Abstract
The anterior cingulate cortex (ACC) plays a key role in cognition, motor function, and emotion processing. However, little is known about how traumatic brain injury (TBI) affects the ACC system. Our purpose was to compare, by functional magnetic resonance imaging (fMRI) studies, the patterns of cortical activation in patients with cognitive impairment after TBI and those of normal subjects. Cortical activation maps of 11 right-handed healthy control subjects and five TBI patients with cognitive impairment were recorded in response to a Stroop task during a block-designed fMRI experiment. Statistical parametric mapping (SPM99) was used for individual subjects and group analysis. In TBI patients and controls, cortical activation, found in similar regions of the frontal, occipital, and parietal lobes, resembled patterns of activation documented in previous neuroimaging studies of the Stroop task in healthy controls. However, the TBI patients showed a relative decrease in ACC activity compared with the controls. Cognitive impairment in TBI patients seems to be associated with alterations in functional cerebral activity, especially less activation of the ACC. These changes are probably the result of destruction of neural networks after diffuse axonal injury and may reflect cortical disinhibition attributable to disconnection or compensation for an inefficient cognitive process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Follow-up studies of patients with traumatic brain injury (TBI) disclosed cognitive dysfunction even in patients with good neurological recovery [1–5]. In spite of advances in acute care and rehabilitation, these deficits interfere with the rehabilitation process, social re-integration, and the ability to function independently.
Several neuroimaging studies revealed structural, cerebral blood flow, and metabolic abnormalities following TBI [2, 3], such as corpus callosal atrophy [4] and anterior temporal dysfunction [5]. However, these studies assessed the patient at resting condition, a status in which neural activity is different from task-related neural activity. Positron-emission tomography (PET) or functional magnetic resonance imaging (fMRI) has been applied to assess changes in neural circuitry in response to specific tasks with well-described functional neuroanatomical characteristics [6]. For example, in an fMRI study of working memory, TBI patients showed increased activation of right frontal regions and generally increased dispersion of activation [7]. In a PET study, they manifested reduced frontal activation during free recall and increased frontal activation during recognition [8]. However, in those studies, working memory tasks that stress these networks were used, and other cognitive functions, such as attention and emotional disorders, were not taken into consideration.
Psychological studies indicated that cognitive impairment was related to frontal and anterior cingulate cortex (ACC) abnormalities [9, 10], and fMRI studies provided information regarding the role of the ACC during the Stroop task [11–15]. This task is a classical experimental paradigm used in cognitive neuroscience to probe attention phenomena [16]. These studies showed that the ACC played a key role in selective attention, motor function, and emotional processing. However, little is currently known about how the ACC system is disrupted by TBI. Statistical analysis showed that the Stroop task was better at discriminating between TBI and control groups than other neuropsychological tests were [17]. To our knowledge, no neuroimaging studies on TBI patients performing the Stroop task have been published to date.
In this fMRI study, we tested our hypothesis that ACC dysfunction contributes to cognitive impairment in individuals with TBI. To this end, we compared the patterns of cortical activation during the Stroop task in TBI patients and healthy subjects.
Methods
Participants
The study population consisted of five patients, three men and two women aged between 24 years and 38 years (mean 29.8±6.4 years), who were making a good neurological recovery after sustaining a severe closed head injury caused by motor vehicle accident. Severe head injury was defined as an initial Glasgow coma scale (GCS) score between 3 and 8 [18]. Initial MRI revealed small focal and diffuse neuropathological consequences, typical of moderate-to-severe TBI, in all patients. There was no evidence of massive contusions, including to the ACC, and none of the patients underwent surgical procedures. All underwent neuropsychological testing by the 30-point mini-mental state examination (MMSE) [19], the Wechsler adult intelligence scale-revised (WAIS-R) [20], and the Wechsler memory scale-revised (WMS-R) [21]; all tests were the Japanese-language version. A trained neuropsychological technician, blind to the MRI findings, administered the neuropsychological tests. These tests and fMRI were performed between 1 year and 7 years after the injury. Clinical and neuropsychological data are summarized in Table 1. Patients were compared with 11 age-matched and education-matched right-handed healthy subjects, seven men and four women aged between 23 years and 35 years (mean 28.1±4.7 years). All control subjects were screened to ensure that they had no history of neurological damage or color-blindness. Written prior informed consent, approved by the Gifu University Medical Review Board, was obtained from all study subjects before inception of this investigation.
