Abstract
It remains controversial whether extracranial–intracranial (EC–IC) arterial bypass surgery leads to a significant increase in brain blood supply, allowing the reversal of regional cerebral hypoperfusion in symptomatic patients with occlusive cerebrovascular disease and hemodynamic impairment. The aim of the present study was to determine the effects of EC–IC bypass surgery on cerebral brain-supplying blood volume flow (BVF; ml/min) from a purely hemodynamic point of view, using 2D cine phase-contrast MR imaging. Twenty-five patients with symptomatic, unilateral internal carotid artery (ICA) occlusion and hemodynamic compromise received EC–IC arterial bypass surgery. All patients underwent quantitative BVF measurements of brain-supplying arteries preoperatively and postoperatively, including the direct BVF measurement in the established EC–IC bypass after surgery. Preoperatively, total brain BVF was reduced in comparison to normal controls (595±89 vs 663±49 ml/min; [mean±SEM]; p=0.039). Mean BVF through the EC–IC bypass reached 84±32 ml/min (range: 14–177 ml/min), leading to a significant net increase in total BVF of 78±43 ml/min (range: 7–136 ml/min) when compared with BVF prior to surgery (p<0.001), with resulting postoperative BVF reaching values obtained in normal controls. EC–IC arterial bypass surgery increases total brain blood supply, allowing restoration of local perfusion in hemodynamically compromised brain tissue in patients with symptomatic ICA occlusion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The critical decrease of regional cerebral perfusion due to insufficient blood supply distal to an occluded parent blood vessel is known to be causative for transient ischemic attack or definite infarction in patients with occlusive cerebrovascular disease. In contrast to asymptomatic carotid occlusion [1], these patients show elevated oxygen extraction fraction (OEF) [2, 3, 4] as a sign of hemodynamic impairment, low regional cerebral blood flow (rCBF) at rest and significantly compromised vascular reserve capacity [5, 6, 7, 8]. In addition, changes in brain metabolism are observed within the dependent vascular territory [9, 10]. Based on these findings, extracranial–intracranial (EC–IC) arterial bypass surgery, at least hypothetically, appears to be ideal for improvement of cerebral perfusion within the territory distal to the arterial occlusion, so that the concept of blood flow restoration and compensation is the pillar on which the concept of EC–IC bypass surgery is based. In 1985 an international study of EC–IC arterial bypass surgery [11] demonstrated no benefit from EC–IC arterial anastomosis for patients with cerebrovascular disease including internal carotid artery (ICA) occlusion. Interest in the use of EC–IC bypass surgery, however, has been rekindled, because some patients with occlusion of the internal carotid artery have poor collateralization and high risk for recurrent or chronic ischemia, whereas other patients with internal carotid-artery occlusion do have sufficient collateralization with adequate perfusion and low probability for stroke. A restricted surgical treatment based on sufficient preoperative evaluation of patient candidates for EC–IC bypass surgery might improve the usefulness of EC–IC bypass surgery. Supporting this view, there was substantial criticism of the international EC–IC bypass trial [12, 13], which demonstrated no value of bypass surgery in any of the subgroups of patients included in the study, in particular regarding the patient selection, including the methods and techniques of preoperative investigations available at that time.
In the literature there is scant data available on direct EC–IC bypass flow. Quantitative flow data in conventional direct arterial EC–IC bypasses—arterial anastomosis between the superficial temporal artery, frontal or parietal branch, and the middle cerebral artery (M2 or M3 segments)—are only measured in a very few number of patients with different techniques [14, 15, 16]. In these measurements blood flow ranges 15–25 ml/min, which must be considered insufficient, in particular in comparison with blood flow values of about 250 ml/min in a normal internal carotid artery. Technically more difficult, so-called high-capacity bypasses, performed as venous grafts with more proximal-located anastomosis (M1 and proximal M2 segments), demonstrate blood flow values of 40–150 ml/min [16, 17].
