient suffered from a spontaneous post-operative intracranial haemorrhage in the left hemisphere and died. Review of the literature, technical considerations for bilateral CCF and complication are discussed.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
The vascular type of Ehlers–Danlos syndrome (EDS 4) is a rare genetic disease characterised by thin, translucent skin, easy bruising, characteristic facial appearance, and arterial, bowel and/or uterine fragility. The diagnosis of EDS 4, vascular type, is based on compatible clinical findings and can be confirmed by biochemical testing. Biochemical studies in affected individuals demonstrate abnormal electrophoretic mobility and abnormal efficiency of secretion of type III procollagen in cultured dermal fibroblasts resulting from mutations in the COL3A1 gene (chromosomal locus 2q31) [1].
Vascular complications of EDS 4 are increased risks for arterial rupture, especially CCF. From a neuroradiological perspective, EDS 4 may be the most dangerous angiodysplasia to treat because of the many difficulties and hazards associated with the endovascular treatment.
Case report
A 40-year-old woman presented in 1994 with a right CCF following a minor trauma. The right CCF was successfully treated by detachable balloon, resulting in the preservation of the carotid artery [2]. She had a family history of cardiovascular disease, and her older brother had died at the age of 40 years from a spontaneous dissection of a subclavian artery aneurysm. She noticed a thin translucent skin with a tendency to bruising. The diagnosis of EDS 4 was made, based on her clinical inspection, familial history and her angiographic findings. The patient had had no complaints for 8 years until 2002, when she began to develop spontaneous pulsatile and progressive headaches and left sixth nerve palsy. Clinical examination did not find any chemosis or exophthalmia. Non-invasive magnetic resonance (MR) imaging with MR angiogram disclosed dilatation of the superior ophthalmic vein and angiodyplasia of the carotid artery (Fig. 1). After informed consent had been obtained, a cerebral angiogram was performed under general anaesthesia. A five French introducer was inserted into the femoral artery and cautious catherisation performed. The left carotid angiogram revealed a CCF with no opacification of the intracranial vasculature (Fig. 2). The entire left hemisphere was fed by the right internal carotid through the anterior communicating artery. A decision was made not to use a balloon because of the importance of the stenosis of the cervical carotid. A five French guide catheter was placed below the left carotid bifurcation, and cautious catheterisation was done with a 2.3 French Rapid Transit infusion catheter (Cordis Endovascular Systems, USA) with a J type 0.16 in. guide wire (Terumo Corporation, Japan). Selective occlusion of the cavernous sinus was performed (Fig. 3) with a detachable coil system (Cook, Willian Cook, Europe), which resulted in total closure of the fistula with preservation of the patency of the internal carotid artery (Fig. 4). No anticoagulation was used during the procedure. Postoperatively, the patient awoke from general anaesthesia with a mild right hemiplegia. A CT scan revealed a left frontal haematoma and intraventricular clot (Fig. 5). A decision was made to shunt the ventricles. Unfortunately, the patient did not recover and died on day 7 at the age of 49.
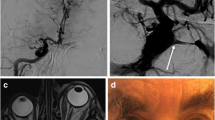
a–c Non-invasive assessment of the left CCF, TOF MR angiogram of the cycle of Willis (a) showing the abnormal and dilated left superior ophthalmic vein. The MR angiogram with gadolinium enhancement of the right (b) and left (c) carotid artery, lateral view, angiodysplasia of the cervical arteries with dilatation and stenosis
a, b Left carotid artery angiogram, antero-posterior view (a) and lateral view (b). There is no opacification of the normal intracranial vascularisation but there is a left, high-flow, carotido-cavernous fistula with opacification of the superior ophthalmic vein and cortical venous reflux in the supra- tentorial and infra-tentorial arachnoid vein. The left hemisphere vascularisation is fed by the right carotid artery through the anterior communicating artery (not shown)
Discussion
As first described by Barabas in 1967 [3], the only form of EDS associated with an increased risk of death is the vascular type, termed Ehlers–Danlos type IV (EDS 4). In a cohort of 419 patients with EDS 4, Pepin et al. [1] reported 44 arterial complications of the central nervous system, with the most frequent being CCFs. Several authors [4–8] have emphasised the difficulties and hazards associated with endovascular treatment of CCFs, especially in EDS 4. It is crucial that the diagnosis of EDS 4 can be done “on call” from the previous medical and family history of the patient, symptoms of vascular rupture or dissection, characteristic ease of bruising and facial appearance. As soon as the diagnosis is made the patient should be transferred to the nearest neuro-interventional team assigned to the disease, ensuring that the team has the technical experience to proceed with the treatment.