Task and design
We used a modified Japanese-language version of the Stroop test. The following two variants of the Stroop task were assigned [13]: (1) the color-naming Stroop task, in which the displayed word named the ink color of the word, and selected name color cards (e.g., the word “yellow” printed in green selects the green card); (2) the word-naming Stroop task, in which the displayed word named a color that was different from that of its ink color, and selected name color cards (e.g., the word “blue” printed in red selects the blue card). The colors used were red, blue, green, and yellow. The stimuli were shown by fiber-optic glasses (Silent Vision 4000, Avotec, USA). Each stimulated block consisted of 15 trials, presented at a rate of one trial every 2 s. The subjects responded by pressing right/left buttons in response to color-congruent/word-congruent targets [12]. A scanner was used to obtain accuracy data for all subjects. We were not able to record reaction time data during scanning.
Functional MRI procedure
We used a blocked fMRI design that involved presentation of a 30-s rest condition followed by a 30-s activation condition. This cycle was repeated three times over the course of 3 min. The resting baseline reference task was a standard condition during which subjects were instructed to lie still and remain quiet with their eyes open [22]. Functional and anatomical imaging was performed on a 1.5 T clinical scanner (Signa, GE Medical Systems, Milwaukee, Wis., USA) with a standard head coil. Blood oxygenation level-dependent (BOLD) functional images parallel to the bicommissural plane were acquired with single-shot echo planar sequences (repetition time 3,000 ms, echo time 50 ms, flip angle 90°, acquisition matrix 64×64, field of view 24 cm, 15 slices, slice thickness 7 mm, slice gap 1.5 mm). In addition, high-resolution T1-weighted three-dimensional spoiled gradient echo (SPGR) anatomical images were obtained (repetition time 7.2 ms, echo time 1.5 ms, flip angle 10°, acquisition matrix 256×256, field of view 24 cm, 160 slices, slice thickness 1 mm). During each cognitive task condition, 60 images per slice were acquired in 210 s (total = 960 images).
Data analysis
Post-processing was done on a Microsoft workstation using SPM99 (The Welcome Department of Neurology, University College London, UK) implemented in Matlab (Mathworks, Sherborn, Mass., USA). Realignment for motion correction, normalization, and deformation was performed by using the standard brain template from the Montreal Neurological Institute (MNI) and converting to the space of the stereotactic atlas of Talairach and Tournoux [23]. Smoothing was at 10-mm thickness; for data analysis we used thresholds of P< 0.05 (corrected) for individual subjects.
Using these data we calculated group activation maps by pooling the data for each condition at thresholds of P< 0.001 (uncorrected). The output from each statistical analysis is a statistical parametric map or a three-dimensional image.
Results
Behavioral data
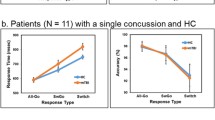
Control subjects and TBI patients did not differ significantly with respect to gender, age, and years of education. Although the TBI patients did generally as well as the control subjects, they manifested a poor memory for recent events and had trouble with their jobs. Although the patients were able to perform the Stroop task, they made more errors than the controls; however, there was not a significant difference between the two groups (P=0.51).
Imaging data
Follow-up MRI scans showed cerebral abnormalities in two patients. Patient 4 in Table 1 had a small contusion scar at the bilateral frontal cortices and patient 1 had a slight subdural effusion in the right frontal region. There was no evidence of abnormalities in the other three patients.
The location of significant increases in the BOLD signal during the modified Stroop task is shown in Table 2 and Fig. 1. In TBI patients and controls, cortical activation was found in similar regions of the frontal [Broadmann’s area (BA) 6, 44, 46], occipital (BA 19, 37), and parietal (BA 7, 40) lobes and resembled the patterns of activation documented in previous neuroimaging studies of the Stroop task in healthy non-Japanese controls [12, 13, 15], indicating that our modified Japanese version of the computed Stroop task can be used to evaluate cerebral recruitment in Japanese subjects.
Maximum-intensity projections of the statistical parametric maps during the Stroop task for TBI patients (bottom) and healthy controls (top) (P< 0.001, uncorrected). For localization of activation see Table 1
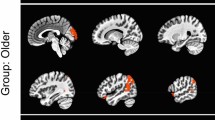
As shown in Fig. 2, compared to the control group, the TBI group displayed more relatively decreased cerebral activation in the ACC (BA 32).
Location of the cognitive division of the ACC (ACcd) on coronal slices (y=+18 mm). The absence of activity in TBI patients (right) and significant activity in control subjects (left) is superimposed on group-averaged Talairach and Tournoux transformed high-resolution structural scans [23]
Discussion
Using fMRI studies, we compared the pattern of cortical activation in patients with TBI and healthy control subjects. Although cognitive impairment may be subclinical and discrete in TBI patients, it can pose considerable challenges in their social re-integration [1]. Our results provide results of our interrogation of cortical physiology after head injury.