So far, however, the potential of EC–IC bypass surgery to reverse hypoperfusion remains controversial. Various studies investigating the effects of bypass surgery on cerebral hemodynamics demonstrated restitution of rCBF, OEF and/or cerebral blood volume (CBV), cerebral metabolic rate of oxygen and glucose (CMRO2 and CMRGlu), when using positron emission tomography (PET) [18, 19, 20, 21]. In addition, restoration of cerebrovascular reserve capacity (CVRC) alone [22] or rCBF and CVRC [6, 23, 24] was observed after surgery when applying Xenon-washout techniques.
On the other hand, there is also evidence from PET studies that cerebral perfusion remains unchanged after cerebral revascularization, suggesting the inability of the bypass to restore perfusion. One explanation, in the light of a patent bypass, could be that bypass surgery leads to redistribution of blood volume flow within the hemodynamically compromised vascular territory only, leaving global perfusion unchanged despite a patent anastomosis.
In order to shed more light on the efficacy of bypass surgery, the aim of the present study was to determine blood volume flow (BVF) in brain-supplying arteries in patients with unilateral ICA occlusion and hemodynamic impairment, before and after surgical revascularization. Previous studies addressed the usefulness of EC–IC bypass surgery and blood flow measurements in a small number of conventional EC–IC bypasses (15–25 ml/min) and the results of venous graft bypasses (40–150 ml/min). The present study primarily focused on the direct blood volume flow measurement in conventional EC–IC bypasses performed in carefully selected patients for bypass surgery with unilateral internal carotid artery occlusion and hemodynamic impairment. Secondary focus was put on the blood flow changes in the contralateral internal carotid artery and the basilar artery feeding as collaterals.
Methods
The study protocols were approved by the local ethics committee. Twenty-five patients (18 male, seven female, 55±12 years) with either transient or minor retinal ischemic attacks or minor stroke and angiographically proven occlusion of one ICA, who fulfilled the criteria for application of EC–IC arterial bypass surgery and who showed no angiographically visible collateral flow via the ophthalmic artery or leptomeningeal vessels, were included in this study. In addition, 16 healthy volunteers (eight male, eight female, 56±11 years), served as controls for BVF measurements.
Indication for EC–IC arterial bypass surgery and surgical procedure
Only patients younger than 70 years, with normal cranial computerized tomography (CCT) or evidence of border-zone-infarction underwent evaluation procedures for EC–IC bypass surgery. The indication for bypass surgery in these patients with symptomatic ICA occlusion was based upon the quantitative assessment of rCBF and CVRC [24]. In brief, patients were considered candidates for surgical revascularization if rCBF augmentation in response to acetazolamide was either less than 30% (i.e., reduced CVRC) or paradoxically decreased (i.e., “steal-phenomenon”) [25]. Functional rCBF studies were performed after diagnostic angiography by means of stable Xenon-enhanced CT (4.5 min wash-in protocol, 30% Xe, 60% O2). Determination of CVRC was done using the following equation:
Here, rCBFstim=stimulated rCBF 15 min after acetazolamide i.v.; rCBFbase=rCBF at rest; rCBF (ml/100 g/min).
The neurosurgical procedure consisted of the establishment of a direct anastomosis between the frontal or parietal branch of the superficial temporal artery (STA; donor vessel) and a cortical (recipient) vessel of the middle cerebral artery (MCA—M2 or M3 segment) via a standard temporal craniotomy [26, 27].
Angiography
In all patients, brain-supplying vessels and STA–MCA anastomosis were investigated by selective intra-arterial angiography before and after surgical procedure, in order to assure ICA occlusion, as well as to exclude collateral flow via the ophthalmic artery and leptomeningeal vessels preoperatively and bypass patency postoperatively.
MRI and MRA studies
MRI, MRA and blood volume flow studies were performed using a 1.5-T MR unit (Magnetom Vision, Siemens, Erlangen, Germany). All patients and control subjects underwent identical MR protocols using a circular polarized head coil and a Helmholtz neck coil. After the acquisition of T1-weighted scout images (TR: 545 ms; TE: 15 ms; slice thickness: 4 mm; slice gap: 0.6 mm) to gain anatomical reference information, quantitative assessment of BVF (ml/min) was performed in the common carotid arteries (CCA; CCAipsi=ipsilateral to ICA occlusion, CCAcontra=contralateral to ICA occlusion), internal carotid arteries (ICAcontra=ICA contralateral to ICA occlusion) and basilar artery (BA). In addition, postoperative studies included BVF measurements within the established EC–IC bypass (BY).