With regard to our patient, although we do not suspect any iatrogenic complication from the puncture or from the catheterisation, we believe that the intracranial haemorrhagic complication was secondary to the reperfusion phenomena and dysautoregulation of the cerebral flow. On the post-embolisation angiogram early venous filling was observed on the capillary phase at the junction of the middle cerebral–anterior cerebral territory (Fig. 4). To our knowledge there are few reports of this type of complication following conservative treatment of CCFs. Even if permanent occlusion of the internal carotid artery and of the CCF has a higher chance of success, our case illustrates that choice is difficult between conservative and elective treatment. Elective treatment could be preferable, since it is known that EDS 4 is widespread and can occur on contro-lateral vessels as well. A possible contributing factor to the reperfusion phenomenon was the 3 months’ delay in treatment following the onset of headache. Before the embolisation, all the flow of the left carotid artery was “stolen” by the fistula (Fig. 2), and there was a delay between the two hemisphere at the venous phase of the right carotid artery angiogram, without any clinical event. We believe that the previous haemodynamic conditions led to impairment of the autoregulation and to a normal pressure perfusion breakthrough type of complication. In retrospect, we should have performed a study of cerebro-vascular reactivity before treatment. Cortical venous reflux was also present on the initial angiogram, but there is no reason to presume that one of these cortical veins would rupture during the coiling of the cavernous sinus.
Technically, the improvement in the endovascular technique allows us to use smaller delivery devices (five French introducer and guiding catheter instead of the previous 6–8 French guiding catheter) and the softness of the tip of the micro-guidewire prevents dissection and perforation. Diagnostic angiographies are associated with 22%–35% of morbidity and up to 12% mortality [6] in EDS 4. Because interventional therapy carries a high risk of morbidity (50%) and mortality (25%) [5], a well-trained team, aware of the disease, should be dedicated to endovascular treatment. Bilateral CCF is rare, and this case is the only one on our local database [9]. Halbach et al. [5] reported two patients who had a bilateral CCF out of four EDS 4 patients. The event supports the selective treatment of the fistula, preserving the patency of the internal carotid. The most frequent endovascular treatment of CCF is the trans-arterial balloon occlusion, which has a lower success rate than that for the usual post-traumatic CCF (50%). The venous approach has been advocated to avoid arterial rupture or dissection [7, 8]. Even if, to date, there is no consensus for the CCF treatment in EDS 4, we must avoid direct puncture of the internal carotid artery (ICA) and hyperinflation of the detachable balloon; most of the time it is possible to use a five French guiding catheter and the softest micro-wire.
In conclusion, we report a fatal outcome of endovascular treatment for CCF in EDS 4. Even if preservation of the ICA is possible, we should also be aware of intracranial haemorrhagic complications postoperatively following the restoration of a normal flow in a previously “under-perfused” territory. This tragic case supports Barabas’s suggestion that “knowledge of the pathology should prevent complications”.
References
Pepin M, Schwarze U, Superti-Furga A, Byers PH (2000) Clinical and genetic features of Ehlers–Danlos syndrome type IV, the vascular type. N Engl J Med 342:673–680
Forlodou P, de Kersaint-Gilly A, Pizzanelli J, Viarouge MP, Auffray-Calvier E (1996) Ehlers–Danlos syndrome with a spontaneous carotidocavernous fistula occluded by detachable balloon: case report and review of the literature. Neuroradiology 38:595–597
Barabas AP (1967) Heterogeneity of the Elhers–Danlos syndrome: description of three clinical types and a hypothesis to explain the basic defect. BMJ 2:612–613
Debrun GM, Aletich VA, Miller NR, DeKeiser RJW (1996) Three cases of spontaneous direct carotid cavernous fistulas associated with Elhers–Danlos syndrome type IV. Surg Neurol 46:247–252
Halbach VV, Higashida RT, Dowd CF, Barnwell SL, Hieshima GB (1990) Treatment of carotid cavernous fistulas associated with Elhers–Danlos syndrome. Neurosurgery 26:1021–1027
Horowitz MB, Purdy PD, Valentine RJ, Morrill K (2000) Remote vascular catastrophes after neurovascular interventional therapy for type 4 Ehlers–Danlos Syndrome. AJNR Am J Neuroradiol 21:974–976
Chuman H, Trobe JD, Petty EM, Schwarze U, Pepin M, Byers PH, Deveikis JP (2002) Spontaneous direct carotid–cavernous fistula in Elhers–Danlos syndrome type IV: two case reports and a review of the literature. J Neuroophthalmol 22:75–81
Kanner AA, Maimon S, Rappaport ZH (2000) Treatment of spontaneous carotid–cavernous fistula in Elhers–Danlos syndrome by transvenous occlusion with Guglielmi detachable coils. Case report and review of the literature. J Neurosurg 93:689–692
Desal H, Leaute F, Auffray-Calvier E, Martin S, Guillon B, Robert R, de Kersaint-Gilly A (1997) Direct carotido-cavernous fistula. Clinical, radiological and therapeutic studies in 49 cases. J Neuroradiol 24:141–154
Acknowledgments
To Gregory Krolczyk for his friendly editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Desal, H.A., Toulgoat, F., Raoul, S. et al. Ehlers–Danlos syndrome type IV and recurrent carotid-cavernous fistula: review of the literature, endovascular approach, technique and difficulties. Neuroradiology 47, 300–304 (2005). https://doi.org/10.1007/s00234-005-1378-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-005-1378-4