The cingulate cortex is comprised of the anterior and posterior cortices, each of which possesses different thalamic and cortical connections [9]. The ACC can be divided into discrete anatomic and behavioral subdivisions: the affective division (ACed) and the cognitive division (ACcd) [14]. The ACed includes areas 25, 33, and rostal area 24 and plays a role in emotion and motivation; the ACcd includes caudal areas 24 and 32 and plays a role in complex cognitive/attentional processing [10, 11, 14]. Neuroimaging studies have shown that the ACcd is activated by numerous cognitive/attentional tasks including Stroop tasks, divided attention tasks, and working memory tasks [12, 24, 25]. The ACcd is vitally important for the proper and efficient functioning of frontostriatal attention networks.
One key region of working memory is the prefrontal cortex, and, in patients with TBI, the frontal cortices tend to be damaged both structurally and functionally [5, 25]. In a functional PET study, TBI patients showed increased activity in the ACC and in frontal and occipital lobe activity during working memory tasks [26]. fMRI study of TBI patients showed higher levels of activation under the high processing load condition than under the low condition [27]. This extensive activation under working memory load may represent a response to increased task difficulty [24]. However, our TBI patients showed less ACcd activation than the controls. Decreased activation of the ACcd has been noted in patients with attention-deficit/hyperactivity disorder (ADHD) who manifested performance deficits in the Stroop task [28]. Even patients able to keep up with the processing demands did so less efficiently for a given level of performance accuracy than poorly performing subjects [29]. However, under-activation in a poorly performing subject may reflect failure to fully engage in the task when it exceeds the subject’s processing capacity [30].
Brain function is now interpreted on the basis of neural networks rather than separate anatomical structures. In our TBI patients, cerebral activation was more regionally dispersed and diminished in the prefrontal and parietal cortex. This decrease may be the result of a decrease in the functional and structural connectivity of parietal and prefrontal regions [31, 32]. It has been shown that the ACcd keeps up with the lateral prefrontal cortex, parietal cortex, and lower motor area [10]. However, it is not known whether it is the sole dominant region for cognitive function, attentional selection, and processing. ACcd dysfunction in a parallel-distributed network is not eliminated, as apparent frontostriatal deficits may be the downstream effects of ACcd dysfunction [14, 33, 34]. At any rate, these changes are probably the result of diffuse axonal injury and may reflect either cortical disinhibition attributable to disconnection or compensation for an inefficient cognitive process.
Several limitations of this study deserve mention. First, our results may not be applicable to older and more severely disabled patients who cannot undergo fMRI. Second, our participants were not subjected to detailed neuropsychological testing of mental status, visual memory, reaction time, and language function testing. Although psychological assessments can yield important information, they fail to reveal the neural substrates or pathways. fMRI scans make it possible to graphically interrogate the cortical physiology under various stimulation paradigms. Third, we utilized a block design fMRI technique rather than an event-related fMRI approach. Event-related designs allow the extraction of relative timing information on the onset of activity in different neural substrates as well as the duration of cognitive processing during a task, offering new opportunities for cognitive studies [35]. Further investigations utilizing an event-related design are necessary for the full explication of our findings. Lastly, our study population consisted of a relatively small number of subjects, and additional experiments with a larger sample size and a randomized design are necessary.
Conclusions
Cognitive impairment in TBI patients appears to be associated with alterations in functional cerebral activity and, especially, with decreased activation of the ACC. Decreased activation in the ACcd regions may represent evidence of cognitive impairment.