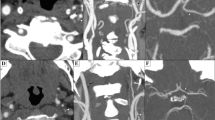
A dynamic 2D cine phase-contrast MR technique was applied, using an ECG-triggered, fast radiofrequency spoiled gradient-echo sequence with the following parameters: TR 28 ms; TE 5 ms; flip angle 30o; FOV 220 mm; matrix 192×256; one acquisition. Velocity encoding was set between 40 cm/s and 250 cm/s, based on blood flow velocity in the measured vessel. Depending on the patient’s heart rate 25–35 single 2D phase-contrast images were acquired within the cardiac cycle (time resolution 28 ms). All blood flow measurements were performed perpendicular to the course of the arteries studied, where section positioning was performed approximately 3–4 cm proximal to the carotid artery bifurcation for the CCA measurement and through the C3 segment of the ICA to measure ICA blood volume flow. BA blood flow measurement was performed in the upper BA, between the origins of the anterior inferior cerebellar artery and the superior cerebellar artery. BVF studies of the EC–IC bypasses were done within the straight segment of the distal STA closest to the STA–MCA anastomosis (Fig. 1). EC–IC bypass measurement localizations were performed using extensive plan scanning in various directions, including the results of 3D time-of-flight (TOF) MR angiography data sets with maximum-intensity projection (MIP) reconstruction of the EC–IC bypasses (Fig. 1).
Evaluation of BVF in a right-sided extracranial–intracranial (EC–IC) arterial bypass. Digital subtraction angiography (DSA) images in lateral a and frontal projection b and radiograph image c of a right-sided EC–IC bypass filling the right MCA territory. Corresponding time-of-flight MRA of the same patient shows right-sided STA–MCA anastomosis with EC–IC bypass filling the right MCA territory (d, e). 2D cine phase-contrast BVF measurement was performed in the straight portion of the superficial temporal artery (STA, frontal branch) (d, arrow). Single section of the 3D MRA data set f, amplitude image g, and single 2D phase-contrast image h, demonstrating BVF measurement plane, placed perpendicularly to the flow in STA branch to be measured (arrows). EC–IC bypass is demonstrated with arrows. Blood flow velocity in systolic-diastolic modulation i in this bypass resulting from dynamic 2D cine phase-contrast MR imaging. BVF was calculated by integration over the vessel lumen [31] (STA superficial temporal artery, MCA middle cerebral artery, BVF blood volume flow)
Blood flow velocities were measured in the middle of the vessel lumen with a 1-pixel region of interest (ROI). BVF data were obtained by integration of defined ROIs over the lumen of the vessel investigated. Total BVF in patients was calculated by adding BVF via the ICAcontra and BA, preoperatively, and the ICAcontra, BA and the BY, postoperatively. In controls, calculation of mean BVF through ICA and CCA was based on the average BVF obtained for both sides. Therefore, computation of total BVF in normal subjects was done as follows: BVF=ICAleft+ICAright+BA.
Values for BVF were calculated as mean and standard deviation (mean±SEM) in ml/min. Statistical evaluation of data was based on paired t-test for normally distributed data samples.
Results
Sufficient preoperative and postoperative studies were obtained in all patients. Postoperative MR studies were carried out within 10 days post surgery, after the documentation of anastomosis patency by means of conventional cerebral angiography. Reliable reference values were acquired from all healthy volunteers.
Normal volunteers
Assessment of BVF in 16 normal subjects led to the following values: CCA: 435±47 ml/min (range: 369–523 ml/min); ICA: 247±32 ml/min (range: 190–315 ml/min) and BA: 169±12 ml/min (range: 159–189 ml/min). Thus, the calculated total mean brain BVF approached 663±49 ml/min (range: 594–734 ml/min; Fig. 2).
Preoperative measurements
BVF values in the 25 consecutive selected patients for EC–IC bypass surgery obtained within the ICAcontra reached 356±63 ml/min (range: 250–482 ml/min), whereas BA BVF was 238±71 ml/min (range: 125–370 ml/min). BVF approached 275±75 ml/min (range: 113–504 ml/min) for the CCAipsi, while studies within the CCAcontra yielded 523±86 ml/min (range: 261–706 ml/min). Despite significantly increased BVF via the BA (238±71 ml/min vs 169±12 ml/min in controls; p<0.001) and the contralateral ICAcontra (356±63 ml/min vs 247±32 ml/min in controls, p<0.001), preoperative total brain BVF (595±89 ml/min (range: 399–848 ml/min)) was significantly lower than in healthy controls (p=0.039) (Fig. 2).
Preoperative BVF values (mean and SEM; ml/min) in symptomatic patients with unilateral internal carotid artery occlusion selected for EC–IC bypass surgery (white bars) in comparison with normal controls (black bars). (ICA internal carotid artery; BA basilar artery, BVF blood volume flow) Bars indicate BVF within the arteries studied and show calculated total brain supplying BVF (t-BVF). Almost in proportion to increased BVF in the contralateral ICA and the BA, total brain-supplying BVF is decreased in selected patients with unilateral ICA occlusion in comparison with normal controls (two times ICA BVF). (**statistically significant difference between groups with p<0.05)
Postoperative measurements
Measurements of BVF within the 25 patients with angiographically patent EC–IC anastomosis reached a mean bypass flow of 84±32 ml/min, ranging from 14–177 ml/min (Fig. 3). There was no significant correlation between total preoperative BVF and the BVF via the new established anastomosis (r2=0.00964 ; p=0.641). BVF within the contralateral ICAcontra (352±65 ml/min; range: 220–490 ml/min), the BA (237±77 ml/min; range: 124–387 ml/min and the contralateral CCAcontra (520±87 ml/min; range: 265–699 ml/min) remained unchanged after surgery, whereas CCAipsi BVF (336±84 ml/min; range: 132–487 ml/min) was significantly increased after surgery compared with preoperative measurements (p<0.001). Total BVF following surgery was 673±102 ml/min (range: 478–954 ml/min), yielding a net increase in total BVF of 78±43 ml/min, extending between 7 ml/min and 136 ml/min, (p<0.001; Fig. 4). Consequently, postoperative total BVF reached the BVF values of healthy volunteers, suggesting restoration of cerebral blood supply by EC–IC bypass surgery.
BVF (mean and SEM; ml/min) in established EC–IC anastomosis in 25 patients; open circle individual BVF values; bold horizontal line mean BVF in patient group; box plot shows 25th percentile, median and 75th percentile (horizontal lines), error bars represent standard deviation (BVF blood volume flow)
Changes in total brain blood volume flow (t-BVF, mean and SEM; ml/min) after EC–IC bypass surgery; stacked bars show preoperative (PRE-OP) and postoperative (POST-OP) BVF in arteries studied (ICA internal carotid artery; BA basilar artery, BY EC–IC bypass; **statistically significant increase in calculated total BVF after surgery with p<0.05)
Discussion
In the present study we have characterized and determined the direct effects of standard EC–IC (STA–MCA) arterial bypass surgery on BVF in patients with hemodynamic impairment due to unilateral ICA occlusion. The principle findings of the study are:
-
1.
Preoperatively, total BVF was reduced in the selected, symptomatic EC–IC bypass surgery patients with unilateral ICA occlusion, compared with healthy controls
-
2.
An extracranial–intracranial arterial bypass does not lead to a significant decrease in BVF in the BA and contralateral ICA
-
3.