References
Levin HS, Gary HE Jr, Eisenberg HM, Ruff HM, Barth JT, Kreutzer J, High WM Jr, Portman S, Foulkes MA, Jane JA, Marmarou A, Marshall LF (1990) Neurobehavioral outcome 1 year after severe head injury. Experience of the traumatic coma bank data. J Neurosurg 73:699–709
Levin HS, Williams DH, Eisenberg HM, High WM Jr, Guinto FC Jr (1992) Serial MRI and neurobehavioural findings after mild to moderate closed head injury. J Neurol Neurosurg Psychiatry 55:255–262
Stamatakis EA, Wilson JTL, Hadley DM, Wyper DJ (2002) SPECT imaging in head injury interpreted with statistical parametric mapping. J Nucl Med 43:217–226
Levin HS, Williams DH, Valastro M, Eisenberg HM, Crofford MJ, Handel SF (1990) Corpus callosal atrophy following closed head injury: detection with magnetic resonance imaging. J Neurosurg 73:77–81
Langfitt TW, Obrist WD, Alavi A, Grossman RI, Zimmerman R, Jaggi J, Uzzell B, Reivich M, Patton DR (1986) Computerized tomography, magnetic resonance imaging, and positron emission tomography in the study of brain trauma. Preliminary observations. J Neurosurg 64:760–767
D’Esposito M (2000) Functional neuroimaging of cognition. Semin Neurol 4:487–498
Christodoulou C, DeLuca J, Ricker JH, Madigan NK, Bly BM, Lange G, Kalnin AJ, Liu WC, Steffener J, Diamond BJ, Ni AC (2001) Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. J Neurol Neurosurg Psychiatry 71:161–168
Ricker JH, Muller RA, Zafonte RD, Black KM, Millis SR, Chugani H (2001) Verbal recall and recognition following traumatic brain injury. A [O-15]-water positron emission tomography study. J Clin Exp Neuropsychol 23:196–206
Vogt BA, Finch DM, Olson CR (1992) Functional heterogeneity in cingulate cortex. The anterior executive and posterior evaluation regions. Cereb Cortex 2:435–443
Devinsky O, Morrell MJ, Vogt BA (1995) Contributions of anterior cingulate cortex to behavior. Brain 118:279–306
Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL (1998) The emotional counting Stroop paradigm. A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 44:1219–1228
Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL (1998) The counting Stroop. An interference task specialized for functional neuroimaging—validation study with functional MRI. Hum Brain Mapp 6:270–282
Leung HC, Skudlarski P, Gatenby JC (2000) An event-related functional MRI study of the Stroop color word interference task. Cereb Cortex 10:552–560
Bush G, Luu P, Posner MI (2000) Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222
Gruber SA, Rogowska J, Holocomb P, Soraci S, Yurgelun-Todd D (2002) Stroop performance in normal control subjects. An fMRI study. Neuroimage 16:349–360
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662
Bate AJ, Mathias JL, Crawford JR (2001) Performance on the test of every attention and standard tests of attention following severe traumatic brain injury. Clin Neuropsychol 15:405–422
Teasdale G, Jannett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet:ii:81–84
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Wechsler D (1985) The Wechsler adult intelligence scale-revised. Psychological Corp., New York
Wechsler D (1987) The Wechsler memory scale-revised. The Psychological Corp., San Antonio
Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Younkin D, Rosen AD, Skolnick BE, Reivich M (1982) Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science 217:659–661
Talairach J, Tournoux P (1988) A co-planar stereotactic atlas of a human brain. Thieme, Stuttgart
Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD (1997) Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia 35:1373–1380
Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC (1997) A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5:49–62
Levine B, Cabeza R, Mclntosh AR, Black SE, Grady CL, Stuss DT (2002) Functional reorganization of memory after traumatic brain injury: a study with H 152 O positron emission tomography. J Neurol Neurosurg Psychiatry 73:173–181
McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, Mamourian AC, Weaver JB, Yanofsky N (1999) Brain activation during working memory 1 month after mild traumatic brain injury. A functional MRI study. Neurology 53:1300–1308
Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J (1999) Anterior cingulated cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting Stroop. Biol Psychiatry 45:1542–1552
Weiss EM, Golaszewski S, Mottaghy FM, Hofer A, Hausmann A, Kemmler G, Kremser C, Brinkhoff C, Felber SR, Fleischhacker WW (2003) Brain activation patterns during a selective attention test. A functional MRI study in healthy volunteers and patients with schizophrenia. Psychiatry Res 123:1–15
Bullmore E, Brammer M, Williams SC, Curtis V, McGuire P, Morris R, Murray R, Sharma T (1999) Functional MR imaging of confounded hypofrontality. Hum Brain Mapp 8:86–91
Cabeza R, McIntosh AR, Tulving E, Nyberg L, Grady CL (1997) Age-related differences in effective neural connectivity during encoding and recall. Neuroreport 8:3479–3483
Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thronton AE, Acker JD (1997) Selective aging of the human cerebral cortex observed in vivo. Differential vulnerability of the prefrontal gray matter. Cereb Cortex 7:268–282
Cohen JD, Dunbar K, McClelland JL (1990) On the control of automatic processes. A parallel disturbed processing account of the Stroop effect. Psychol Rev 97:332–361
Goldman-Rakic PS (1988) Topography of cognition. Parallel distributed networks in primate association cortex. Ann Rev Neurosci 11:137–156
Menon RS, Kim SG (1999) Spatial and temporal limits in cognitive neuroimaging with fMRI. Trends Cogn Sci 3:207–216
Acknowledgment
The authors thank Mr. Naoki Hirata at GE Yokogawa Medical Systems, Ltd., and Mr. Yukinori Kasuya for technical assistance and Ms. Ursula A. Petralia for editing the manuscript. This work was supported and approved by the Gifu Prefecture Brain Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soeda, A., Nakashima, T., Okumura, A. et al. Cognitive impairment after traumatic brain injury: a functional magnetic resonance imaging study using the Stroop task. Neuroradiology 47, 501–506 (2005). https://doi.org/10.1007/s00234-005-1372-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-005-1372-x