Based on the above, an improvement in total brain BVF can be achieved by surgical revascularization
The cerebral perfusion in patients with occlusive cerebrovascular disease depends, critically, upon the extent of intracranial collateral pathways, which guarantee sufficient supply of blood within the dependent vascular territory distal to the occlusion [28]. Thus, insufficient cerebral collateralization leads to a decrease in cerebral perfusion pressure. This is well-compensated by vasodilation maintaining rCBF in most patients with ICA occlusion. However, in some patients clinical symptoms occur, indicating exhaustion of cerebral compensatory mechanisms. This is due to either an increased demand or to further reduction in cerebral perfusion pressure. This condition, however, cannot be sufficiently predicted by studying the pattern of cerebral blood distribution by means of cerebral angiography alone [29]. Thus, in addition to clinical diagnosis and angiography, characterization of hemodynamic impairment requires functional blood flow studies.
Dynamic 2D cine phase-contrast MR techniques, which allow the quantitative measurement of BVF in cerebral vessels, represent established techniques with a noninvasive approach to the assessment of cerebral hemodynamics [30, 31]. Using this technique, we were able to demonstrate a significant reduction in total BVF in the patient group selected for EC–IC bypass surgery prior to surgery in comparison with controls, despite a compensatory increase in ICAcontra and BA blood flow. This finding was indicative of inadequate functional collateral blood supply after ICA occlusion, resulting in hemodynamic impairment. In contrast to the present study, earlier investigation [31] failed to demonstrate a significant reduction in total BVF in patients with unilateral ICA occlusion. In that previous study, however, patient selection was based on clinical and angiographic findings only, not assuring the cerebral perfusion status, which may explain these different results. From our point of view, the assessment of BVF represents a novel diagnostic tool, which, in line with clinical signs, symptoms, angiographic findings and MR spectroscopy [30] might help to identify patients with hemodynamic compromise. However, in order to prove this assumption, further comparative investigations have to be performed focusing on the predictability of rCBF and CVRC by means of BVF measurements in patients with occlusive cerebrovascular disease.
Since local cerebral compensatory mechanisms are exhausted in symptomatic carotid occlusion due to hemodynamic insufficiency, any additional blood supply to the hemodynamically impaired vascular territory should increase cerebral perfusion pressure and, therefore, improve the cerebral hemodynamic state [24]. If this holds true, extracranial–intracranial arterial bypass surgery should lead to an increase in total BVF, reaching volume flows close to those of normal controls. In the present study, standard STA–MCA bypass surgery appeared to be effective for this indication. EC–IC bypass surgery led to a marked increase in total BVF the first days after operation, where the net improvement in BVF of approximately 80 ml/min was close to the flow values obtained within the bypass (84±32 ml/ min). Thus, despite a tendency towards a decreased BVF via the BA and ICAcontra after surgery (Fig. 4), simple redistribution of BVF within the brain-supplying arteries, which had been previously suggested by PET studies [20], did not occur. Our results, rather, support the results of studies that showed an increase in rCBF and an improvement in CVRC and, therefore, cerebral perfusion pressure after EC–IC bypass surgery [22, 23, 24]. In an earlier study [31], a reduction of BVF within both MCAs was found in symptomatic patients with unilateral carotid occlusion, which might be indicative of potential redistribution of blood within the basal collateral system after surgery. Preoperatively in our patients, rCBF and CVRC were within normal limits inside the vascular territory contralateral to the occluded ICA. Consequently, a significant reduction in BVF within the related MCA appears to be unlikely. We therefore believe that the observed net increase in BVF after bypass surgery almost exclusively contributes to the restoration of rCBF and CVRC via an increase in cerebral perfusion pressure within the hemodynamically compromised vascular territory.
In summary, the present study demonstrates, from a purely hemodynamic point of view, that a substantial improvement in total brain blood supply can be achieved with standard STA–MCA arterial bypass surgery in selected symptomatic patients with occlusive cerebrovascular disease. Thus, bypass procedures are suitable for the restoration of regional cerebral blood supply for this indication. However, follow-up investigations of BVF have to be applied in line with functional blood flow studies and clinical examinations, in order to prove the long-term efficacy of extracranial–intracranial arterial bypass surgery in the treatment of hemodynamic compromise in occlusive cerebrovascular disease. New clinical trials have been proposed [32] and must be evaluated for the overall usefulness of EC–IC arterial or venous bypass surgery for the treatment of carefully selected patients with internal carotid artery occlusion.
References
Powers WJ, Derdeyn CP, Fritsch SM, Carpenter DA, Yundt KD, Videen TO, Grubb RL Jr (2000) Benign prognosis of never-symptomatic carotid occlusion. Neurology 54:878–882
Grubb RL Jr, Derdeyn CP, Fritsch SM, Carpenter DA, Yundt KD, Videen TO, Spitznagel EL, Powers WJ (1998) Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion [see comments]. JAMA 280:1055–1060
Derdeyn CP, Yundt KD, Videen TO, Carpenter DA, Grubb RL, Jr, Powers WJ (1998) Increased oxygen extraction fraction is associated with prior ischemic events in patients with carotid occlusion. Stroke 29:754–758
Yamauchi H, Fukuyama H, Nagahama Y, Nabatame H, Ueno M, Nishizawa S, Konishi J, Shio H (1999) Significance of increased oxygen extraction fraction in five-year prognosis of major cerebral arterial occlusive diseases. J Nucl Med 40:1992–1998
Yonas H, Smith HA, Durham SR, Pentheny SL, Johnson DW (1993) Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg 79:483–489
Ishikawa T, Houkin K, Abe H, Isobe M, Kamiyama H (1995) Cerebral haemodynamics and long-term prognosis after extracranial-intracranial bypass surgery. J Neurol Neurosurg Psychiatry 59:625–628
Klijn CJ, Kappelle LJ, Tulleken CA, van Gijn J (1997) Symptomatic carotid artery occlusion. A reappraisal of hemodynamic factors [see comments]. Stroke 28:2084–2093
Piepgras A, Leinsinger G, Kirsch CM, Schmiedek P (1994) STA-MCA bypass in bilateral carotid artery occlusion: clinical results and long-term effect on cerebrovascular reserve capacity. Neurol Res 16:104–107
Tsuchida C, Kimura H, Sadato N, Tsuchida T, Tokuriki Y, Yonekura Y (2000) Evaluation of brain metabolism in steno-occlusive carotid artery disease by proton MR spectroscopy: a correlative study with oxygen metabolism by PET [see comments]. J Nucl Med 41:1357–1362
van der Grond J, Balm R, Kappelle LJ, Eikelboom BC, Mali WP (1995) Cerebral metabolism of patients with stenosis or occlusion of the internal carotid artery. A 1H-MR spectroscopic imaging study. Stroke 26:822–828
The EC-IC bypass study group (1985) Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. N Engl J Med 313:1191–1200
Ausman JI, Diaz FG (1986) Critique of the extracranial-intracranial bypass study. Surg Neurol 26:218–222
Day LD, Rhoton AL, Little JR (1986) The extracranial-intracranial bypass study. Surg Neurol 26:222–226
Gilsbach JM (1983) Intraoperative Doppler sonography in neurosurgery. Springer, Berlin Heidelberg New York
Spetzler RF, Chater N (1976) Microvascular bypass surgery. Part 2: Physiological studies. J Neurosurg 45:508–513
Sekhar L, Kalavakonda C (1999) Saphenous vein and radial artery grafts in the management of skull base tumors and aneurysms. In: Spetzler RF, Schmiedek P (eds) Operative techniques in neurosurgery, extra-intracranial bypass surgery, vol 2. Saunders, Philadelphia, pp 129–141
van der Zwan A, Tulleken CA, Hillen B (2001) Flow quantification of the non-occlusive excimer laser-assisted EC-IC bypass. Acta Neurochir (Wien) 143:647–654
Takagi Y, Hashimoto N, Iwama T, Hayashida K (1997) Improvement of oxygen metabolic reserve after extracranial-intracranial bypass surgery in patients with severe haemodynamic insufficiency. Acta Neurochir (Wien) 139:52–56; discussion 56–57
Gibbs JM, Wise RJ, Thomas DJ, Mansfield AO, Russell RW (1987) Cerebral haemodynamic changes after extracranial-intracranial bypass surgery. J Neurol Neurosurg Psychiatry 50:140–150
Powers WJ, Martin WR, Herscovitch P, Raichle ME, Grubb RL Jr (1984) Extracranial-intracranial bypass surgery: hemodynamic and metabolic effects. Neurology 34:1168–1174
Kobayashi H, Kitai R, Ido K, Kabuto M, Handa Y, Kubota T, Yonekura Y (1999) Hemodynamic and metabolic changes following cerebral revascularization in patients with cerebral occlusive diseases. Neurol Res 21:153–160
Yamashita T, Kashiwagi S, Nakano S, Takasago T, Abiko S, Shiroyama Y, Hayashi M, Ito H (1991) The effect of EC-IC bypass surgery on resting cerebral blood flow and cerebrovascular reserve capacity studied with stable XE-CT and acetazolamide test. Neuroradiology 33:217–222
Holzschuh M, Brawanski A, Ullrich W, Meixensberger J (1991) Cerebral blood flow and cerebrovascular reserve 5 years after EC-IC bypass. Neurosurg Rev 14:275–278
Schmiedek P, Piepgras A, Leinsinger G, Kirsch CM, Einheupl K (1994) Improvement of cerebrovascular reserve capacity by EC-IC arterial bypass surgery in patients with ICA occlusion and hemodynamic cerebral ischemia. J Neurosurg 81:236–244
Vorstrup S, Brun B, Lassen NA (1986) Evaluation of the cerebral vasodilatory capacity by the acetazolamide test before EC-IC bypass surgery in patients with occlusion of the internal carotid artery. Stroke 17:1291–1298
Yasargil MG (1967) Experimental small vessel surgery in the dog including patching and grafting of cerebral vessels and the formation of functional extra-intracranial shunts. In: Donaghy RMP, Yasargil MG (eds) Micro-vascular surgery. Georg Thieme Verlag, Stuttgart, Germany, pp 87–126
Vajkoczy P, Horn P, Schmiedek P (1999) Standard superficial temporal artery–middle cerebral artery bypass surgery in hemodynamic cerebral ischemia: indication and technique. Oper Techn Neurosurg 2:106–116
Macchi C, Catini C, Federico C, Gulisano M, Pacini P, Cecchi F, Corcos L, Brizzi E (1996) Magnetic resonance angiographic evaluation of circulus arteriosus cerebri (circle of Willis): a morphologic study in 100 human healthy subjects. Ital J Anat Embryol 101:115–123
Derdeyn CP, Shaibani A, Moran CJ, Cross DT 3rd, Grubb RL Jr, Powers WJ (1999) Lack of correlation between pattern of collateralization and misery perfusion in patients with carotid occlusion. Stroke 30:1025–1032
Klijn CJ, Kappelle LJ, van Der Grond J, Algra A, Tulleken CA, van Gijn J (2000) Magnetic resonance techniques for the identification of patients with symptomatic carotid artery occlusion at high risk of cerebral ischemic events. Stroke 31:3001–3007
van Everdingen KJ, Klijn CJ, Kappelle LJ, Mali WP, van der Grond J (1997) MRA flow quantification in patients with a symptomatic internal carotid artery occlusion. The Dutch EC-IC Bypass Study Group. Stroke 28:1595–1600
Adams HP Jr, Powers WJ, Grubb RL Jr, Clarke WR, Woolson RF (2001) Preview of a new trial of extracranial-to-intracranial arterial anastomosis: the carotid occlusion surgery study. Neurosurg Clin N Am 12:613–624
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neff, K.W., Horn, P., Dinter, D. et al. Extracranial–intracranial arterial bypass surgery improves total brain blood supply in selected symptomatic patients with unilateral internal carotid artery occlusion and insufficient collateralization. Neuroradiology 46, 730–737 (2004). https://doi.org/10.1007/s00234-004-1252-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-004-1252-9